Assessment of Chokeberry Powders Quality Obtained Using an Innovative Fluidized-Bed Jet Milling and Drying Method with Pre-Drying Compared with Convection Drying
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Water Activity (aw)
2.2.2. Dry Matter Content/Moisture Content
2.2.3. Particle Classification
2.2.4. Preparation of Extracts for Analysis of Antioxidant Properties and Total Polyphenol Content
2.2.5. Determination of Antioxidant Properties
2.2.6. Total Polyphenol Content
2.2.7. Content and Separation of Individual Polyphenolic Compounds
2.2.8. Vitamin C
2.2.9. Microbiology
2.2.10. Sensory Analysis
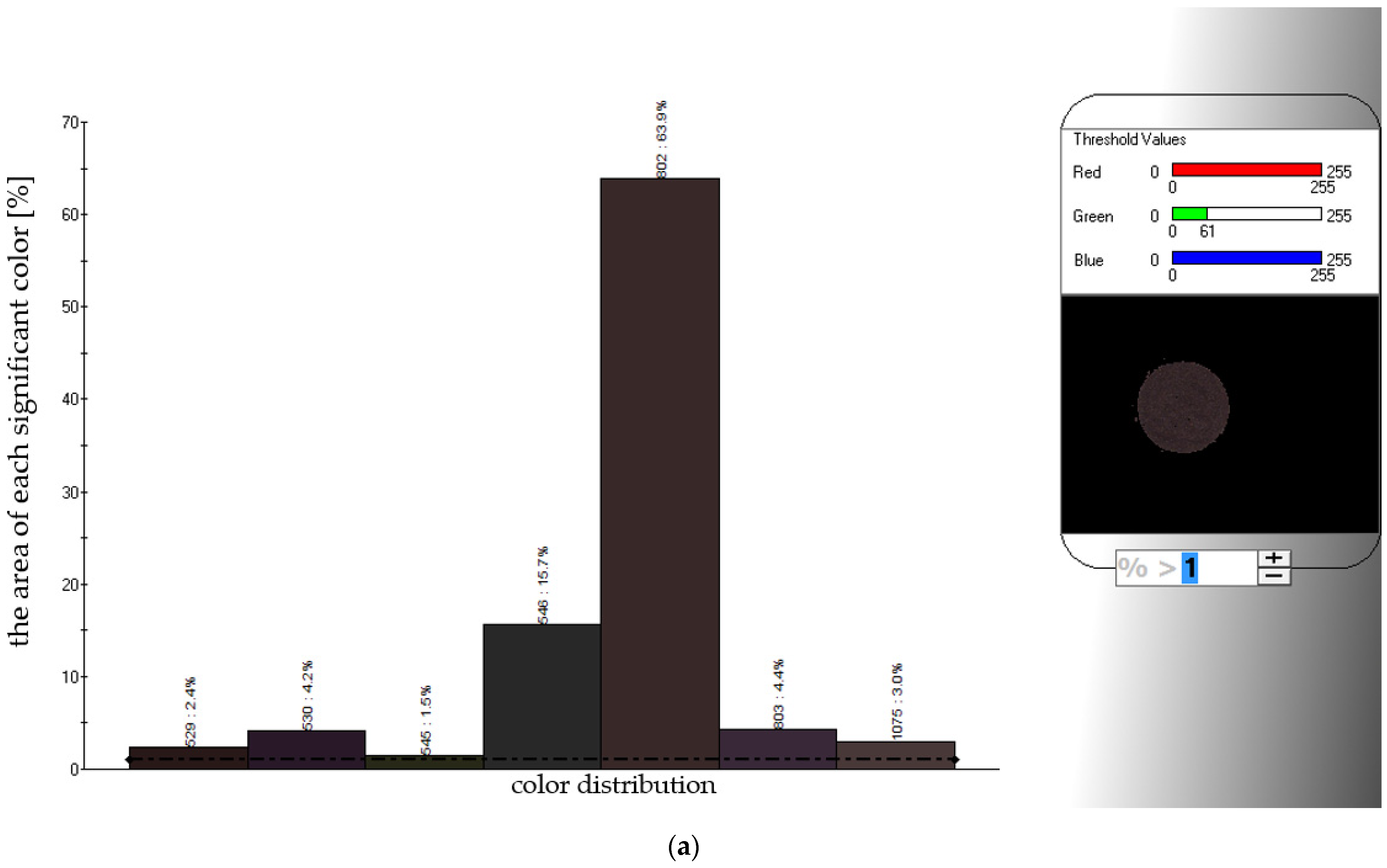
2.2.11. Instrumental Color Measurement
2.2.12. Statistical Analysis
3. Results and Discussion
3.1. Comparison of the Microbiological Quality and Bioactive Compounds’ Content in Chokeberry Powders, Obtained by the FBJD Method, with Fruit Pre-Drying at Different Temperatures
3.1.1. Microbiology Quality of FBJD Powders Obtained from Fruits Subjected to Pre-Drying at Different Temperatures
3.1.2. The Content of Bioactive Compounds in Raw Chokeberry and in Chokeberry FBJD Powders Obtained from Fruits Subjected to Pre-Drying at Different Temperatures
3.2. Comparison of Powders Obtained by the FBJD Method with Pre-Drying of Fruits at 70 °C with those Obtained by Drying Chokeberry Fruits Using CD
3.2.1. Comparing the Sensory Quality of CD Powders Prepared by Drying Fruits to Different aw and Moisture Content
3.2.2. Comparing the Content of Bioactive Compounds, Sensory Quality, and Color of Pre-Dried Powders (at 70 °C) Obtained by the FBJD Method with the Powders Obtained by the CD Method at 70 °C
3.2.3. Comparing Sensory Quality and Color of CD and FBJD Powders
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, antioxidant efficacies, and health effects—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, A.; Świderski, F.; Rakowska, R.; Hallmann, E. The functional properties of chokeberry and kale powders obtained by an innovative method of fluidised-bed jet milling with drying compared to freeze drying. Int. J. Food Eng. 2017, 13. [Google Scholar] [CrossRef]
- Regulation no 1169/2011 of the European Parliament and of the Council (UE) of 25 October 2011 on the Provision of Food Information to Consumers. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32011R1169 (accessed on 1 December 2020).
- Piotrowska-Kempisty, H.; Nowicki, M.; Jodynis-Liebert, J.; Kurpik, M.; Ewertowska, M.; Adamska, T.; Oszmiański, J.; Kujawska, M. Assessment of Hepatoprotective Effect of Chokeberry Juice in Rats Treated Chronically with Carbon Tetrachloride. Molecules 2020, 25, 1268. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Wiloch, M.; Zawada, K.; Cyplik, W.; Kujawski, W. Evaluation of Antioxidant and Anti-Inflammatory Activity of Anthocyanin-Rich Water-Soluble Aronia Dry Extracts. Molecules 2020, 25, 4055. [Google Scholar] [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef]
- Sadowska, A.; Świderski, F.; Rakowska, R.; Hallmann, E. Comparison of quality and microstructure of chokeberry powders prepared by different drying methods, including innovative fluidised bed jet milling and drying. Food Sci. Biotechnol. 2019, 28, 1073–1081. [Google Scholar] [CrossRef]
- Si, X.; Chen, Q.; Bi, J.; Wu, X.; Yi, J.; Zhou, L.; Li, Z. Comparison of different drying methods on the physical properties, bioactive compounds and antioxidant activity of raspberry powders. J. Sci. Food Agric. 2016, 96, 2055–2062. [Google Scholar] [CrossRef]
- Sadowska, A.; Rakowska, R.; Świderski, F.; Kulik, K.; Hallmann, E. Properties and microstructure of blackcurrant powders prepared using a new method of fluidized-bed jet milling and drying versus other drying methods. CyTA J. Food 2019, 17, 439–446. [Google Scholar] [CrossRef]
- Samoticha, J.; Wojdyło, A.; Lech, K. The influence of different the drying methods on chemical composition and antioxidant activity in chokeberries. LWT Food Sci. Technol. 2016, 66, 484–489. [Google Scholar] [CrossRef]
- Lopez-Nicolas, J.M.; Garcıa-Carmona, F. Enzymatic and Nonenzymatic Degradation of Polyphenols. In Fruit and Vegetable Phytochemicals Chemistry, Nutritional Value, and Stability; Rosa, L.A., Alvarez-Parrilla, E., González-Aguilar, G.A., Eds.; Blackwell Publishing: Hoboken, NJ, USA, 2010; pp. 101–129. [Google Scholar] [CrossRef]
- Ratti, C. Hot air anf freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Reyes, A.; Vega, R.; García, G. Drying sawdust in a pulsed fluidized bed. Dry. Technol. 2008, 26, 476–486. [Google Scholar] [CrossRef]
- Beaudry, C.; Raghavan, G.S.V.; Rennie, T.J. Microwave finish drying of osmotically dehydrated cranberries. Dry. Technol. 2003, 21, 1797–1810. [Google Scholar] [CrossRef]
- Soysal, Y.; Oztekin, S.; Eren, O. Microwave drying of parsley: Modelling, kinetics, and energy aspects. Biosyst. Eng. 2006, 93, 403–413. [Google Scholar] [CrossRef]
- AOAC. Available online: www.aoac.org (accessed on 10 December 2020).
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Hallmann, E.; Kazimierczak, R.; Marszałek, K.; Drela, N.; Kiernozek, E.; Toomik, P.; Matt, D.; Luik, A.; Rembiałkowska, E. The nutritive value of organic and conventional white cabbage (Brassica oleracea L. var.capitata) and anti-apoptotic activity in gastric adenocarcinoma cells of sauerkraut juice produced therof. J. Agric. Food Chem. 2017, 65, 8171–8183. [Google Scholar] [CrossRef]
- PN-EN ISO 4833-1:2013-12. Food Chain Microbiology. Horizontal Method for the Determination of the Number of Microorganisms. Part 1: Enumeration by Flooding Method at 30 Degrees C; ISO: Geneva, Switzerland, 2013. [Google Scholar]
- PN-ISO 21527–2:2009. Microbiology of Food and Feed. Horizontal Method for the Enumeration of Yeasts and Molds. Part 2: Method of Counting Colonies in Products with a Water Activity Less than or Equal to 0,95; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- ISO PN-EN ISO 13299:2016-05. Sensory Analysis-Methodology-General Guidelines for Determining the Sensory Profile; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- BS EN ISO 8589:2010. Sensory Analysis. General Guidance for the Design of Test Rooms; ISO: Geneva, Switzerland, 2010. [Google Scholar]
- Charpe, A.M.; Sedani, S.; Murumkar, R.; Bhad, R.G. Effect of temperature on microbial growth in food during storage. Multilogic Sci. 2019, 8, 56–58. [Google Scholar]
- Witthuhn, R.; Engelbrecht, S.; Joubert, E.; Britz, T. Microbial content of commercial South African high-moisture dried fruits. J. Appl. Microbiol. 2005, 98, 722–726. [Google Scholar] [CrossRef]
- Glowacz, M.; Colgan, R.; Rees, D. The use of ozone to extend the shelf-life and maintain quality of fresh produce. J. Sci. Food Agric. 2015, 95, 662–671. [Google Scholar] [CrossRef]
- Arancibia-Avila, P.; Namiesnik, J.; Toledo, F.; Werner, E.; Martinez-Ayala, A.L.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Gorinstein, S. The influence of different time durations of thermal processing on berries quality. Food Control 2012, 26, 587–593. [Google Scholar] [CrossRef]
- Sanos, P.S.; Silva, M. Retention of Vitamin C in Drying Processes of Fruits and Vegetables—A Review. Dry. Technol. 2012, 26, 1421–1437. [Google Scholar] [CrossRef]
- Bober, I.; Oszmiańki, J. Application of chokeberry pomace forinfusions of fruit teas. Acta Sci. Pol. Technol. Aliment. 2004, 3, 63–72. [Google Scholar]
- Stratil, P.; Klejdus, B.; Kubaänÿ, V. Determination of total content of phenolic compounds and their antioxidant activity in vegetablessevaluation of spectrophotometric methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Lachowicz, S. Effect of the Production of Dried Fruits and Juice from Chokeberry (Aronia melanocarpa L.) on the Content and Antioxidative Activity of Bioactive Compounds. Molecules 2016, 21, 1098. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez-Araque, R.; Rodríguez-Villacres, J.; Urresto-Villegas, J. Electronic nose, tongue and eye: Their usefulness for the food industry. Vitae 2020, 27, 3. [Google Scholar] [CrossRef]
- Konczak, I.; Zhang, W. Anthocyanins-more than nature’s colours. J. Biomed. Biotechnol. 2004, 1, 239–240. [Google Scholar] [CrossRef]
- Coimbra, M.A.; Nunes, C.; Cunha, P.R.; Guine, R. Amino acid profile and Maillard compounds of sun-dried pears. Relation with the reddish brown colour of the dried fruits. Eur. Food Res. Technol. 2011, 233, 637–646. [Google Scholar] [CrossRef]
- Piga, A.; Del Caro, A.; Corda, G. From plums to prunes: Influence of drying parameters on polyphenols and antioxidant activity. J Agric. Food Chem. 2003, 51, 3675–3681. [Google Scholar] [CrossRef]
- ANHTI02—Instrumental Sensory Benchmarking Application to Sausages. Available online: https://www.norlab.com/library/application-note/11578 (accessed on 13 January 2021).
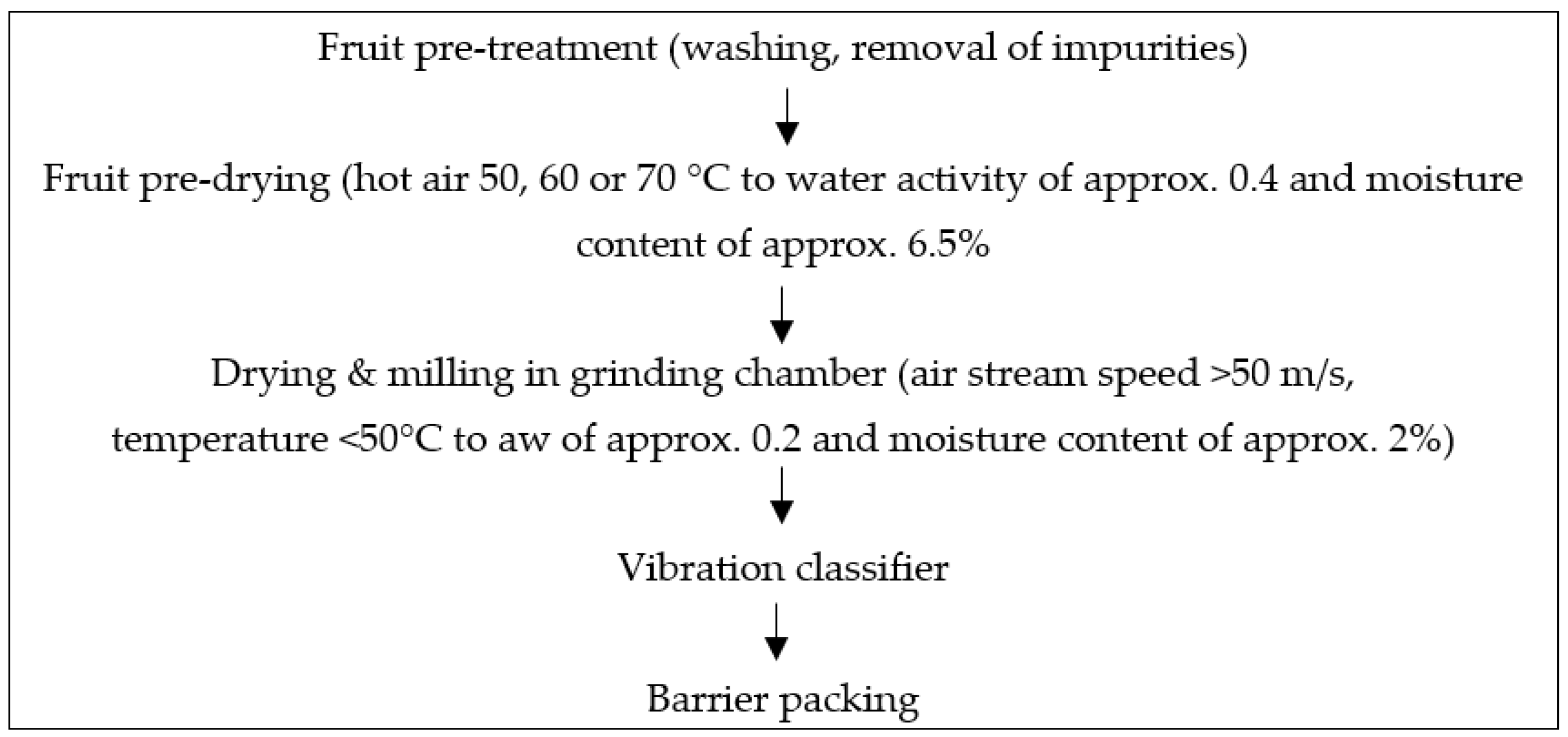




| Pre-Drying Temperatures | 50 °C | 60 °C | 70 °C | ||
|---|---|---|---|---|---|
| Fruits pre-dried using CD method | water activity | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | |
| moisture content (%) | 6.5 ± 0.1 | 6.4 ± 0.1 | 6.5 ± 0.0 | ||
| pre-drying time (h) | 12 | 10 | 8 | ||
| Powders obtained by the FBJD method after pre-drying the fruits using CD method | water activity | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | |
| moisture content (%) | 2.2 ± 0.0 | 2.2 ±0.0 | 2.2 ± 0.1 | ||
| drying and milling time FJBD (min) | 10 | 10 | 10 | ||
| the mesh size of the screens (%) | 630–315 µm | 0 | 0 | 0 | |
| 315–100 µm | 86.7 | 87.4 | 79.5 | ||
| <100 µm | 14.1 | 13.9 | 21.2 | ||
| Drying temperature | 70 °C | ||||
| Powders obtained by the CD method | water activity | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | |
| moisture content (%) | 2.9 ± 0.1 | 3.8 ± 0.0 | 6.3 ± 0.0 | ||
| drying time (h) | 28 | 20 | 16 | ||
| the mesh size of the screens (%) | 630–315 µm | 11.6 | 22.1 | 30.5 | |
| 315–100 µm | 65.0 | 58.4 | 55.2 | ||
| <100 µm | 24.3 | 20.8 | 15.1 | ||
| Samples | Microorganisms (CFU g−1) | Dried Samples Pre-Drying Temp. | Raw Sample | ||
|---|---|---|---|---|---|
| 50 °C | 60 °C | 70 °C | |||
| Fruits raw and pre-dried to water activity of 0.4 using CD method | total number of colonies | 1.0 × 103 | 1.4 × 103 | 4.8 × 102 | 1.1 × 106 |
| yeast | 4.0 × 102 | <10 | <10 | 3.0 × 104 | |
| mould | 5.0 × 10 | <10 | <10 | 2.0 × 103 | |
| Powders obtained by the FBJD method after pre-drying the fruit to water activity of 0.4 | total number of colonies | 1.9 × 104 | 2.6 × 104 | 2.4 × 103 | |
| yeast | 2.2 × 103 | 1.0 × 102 | 50 | ||
| mould | 7.5 × 102 | 80 | 41 | ||
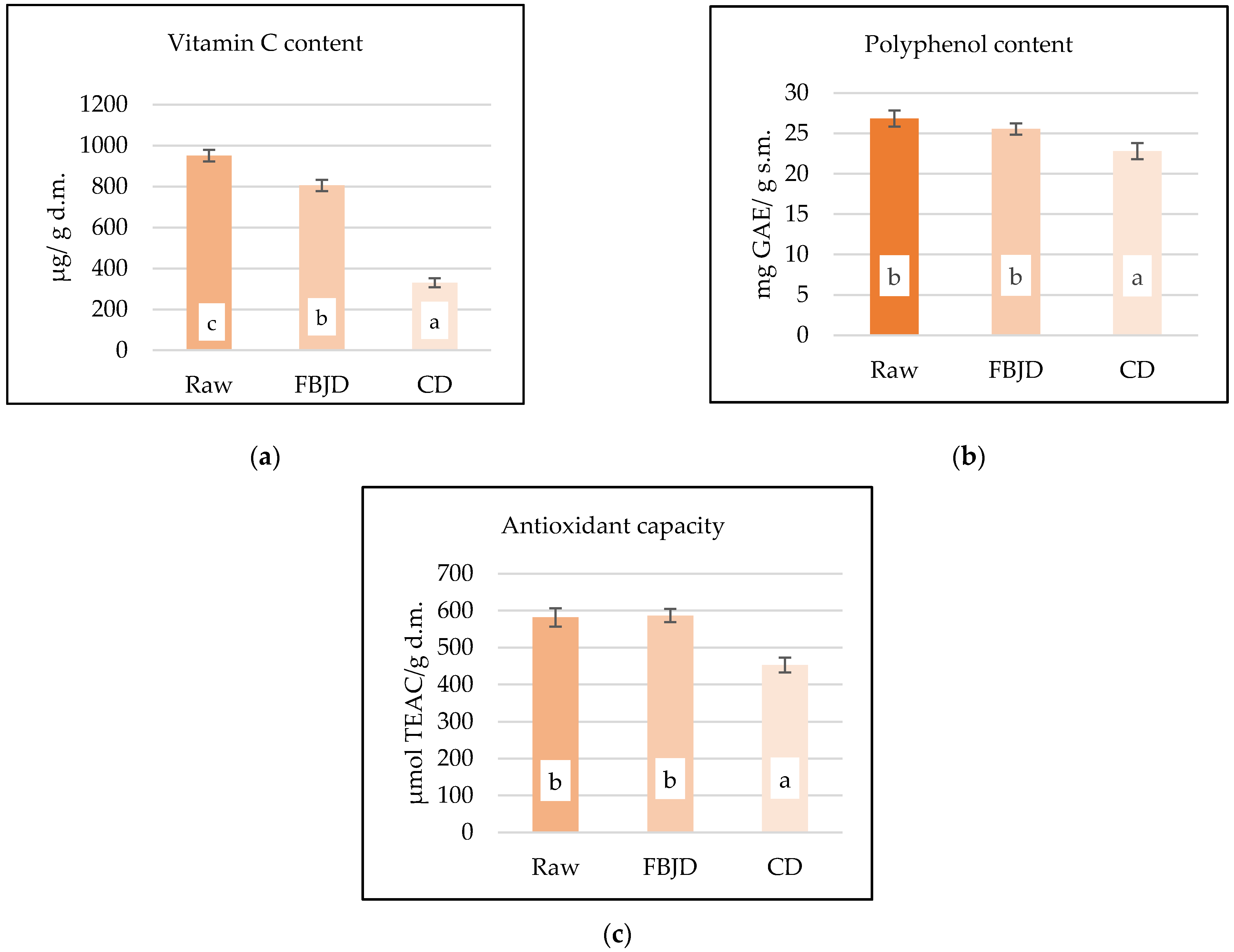
| Bioactive Compounds/ Antioxidant Properties | Dried Samples Pre-Drying Temperatures | Raw Sample | |||
|---|---|---|---|---|---|
| 50 °C | 60 °C | 70 °C | |||
| Vitamin C content | µg/g d.m. | 298.1 ± 40.6 a | 505.2 ± 71.7 b | 805.6 ± 80.3 c | 950.6 ± 95.7 d |
| Polyphenols content | mg GAE/g d.m. | 19.6 ± 1.1 a | 23.0 ± 1.2 b | 25.6 ± 0.7 b | 26.8 ± 0.8 b |
| Antioxidant properties | µM TEAC/g d.m. | 368.6 ± 13.2 a | 595.5 ± 26.2 b | 589.1 ± 13.2 b | 590.4 ± 33.1 b |
| Polyphenols | Dried Samples/Pre-Drying Temperatures | Raw Sample | ||
|---|---|---|---|---|
| 50 °C | 60 °C | 70 °C | ||
| Total polyphenols | 19.1 ± 0.2 a | 20.2 ± 0.2 b | 20.8 ± 0.1 c | 23.3 ± 0.2 d |
| Total phenolic acids | 1.6 ± 0.1 a | 2.2 ± 0.2 b | 3.0 ± 0.1 c | 4.6 ± 0.1 d |
| gallic acid | 0.1 ± 0.0 b | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 1.6 ± 0.0 c |
| chlorogenic acid | 1.2 ± 0.9 a | 1.4 ± 0.9 a | 1.3 ± 0.1 a | 1.6 ± 0.0 b |
| caffeic acid | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| p-coumaric acid | 0.0 ± 0.0 a | 0.8 ± 0.1 b | 1.7 ± 0.1 c | 1.3 ± 0.1 c |
| ferulic acid | 0.2 ± 0.0 b | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a |
| Total flavonoids | 17.5 ± 0.3 a | 18.0 ± 0.3 b | 17.7 ± 0.1 a,b | 19.4 ± 0.2 c |
| Total flavonols | 2.0 ± 0.0 a | 2.4 ± 0.3 b | 2.3 ± 0.1 a,b | 4.0 ± 0.1 c |
| epigallocatechin gallate | 1.1 ± 0.1 a | 1.3 ± 0.3 a | 1.3 ± 0.2 a | 2.7 ± 0.1 b |
| quercetin-3-O-rutinoside | 0.5 ± 0.0 a | 0.6 ± 0.0 a | 0.5 ± 0.0 a | 0.5 ± 0.0 a |
| myricetin | 0.1 ± 0.0 b | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| luteolin | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.2 ± 0.0 b |
| kaempferol | 0.0 ± 0.0 b | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.2 ± 0.0 c |
| quercetin-3-O-glucoside | 0.0 ± 0.0 a | 0.4 ± 0.0 c | 0.4 ± 0.0 b | 0.3 ± 0.0 b |
| kaempferol-3-O-glucoside | 0.0 ± 0.0 c | 0.0 ± 0.0 a | 0.0 ± 0.0 b | 0.0 ± 0.0 b |
| Total anthocyanins | 15.5 ± 0.2 a | 15.5 ± 0.2 a | 15.5 ± 0.2 a | 16.4 ± 0.8 b |
| cyanidin-3,5-di-O-glucoside | 14.4 ± 0.2 a | 14.3 ± 0.2 a | 14.2 ± 0.2 a | 14.7 ± 0.1 a |
| cyanidin-3,5-di-O-arabinoside | 1.2 ± 0.1 a | 1.3 ± 0.0 b | 1.2 ± 0.0 a,b | 1.7 ± 0.0 c |
| Color Parameters | FBJD | CD |
|---|---|---|
| L* (lightness) | 17.4 ± 0.3 b | 14.17 ± 1.0 a |
| a* (redness) | 6.5 ± 0.5 a | 7.9 ± 0.4 b |
| b* (yellowness) | 1.7 ± 0.3 a | 1.4 ± 0.1 a |
| R (redness) | 52.7 ± 0.6 b | 46.3 ± 0.6 a |
| G (greenness) | 38.7 ± 0.6 b | 31.7 ± 0.6 a |
| B (blueness) | 41.0 ± 1.0 b | 34.0 ± 1.0 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadowska, A.; Świderski, F.; Hallmann, E.; Świąder, K. Assessment of Chokeberry Powders Quality Obtained Using an Innovative Fluidized-Bed Jet Milling and Drying Method with Pre-Drying Compared with Convection Drying. Foods 2021, 10, 292. https://doi.org/10.3390/foods10020292
Sadowska A, Świderski F, Hallmann E, Świąder K. Assessment of Chokeberry Powders Quality Obtained Using an Innovative Fluidized-Bed Jet Milling and Drying Method with Pre-Drying Compared with Convection Drying. Foods. 2021; 10(2):292. https://doi.org/10.3390/foods10020292
Chicago/Turabian StyleSadowska, Anna, Franciszek Świderski, Ewelina Hallmann, and Katarzyna Świąder. 2021. "Assessment of Chokeberry Powders Quality Obtained Using an Innovative Fluidized-Bed Jet Milling and Drying Method with Pre-Drying Compared with Convection Drying" Foods 10, no. 2: 292. https://doi.org/10.3390/foods10020292
APA StyleSadowska, A., Świderski, F., Hallmann, E., & Świąder, K. (2021). Assessment of Chokeberry Powders Quality Obtained Using an Innovative Fluidized-Bed Jet Milling and Drying Method with Pre-Drying Compared with Convection Drying. Foods, 10(2), 292. https://doi.org/10.3390/foods10020292








