Volatilome and Bioaccessible Phenolics Profiles in Lab-Scale Fermented Bee Pollen
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Conditions
2.2. Fermentation of BCP
2.3. Volatilome Analysis
2.4. In Vitro Gastrointestinal Batch Digestion
2.5. Analyses of Bioaccessible Phenolics
2.6. Statistical Analysis
3. Results and Discussion
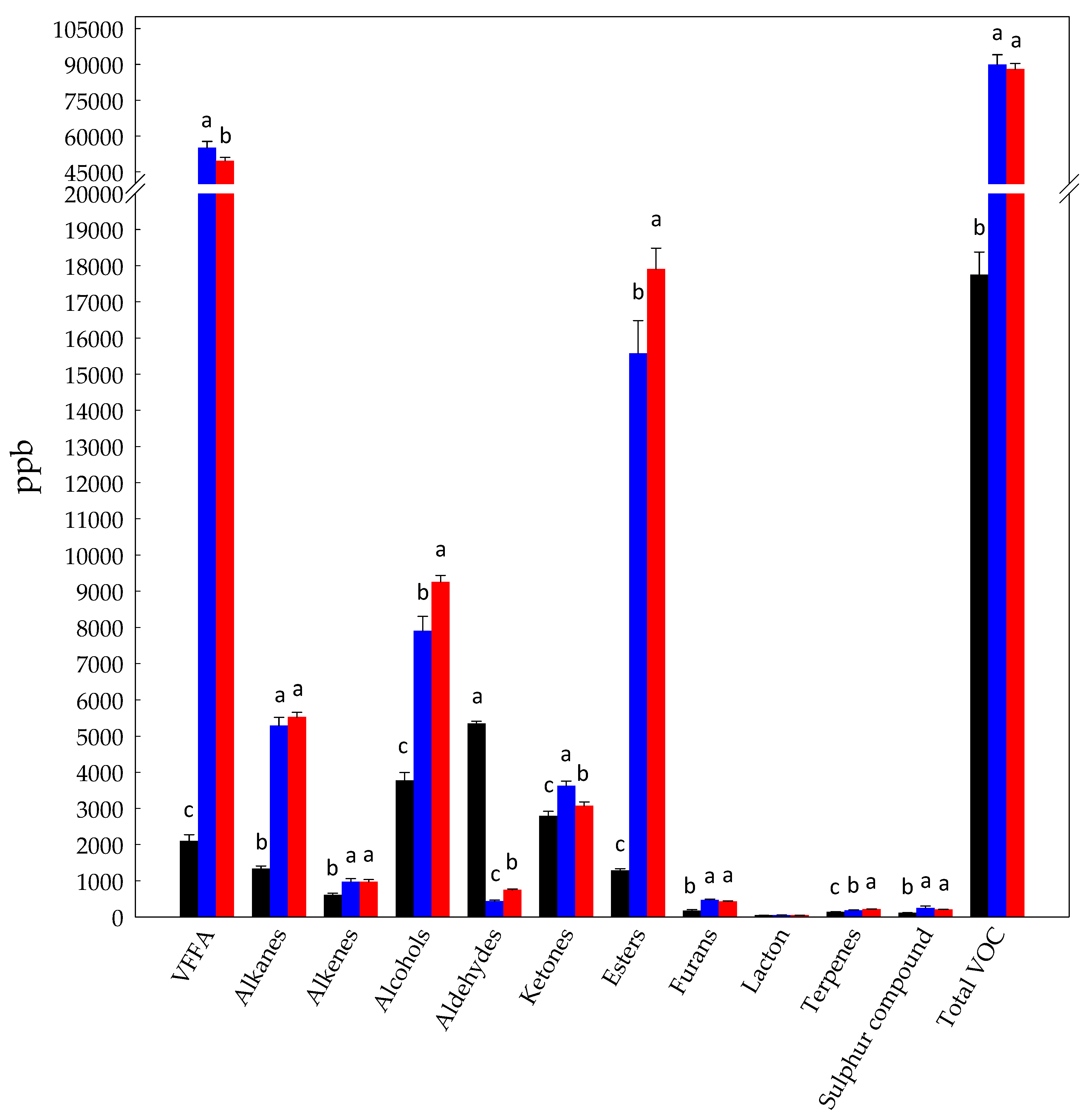
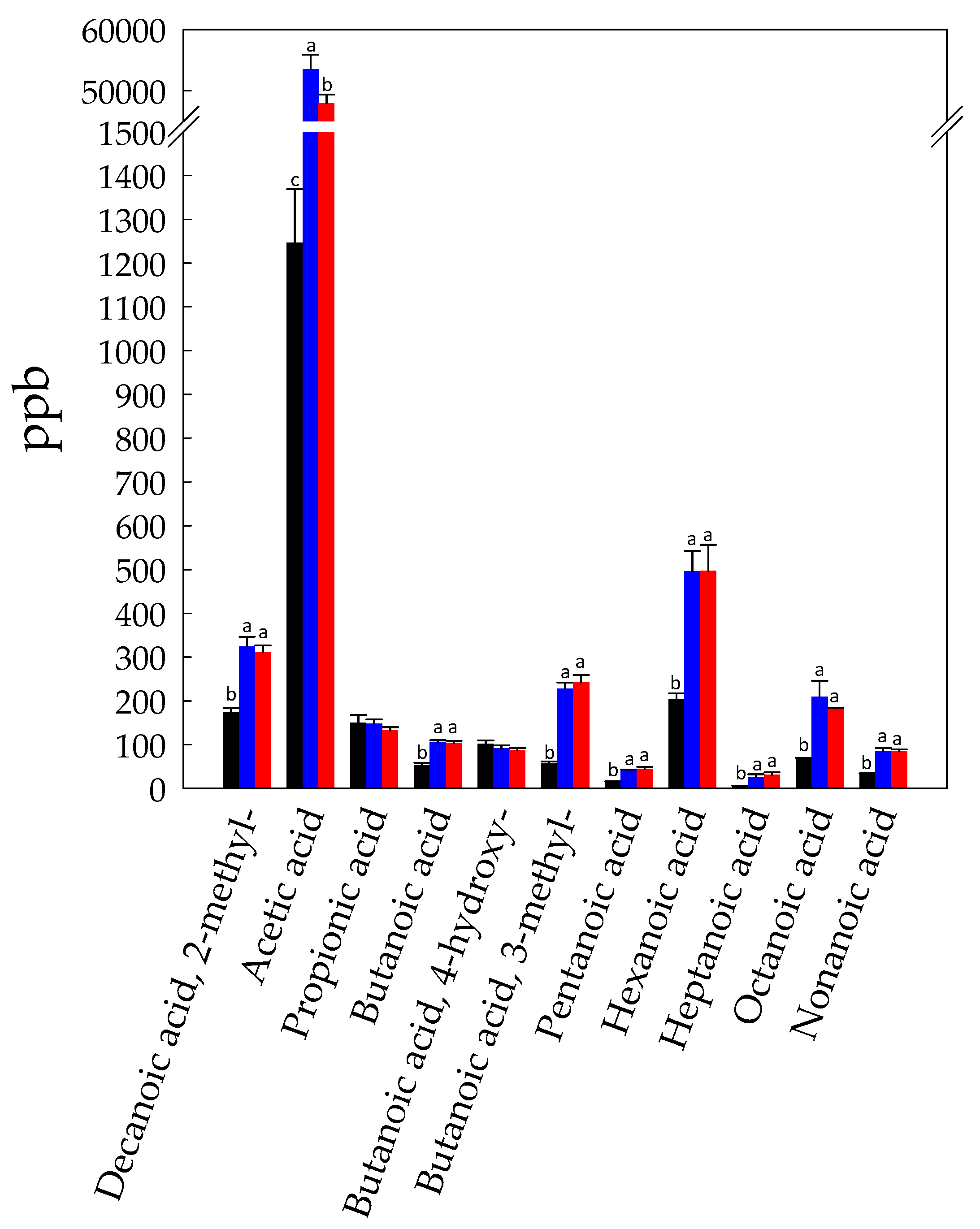
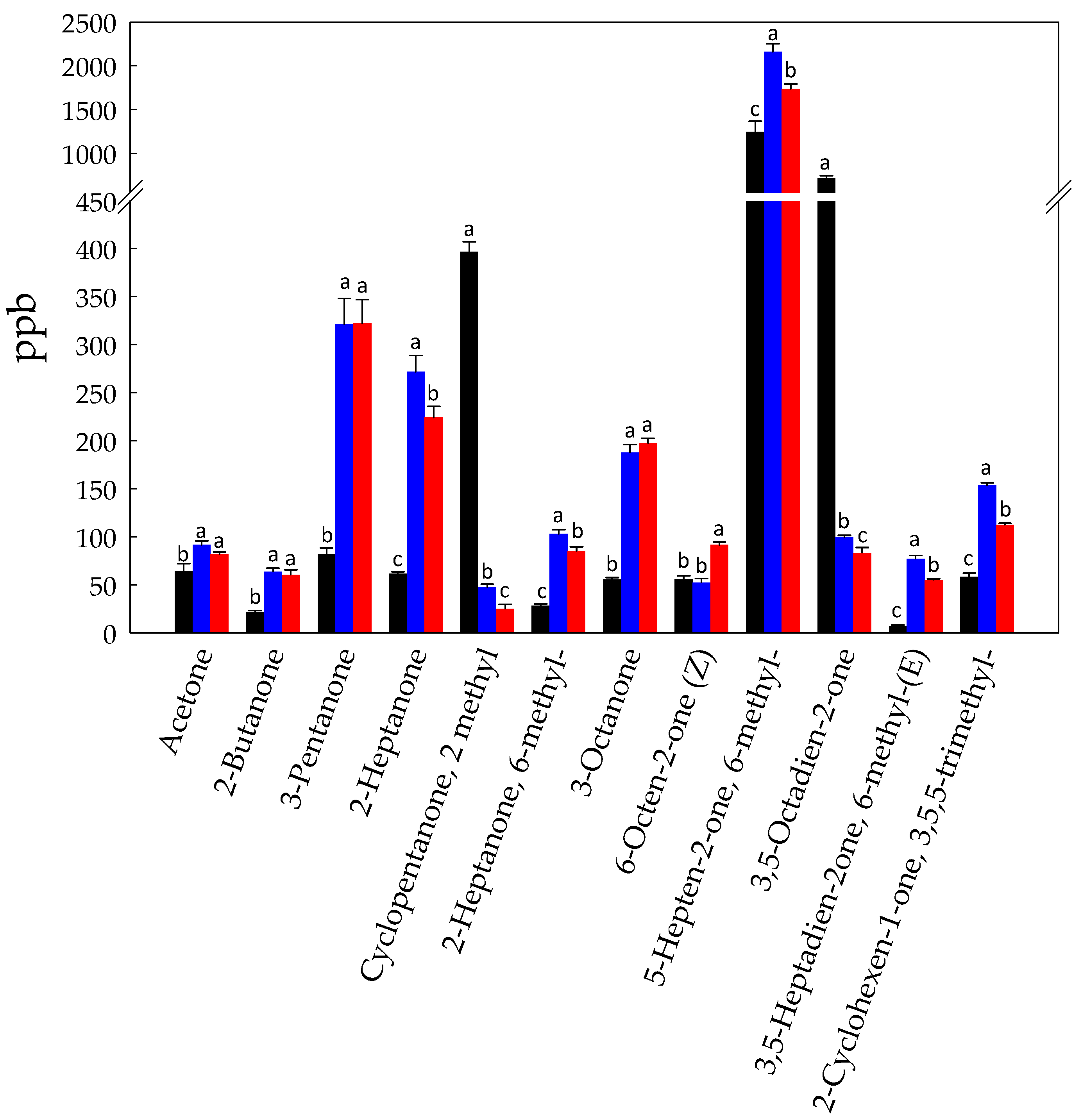
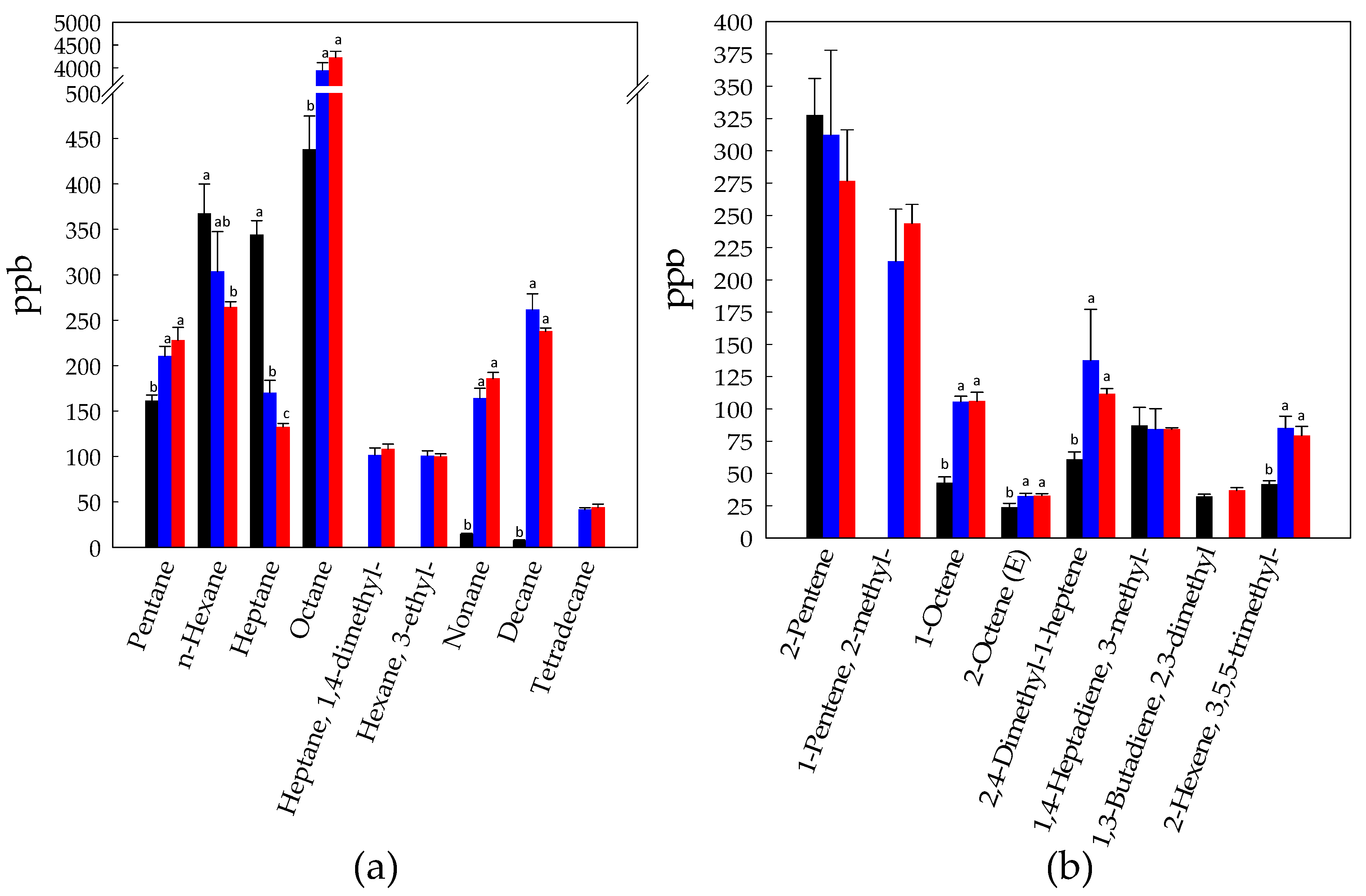
3.1. Volatilome Analysis
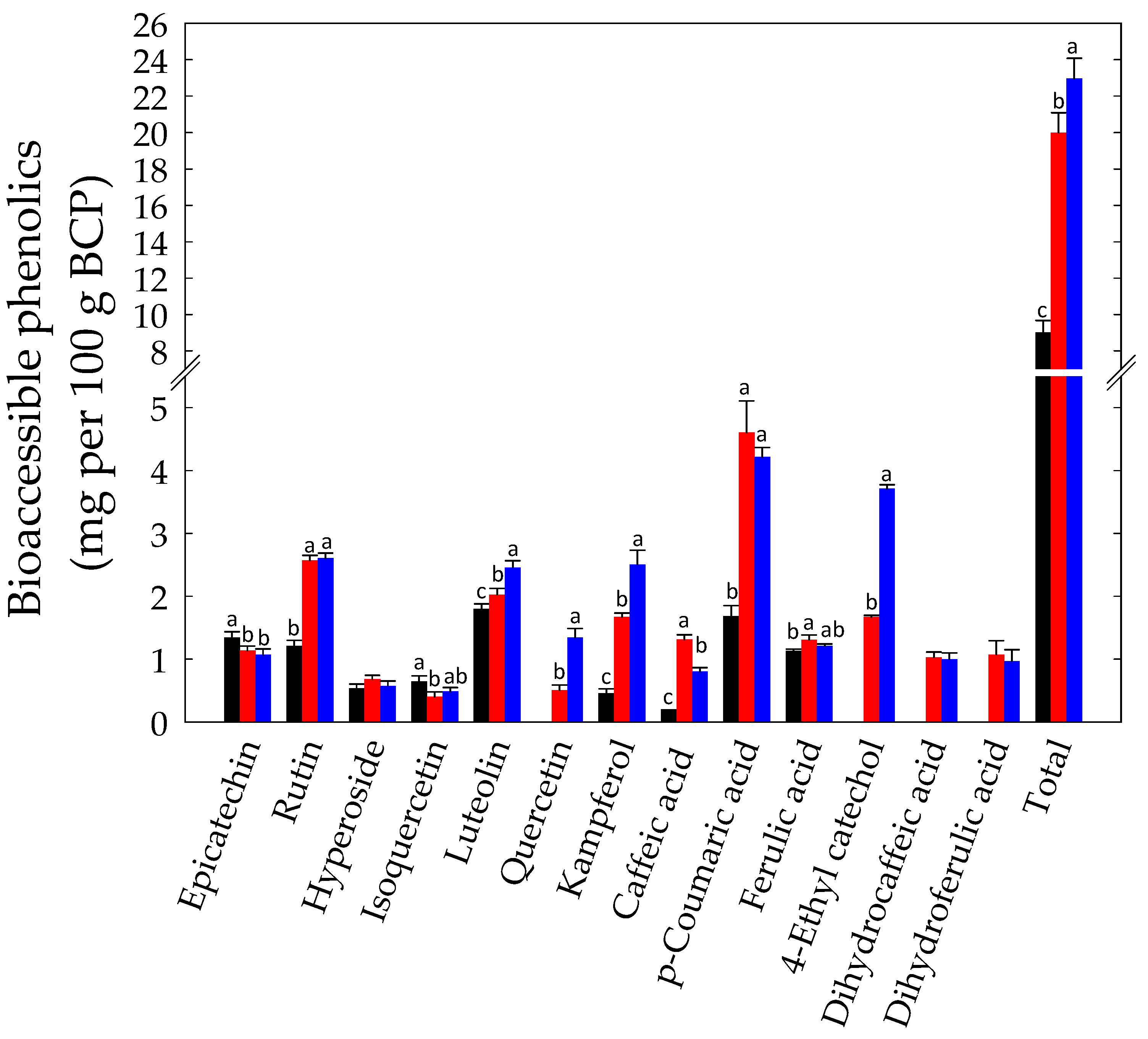
3.2. In Vitro Gastrointestinal Batch Digestion of BCP and Phenolics Bioaccessibility Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thakur, M.; Nanda, V. Composition and functionality of bee pollen: A review. Trends Food Sci. Technol. 2020, 96, 82–106. [Google Scholar] [CrossRef]
- Denisow, B.; Denisow-Pietrzyk, M. Biological and therapeutic properties of bee pollen: A review. J. Sci. Food Agric. 2016, 96, 4303–4309. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Piwowarek, K.; Kot, A.M.; Błażejak, S.; Chlebowska-Śmigiel, A.; Wolska, I. Pollen and bee bread as new health-oriented products: A review. Trends Food Sci. Technol. 2018, 71, 170–180. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Barać, M.B.; Ali Shariati, M.; Tešić, Ž.L.; Pešić, M.B. The application of pollen as a functional food and feed ingredient—The present and perspectives. Biomolecules. 2020, 10, 84. [Google Scholar] [CrossRef]
- Zuluaga-Domínguez, C.; Castro-Mercado, L.; Cecilia Quicazán, M. Effect of enzymatic hydrolysis on structural characteristics and bioactive composition of bee-pollen. J. Food Process. Preserv. 2019, 3, e13983. [Google Scholar] [CrossRef]
- Xu, X.; Sun, L.; Dong, J.; Zhang, H. Breaking the cells of rape bee pollen and consecutive extraction of functional oil with supercritical carbon dioxide. Innov. Food Sci. Emerg. 2009, 10, 42–46. [Google Scholar] [CrossRef]
- Li, L.I.; Liu, Y.W.; Zhao, J.X.; Pei, D.; Duo-Long, D.I.; Wang, N. High-speed shear treatment for cell wall disruption of rape bee pollen. J. Food Sci. 2012, 33, 97–101. [Google Scholar]
- Dong, J.; Gao, K.; Wang, K.; Xu, X.; Zhang, H. Cell wall disruption of rape bee pollen treated with combination of protamex hydrolysis and ultrasonication. Food Res. Int. 2015, 75, 123–130. [Google Scholar] [CrossRef]
- Tuoheti, T.; Rasheed, H.A.; Meng, L.; sheng Dong, M. High hydrostatic pressure enhances the anti-prostate cancer activity of lotus bee pollen via increased metabolites. J. Ethnopharmacol. 2020, 261, 113057. [Google Scholar] [CrossRef]
- Wu, W.; Wang, K.; Qiao, J.; Dong, J.; Li, Z.; Zhang, H. Improving nutrient release of wall-disrupted bee pollen with a combination of ultrasonication and high shear technique. J. Sci. Food Agric. 2019, 99, 564–575. [Google Scholar] [CrossRef]
- Zuluaga, C.M.; Serrato, J.C.B.; Quicazán, M.C. Valorization alternatives of Colombian bee-pollen for its use as food resource—A structured review. Vitae 2014, 21, 237–247. [Google Scholar]
- Gilliam, M.; Prest, D.B.; Lorenz, B.J. Microbiology of pollen and bee bread: Taxonomy and enzymology of molds. Apidologie 1989, 20, 53–68. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Cantatore, V.; Gobbetti, M. Novel solid-state fermentation of bee-collected pollen emulating the natural fermentation process of bee bread. Food Microbiol. 2019, 82, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.E.; Sheehan, T.H.; Mott, B.M.; Maes, P.; Snyder, L.; Schwan, M.R.; Walton, A.; Beryl, M.J.; Corby-Harris, V. Microbial ecology of the hive and pollination landscape: Bacterial associates from floral nectar, the alimentary tract and stored food of honey bees (Apis mellifera). PLoS ONE 2013, 8, e83125. [Google Scholar] [CrossRef] [PubMed]
- Čadež, N.; Fülöp, L.; Dlauchy, D.; Péter, G. Zygosaccharomyces favi sp. nov., an obligate osmophilic yeast species from bee bread and honey. Antonie Van Leeuwenhoek 2015, 107, 645–654. [Google Scholar] [CrossRef]
- Salazar-González, C.; Díaz-Moreno, C. The nutritional and bioactive aptitude of bee pollen for a solid-state fermentation process. J. Apic. Res. 2016, 55, 161–175. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Adaškevičiūtė, V.; Kaškonas, P.; Mickienė, R.; Maruška, A. Antimicrobial and antioxidant activities of natural and fermented bee pollen. Food Biosci. 2020, 34, 100532. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Katilevičiūtė, A.; Kaškonas, P.; Maruška, A. The impact of solid-state fermentation on bee pollen phenolic compounds and radical scavenging capacity. Chem. Pap. 2018, 72, 2115–2120. [Google Scholar] [CrossRef]
- Filannino, P.; Di Cagno, R.; Gobbetti, M. Metabolic and functional paths of lactic acid bacteria in plant foods: Get out of the labyrinth. Curr. Opin. Biotechnol. 2018, 49, 64–72. [Google Scholar] [CrossRef]
- Filannino, P.; Di Cagno, R.; Tlais, A.Z.A.; Cantatore, V.; Gobbetti, M. Fructose-rich niches traced the evolution of lactic acid bacteria toward fructophilic species. Crit. Rev. Microbiol. 2019, 45, 65–81. [Google Scholar] [CrossRef]
- Gambacorta, G.; Trani, A.; Fasciano, C.; Paradiso, V.M.; Faccia, M. Effects of prefermentative cold soak on polyphenols and volatiles of Aglianico, Primitivo and Nero di Troia red wines. Food Sci. Nutr. 2019, 7, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Eid, N.; Enani, S.; Walton, G.; Corona, G.; Costabile, A.; Gibson, G.; Rowland, I.; Spencer, J.P. The impact of date palm fruits and their component polyphenols, on gut microbial ecology, bacterial metabolites and colon cancer cell proliferation. J. Nutr. Sci. 2014, 3, e46. [Google Scholar] [CrossRef] [PubMed]
- Celep, E.; Charehsaz, M.; Akyüz, S.; Acar, E.T.; Yesilada, E. Effect of in vitro gastrointestinal digestion on the bioavailability of phenolic components and the antioxidant potentials of some Turkish fruit wines. Food Res. Int. 2015, 78, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef]
- Bouayed, J.; Deußer, H.; Hoffmann, L.; Bohn, T. Bioaccessible and dialysable polyphenols in selected apple varieties following in vitro digestion vs. their native patterns. Food Chem. 2012, 131, 1466–1472. [Google Scholar] [CrossRef]
- Tlais, A.Z.; Da Ros, A.; Filannino, P.; Vincentini, O.; Gobbetti, M.; Di Cagno, R. Biotechnological re-cycling of apple by-products: A reservoir model to produce a dietary supplement fortified with biogenic phenolic compounds. Food Chem. 2021, 336, 127616. [Google Scholar] [CrossRef]
- Filannino, P.; Tlais, A.Z.; Morozova, K.; Cavoski, I.; Scampicchio, M.; Gobbetti, M.; DiCagno, R. Lactic acid fermentation enriches the profile of biogenic fatty acid derivatives of avocado fruit (Persea americana Mill.). Food Chem. 2020, 317, 126384. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Kaškonas, P.; Maruška, A. Volatile compounds composition and antioxidant activity of bee pollen collected in Lithuania. Chem. Pap. 2015, 69, 291–299. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Ruočkuvienė, G.; Kaškonas, P.; Akuneca, I.; Maruška, A. Chemometric analysis of bee pollen based on volatile and phenolic compound compositions and antioxidant properties. Food Anal. Methods. 2015, 8, 1150–1163. [Google Scholar] [CrossRef]
- Flamini, G.; Tebano, M.; Cioni, P.L. Volatiles emission patterns of different plant organs and pollen of Citrus limon. Anal. Chim. Acta 2007, 589, 120–124. [Google Scholar] [CrossRef]
- Rothschild, M.; Bergström, G.; Wängberg, S.Å. Cannabis sativa: Volatile compounds from pollen and entire male and female plants of two variants, Northern Lights and Hawaian Indica. J. Linn. Soc. Bot. 2005, 147, 387–397. [Google Scholar] [CrossRef]
- Van Vuuren, S.V.; Viljoen, A.M. Antimicrobial activity of limonene enantiomers and 1, 8-cineole alone and in combination. Flavour Frag. J. 2007, 22, 540–544. [Google Scholar] [CrossRef]
- Gong, A.D.; Dong, F.Y.; Hu, M.J.; Kong, X.W.; Wei, F.F.; Gong, S.J.; Zhang, J.B.; Wu, A.B.; Liao, Y.C. Antifungal activity of volatile emitted from Enterobacter asburiae Vt-7 against Aspergillus flavus and aflatoxins in peanuts during storage. Food Control. 2019, 106, 106718. [Google Scholar] [CrossRef]
- Baschieri, A.; Ajvazi, M.D.; Tonfack, J.L.F.; Valgimigli, L.; Amorati, R. Explaining the antioxidant activity of some common non-phenolic components of essential oils. Food Chem. 2017, 232, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.M.; Toub, O.; Chiang, A.; Lo, B.C.; Ohse, S.; Lund, S.T.; Bohlmann, J. The bouquet of grapevine (Vitis vinifera L. cv. Cabernet Sauvignon) flowers arises from the biosynthesis of sesquiterpene volatiles in pollen grains. Proc. Natl. Acad. Sci. USA 2009, 106, 7245–7250. [Google Scholar] [CrossRef] [PubMed]
- Torto, B.; Arbogast, R.T.; Alborn, H.; Suazo, A.; Van Engelsdorp, D.; Boucias, D.; Tumlinson, J.H.; Teal, P.E. Composition of volatiles from fermenting pollen dough and attractiveness to the small hive beetle Aethina tumida, a parasite of the honeybee Apis mellifera. Apidologie 2007, 38, 380–389. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Gobbetti, M. Lactic acid fermentation drives the optimal volatile flavor-aroma profile of pomegranate juice. Int. J. Food Microbiol. 2017, 248, 56–62. [Google Scholar] [CrossRef]
- Cagliari, A.; Margis, R.; dos Santos Maraschin, F.; Turchetto-Zolet, A.C.; Loss, G.; Margis-Pinheiro, M. Biosynthesis of triacylglycerols (TAGs) in plants and algae. Int. J. Plant Biol. 2011, 2, e10. [Google Scholar] [CrossRef]
- Corby-Harris, V.; Snyder, L.A.; Schwan, M.R.; Maes, P.; McFrederick, Q.S.; Anderson, K.E. Origin and Effect of Alpha 2.2 Acetobacteraceae in Honey Bee Larvae and Description of Parasaccharibacterapium gen. nov., sp. nov. Appl. Environ. Microbiol. 2014, 80, 7460–7472. [Google Scholar] [CrossRef]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and its importance to wine aroma—A review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Fariña, L.; Gioia, O.; Gomez, M.E.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased flavour diversity of Chardonnay wines by spontaneous fermentation and co-fermentation with Hanseniaspora vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Engelvin, G.; Feron, G.; Perrin, C.; Molle, D.; Talon, R. Identification of beta-oxidation and thioesterase activities in Staphylococcuscarnosus 833 strain. FEMS Microbiol. Lett. 2000, 190, 115–120. [Google Scholar] [PubMed]
- Gao, P.; Wang, W.; Jiang, Q.; Xu, Y.; Xia, W. Effect of autochthonous starter cultures on the volatile flavour compounds of Chinese traditional fermented fish (Suanyu). Int. J. Food Sci. Technol. 2016, 51, 1630–1637. [Google Scholar] [CrossRef]
- Forney, F.W.; Markovetz, A.J.; Kallio, R.E. Bacterial oxidation of 2-tridecanone to 1-undecanol. J. Bacteriol. 1967, 93, 649–655. [Google Scholar] [CrossRef]
- Park, Y.C.; Shaffer, C.E.H.; Bennett, G.N. Microbial formation of esters. Appl. Microbiol. Biotechnol. 2009, 85, 13. [Google Scholar] [CrossRef]
- Reyes-Sánchez, F.J.; Páez-Lerma, J.B.; Rojas-Contreras, J.A.; López-Miranda, J.; Soto-Cruz, N.Ó.; Reinhart-Kirchmayr, M. Study of the Enzymatic Capacity of Kluyveromyces marxianus for the Synthesis of Esters. J. Mol. Microbiol. Biotechnol. 2019, 29, 1–9. [Google Scholar] [CrossRef]
- Filannino, P.; Gobbetti, M.; De Angelis, M.; Di Cagno, R. Hydroxycinnamic acids used as external acceptors of electrons: An energetic advantage for strictly heterofermentative lactic acid bacteria. Appl. Environ. Microbiol. 2014, 80, 7574–7582. [Google Scholar] [CrossRef]
- Ardö, Y. Flavour formation by amino acid catabolism. Biotechnol. Adv. 2006, 24, 238–242. [Google Scholar] [CrossRef]
- Ravasio, D.; Wendland, J.; Walther, A. Major contribution of the Ehrlich pathway for 2-phenylethanol/rose flavor production in Ashbya gossypii. FEMS Yeast Res. 2014, 14, 833–844. [Google Scholar] [CrossRef]
- Berger, R.G. (Ed.) Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Springer Science & Business Media: Berlin, Germany, 2007. [Google Scholar]
- Mărgăoan, R.; Cornea-Cipcigan, M.; Topal, E.; Kösoğlu, M. Impact of fermentation processes on the bioactive profile and health-promoting properties of bee bread, mead and honey vinegar. Processes 2020, 8, 1081. [Google Scholar] [CrossRef]
- Liu, S.Q.; Holland, R.; Crow, V.L. Esters and their biosynthesis in fermented dairy products: A review. Int. Dairy J. 2004, 14, 923–945. [Google Scholar] [CrossRef]
- Domizio, P.; Romani, C.; Lencioni, L.; Comitini, F.; Gobbi, M.; Mannazzu, I.; Ciani, M. Outlining a future for non-Saccharomyces yeasts: Selection of putative spoilage wine strains to be used in association with Saccharomyces cerevisiae for grape juice fermentation. Int. J. Food Microbiol. 2011, 147, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, J.; Chatterjee, S.; Gamre, S.; Chattopadhyay, S.; Variyar, P.S.; Sharma, A. Analysis of free and bound aroma compounds of pomegranate (Punica granatum L.). LWT-Food Sci. Technol. 2014, 59, 461–466. [Google Scholar] [CrossRef]
- Peana, A.T.; D’Aquila, P.S.; Panin, F.; Serra, G.; Pippia, P.; Moretti, M.D.L. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine 2002, 9, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Seol, G.H. Linalool elicits vasorelaxation of mouse aortae through activation of guanylyl cyclase and K+ channels. J. Pharm. Pharmacol. 2015, 67, 714–719. [Google Scholar] [CrossRef]
- Kim, M.G.; Kim, S.M.; Min, J.H.; Kwon, O.K.; Park, M.H.; Park, J.W.; Ahn, H.I.; Hwang, J.Y.; Oh, S.R.; Lee, J.W.; et al. Anti-inflammatory effects of linalool on ovalbumin-induced pulmonary inflammation. Int. Immunopharmacol. 2019, 74, 105706. [Google Scholar] [CrossRef]
- Ruiz-Moyano, S.; Hernández, A.; Galvan, A.I.; Córdoba, M.G.; Casquete, R.; Serradilla, M.J.; Martín, A. Selection and application of antifungal VOCs-producing yeasts as biocontrol agents of grey mould in fruits. Food Microbiol. 2020, 92, 103556. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Venskutonis, P.R.; Čeksterytė, V. Composition of volatile compounds of honey of various floral origin and beebread collected in Lithuania. Food Chem. 2008, 111, 988–997. [Google Scholar] [CrossRef]
- Mayda, N.; Özkök, A.; Bayram, N.E.; Gerçek, Y.C.; Sorkun, K. Bee bread and bee pollen of different plant sources: Determination of phenolic content, antioxidant activity, fatty acid and element profiles. J. Food Meas. Charact. 2020, 14, 1795–1809. [Google Scholar] [CrossRef]
- Rocchetti, G.; Castiglioni, S.; Maldarizzi, G.; Carloni, P.; Lucini, L. UHPLC-ESI-QTOF-MS phenolic profiling and antioxidant capacity of bee pollen from different botanical origin. Int. J. Food Sci. Tech. 2019, 54, 335–346. [Google Scholar] [CrossRef]
- Li, Q.Q.; Wang, K.; Marcucci, M.C.; Sawaya, A.C.H.F.; Hu, L.; Xue, X.F.; Wu, L.M.; Hu, F.L. Nutrient-rich bee pollen: A treasure trove of active natural metabolites. J. Funct. Foods 2018, 49, 472–484. [Google Scholar] [CrossRef]
- Zuluaga-Dominguez, C.M.; Quicazan, M. Effect of fermentation on structural characteristics and bioactive compounds of bee-pollen based food. J. Apic. Sci. 2019, 63, 209–222. [Google Scholar] [CrossRef]
- Yan, S.; Li, Q.; Xue, X.; Wang, K.; Zhao, L.; Wu, L. Analysis of improved nutritional composition of bee pollen (Brassica campestris L.) after different fermentation treatments. Int. J. Food Sci. Technol. 2019, 54, 2169–2181. [Google Scholar] [CrossRef]
- Zuluaga, C.; Martínez, A.; Fernández, J.; López-Baldó, J.; Quiles, A.; Rodrigo, D. Effect of high pressure processing on carotenoid and phenolic compounds, antioxidant capacity, and microbial counts of bee-pollen paste and bee-pollen-based beverage. Innov. Food Sci. Emerg. Technol. 2016, 37, 10–17. [Google Scholar] [CrossRef]
- Tomás, A.; Falcão, S.I.; Russo-Almeida, P.; Vilas-Boas, M. Potentialities of beebread as a food supplement and source of nutraceuticals: Botanical origin, nutritional composition and antioxidant activity. J. Apic. Res. 2017, 56, 219–230. [Google Scholar] [CrossRef]
- Dranca, F.; Ursachi, F.; Oroian, M. Bee bread: Physicochemical characterization and phenolic content extraction optimization. Foods 2020, 9, 1358. [Google Scholar] [CrossRef]
- Wehling, K.; Niester, C.; Boon, J.J.; Willemse, M.T.M.; Wiermann, R. p-Coumaric acid—A monomer in the sporopollenin skeleton. Planta 1989, 179, 376–380. [Google Scholar] [CrossRef]
- Montgomery, W.; Potiszil, C.; Watson, J.S.; Sephton, M.A. Sporopollenin, a natural copolymer, is robust under high hydrostatic pressure. Macromol. Chem. Phys. 2016, 217, 2494–2500. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Díaz-Rubio, M.E.; Saura-Calixto, F. Non-extractable polyphenols, a major dietary antioxidant: Occurrence, metabolic fate and health effects. Nutr. Res. Rev. 2013, 26, 118–129. [Google Scholar] [CrossRef]
- Fu, Y.J.; Liu, W.; Zu, Y.G.; Tong, M.H.; Li, S.M.; Yan, M.M.; Efferth, T.; Luo, H. Enzyme assisted extraction of luteolin and apigenin from pigeonpea [Cajanuscajan (L.) Millsp.] leaves. Food Chem. 2008, 111, 508–512. [Google Scholar] [CrossRef]
- Filannino, P.; Di Cagno, R.; Addante, R.; Pontonio, E.; Gobbetti, M. Metabolism of fructophilic lactic acid bacteria isolated from the Apis mellifera L. bee gut: Phenolic acids as external electron acceptors. Appl. Environ. Microbiol. 2016, 82, 6899–6911. [Google Scholar] [CrossRef] [PubMed]
- Granato, T.M.; Romano, D.; Vigentini, I.; Foschino, R.C.; Monti, D.; Mamone, G.; Ferranti, P.; Nitride, C.; Iametti, S.; Bonomi, F.; et al. New insights on the features of the vinyl phenol reductase from the wine-spoilage yeast Dekkera/Brettanomyces bruxellensis. Ann. Microbiol. 2014, 65, 321–329. [Google Scholar] [CrossRef]
- Benito, S.; Palomero, F.; Morata, A.; Calderón, F.; Suárez-Lepe, J.A. Factors affecting the hydroxycinnamate decarboxylase/vinylphenol reductase activity of Dekkera/Brettanomyces: Application for Dekkera/Brettanomyces control in red wine making. J. Food Sci. 2009, 74, M15–M22. [Google Scholar] [CrossRef] [PubMed]
- Mosele, J.I.; Macià, A.; Romero, M.P.; Motilva, M.J. Stability and metabolism of Arbutus unedo bioactive compounds (phenolics and antioxidants) under in vitro digestion and colonic fermentation. Food Chem. 2016, 201, 120–130. [Google Scholar] [CrossRef]
- Silva, F.A.M.; Borges, F.; Guimarães, C.; Lima, J.L.F.C.; Matos, C.; Reis, S. Phenolic acids and derivatives: Studies on the relationship among structure, radical scavenging activity, and physicochemical parameters. J. Agric. Food Chem. 2000, 48, 2122–2126. [Google Scholar] [CrossRef]
- Huang, J.; de Paulis, T.; May, J.M. Antioxidant effects of dihydrocaffeicacid in human EA.hy926 endothelial cells. J. Nutr. Biochem. 2004, 15, 722–729. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filannino, P.; Di Cagno, R.; Gambacorta, G.; Tlais, A.Z.A.; Cantatore, V.; Gobbetti, M. Volatilome and Bioaccessible Phenolics Profiles in Lab-Scale Fermented Bee Pollen. Foods 2021, 10, 286. https://doi.org/10.3390/foods10020286
Filannino P, Di Cagno R, Gambacorta G, Tlais AZA, Cantatore V, Gobbetti M. Volatilome and Bioaccessible Phenolics Profiles in Lab-Scale Fermented Bee Pollen. Foods. 2021; 10(2):286. https://doi.org/10.3390/foods10020286
Chicago/Turabian StyleFilannino, Pasquale, Raffaella Di Cagno, Giuseppe Gambacorta, Ali Zein Alabiden Tlais, Vincenzo Cantatore, and Marco Gobbetti. 2021. "Volatilome and Bioaccessible Phenolics Profiles in Lab-Scale Fermented Bee Pollen" Foods 10, no. 2: 286. https://doi.org/10.3390/foods10020286
APA StyleFilannino, P., Di Cagno, R., Gambacorta, G., Tlais, A. Z. A., Cantatore, V., & Gobbetti, M. (2021). Volatilome and Bioaccessible Phenolics Profiles in Lab-Scale Fermented Bee Pollen. Foods, 10(2), 286. https://doi.org/10.3390/foods10020286







