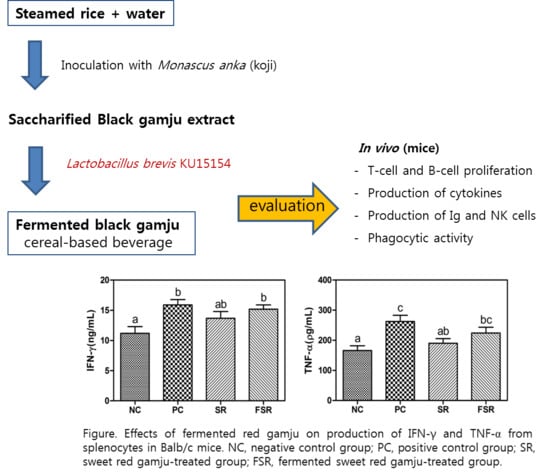
In Vivo Evaluation of Immune-Enhancing Activity of Red Gamju Fermented by Probiotic Levilactobacillus brevis KU15154 in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Strains and Tissue Culturing
2.2. Chemicals and Sample
2.3. Experimental Animal and Treatment
2.4. T- and B-Cell Proliferation
2.5. Cytokine Production
2.6. Real-Time Quantitative PCR (RT-PCR)
2.7. Serum Immunoglobulins (Ig) Production
2.8. Natural Killer (NK) Cell Activity
2.9. Phagocytic Activity
2.10. Statistical Analysis
3. Results
3.1. Body Weight, Food Intake, and Feed Efficiency Ratio (FER)
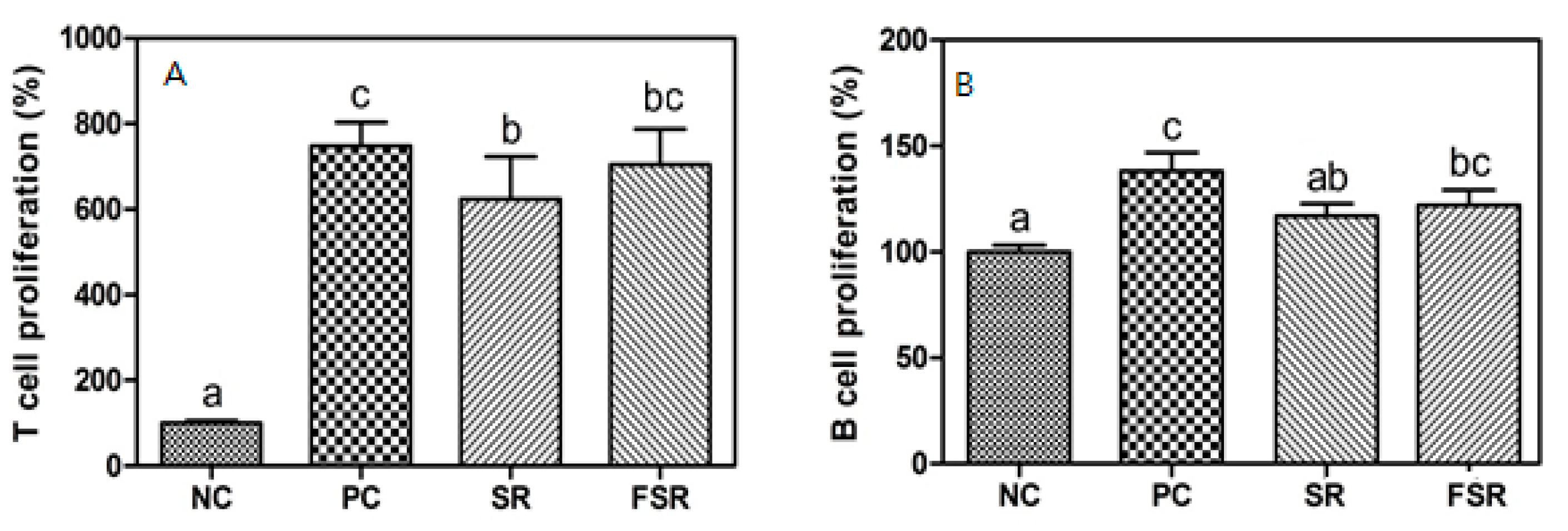
3.2. Proliferation of T- and B-Cells
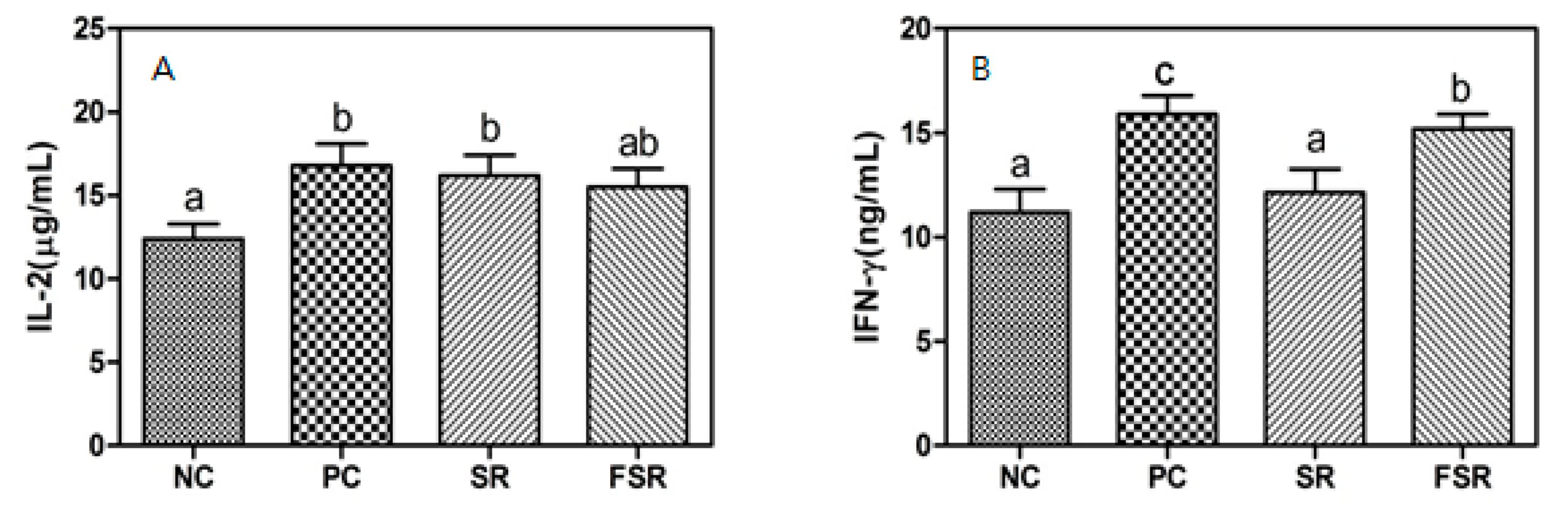
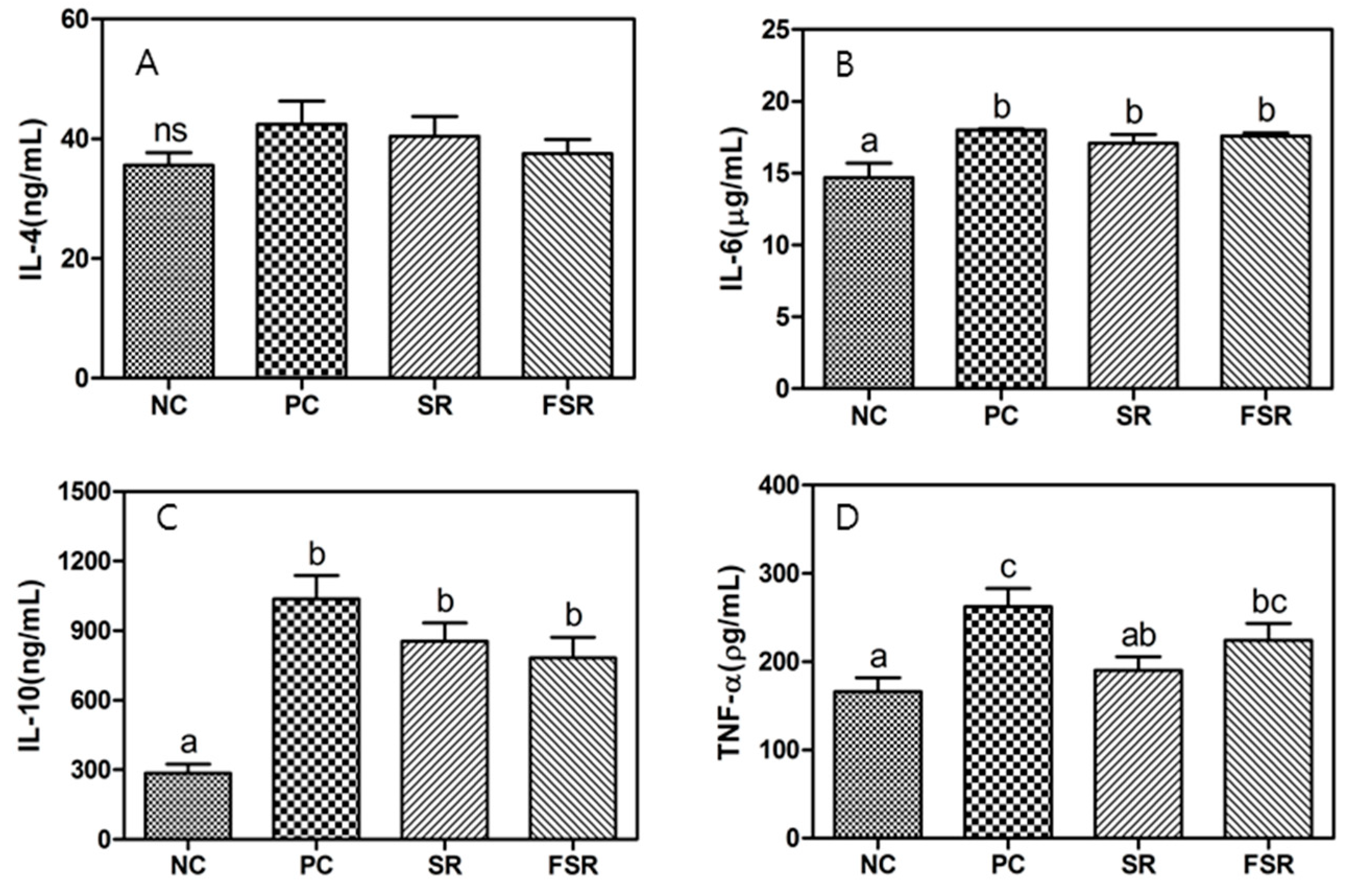
3.3. Production of Cytokines
3.4. Production of Ig
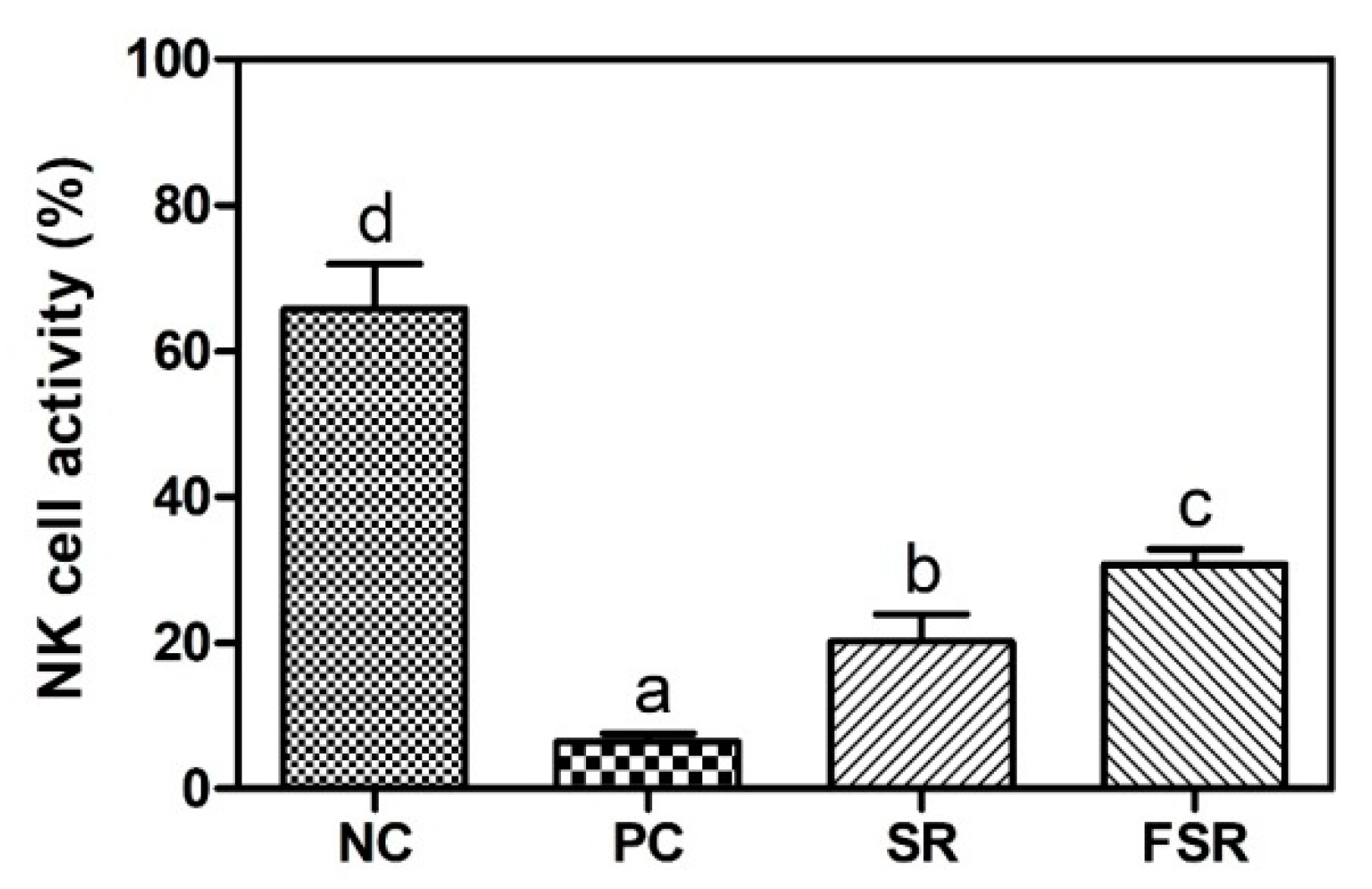
3.5. Activation of NK Cells against Yac-1 Cells
3.6. Phagocytic Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, H.R.; Chung, Y.H.; Yuk, H.G.; Lee, H.; Jang, H.S.; Kim, Y.; Shin, D. Characterization of Lactobacillus plantarum strains isolated from black raspberry and their effect on BALB/c mice gut microbiota. Food Sci. Biotechnol. 2018, 27, 1747–1754. [Google Scholar] [CrossRef]
- Shori, A.B. Influence of food matrix on the viability of probiotic bacteria: A review based on dairy and non-dairy beverages. Food Biosci. 2016, 13, 1–8. [Google Scholar] [CrossRef]
- Kariyawasam, K.; Yang, S.J.; Lee, N.K.; Paik, H.D. Probiotic properties of Lactobacillus brevis KU200019 and synergistic activity with fructooligosaccharides in antagonistic activity against foodborne pathogens. Food Sci. Anim. Resour. 2020, 40, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Bae, W.Y.; Yu, H.S.; Chang, K.H.; Hong, Y.H.; Lee, N.K.; Paik, H.D. Inula britannica fermented with probiotic Weissella cibaria D30 exhibited anti-inflammatory effect and increased viability in RAW 264.7 cells. Food Sci. Biotechnol. 2020, 29, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cho, K.; Kim, J.S.; Jung, H.C.; Kim, B.; Park, M.S.; Ji, G.E.; Cho, J.; Hong, K.S. Probiotic treatment induced change of inflammation related metabolites in IBS-D patients/double-blind, randomized, placebo-controlled trial. Food Sci. Biotechnol. 2020, 29, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.; Moridnia, A.; Mortazavi, D.; Salehi, M.; Bagheri, M.; Sheikhi, A. Kefir: A powerful probiotics with anticancer properties. Med. Oncol. 2017, 34, 183. [Google Scholar] [CrossRef] [PubMed]
- Han, K.J.; Lee, J.E.; Lee, N.K.; Paik, H.D. Antioxidant and anti-inflammatory effect of probiotic Lactobacillus plantarum KU15149 derived from Korean homemade diced-radish kimchi. J. Microbiol. Biotechnol. 2020, 30, 591–598. [Google Scholar] [CrossRef]
- Lim, S.M.; Lee, N.K.; Kim, K.T.; Paik, H.D. Probiotic Lactobacillus fermentum KU200060 isolated from watery kimchi and its application in probiotic yogurt for oral health. Microb. Pathog. 2020, 147, 104430. [Google Scholar] [CrossRef]
- Son, S.H.; Yang, S.J.; Jeon, H.L.; Yu, H.S.; Lee, N.K.; Park, Y.S.; Paik, H.D. Antioxidant and immunostimulatory effect of potential probiotic Lactobacillus paraplantarum SC61 isolated from Korean traditional fermented food, jangajji. Microb. Pathog. 2018, 125, 486–492. [Google Scholar] [CrossRef]
- Peyer, L.C.; Zannini, E.; Arendt, E.K. Lactic acid bacteria as sensory biomodulators for fermented cereal-based beverages. Trends Food Sci. Technol. 2016, 54, 17–25. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Bang, J.; Kim, Y.; Beuchat, L.R.; Ryu, J.H. Reduction of Bacillus cereus spores in sikhye, a traditional Korean rice beverage, by modified tyndallization processes with and without carbon dioxide injection. Lett. Appl. Microbiol. 2012, 55, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Guo, J.; Tu, Y.; Yuan, L. Functional study of C-terminal domain of the thermoacidophilic raw starch-hydrolyzing α-amylase Gt-amy. Food Sci. Biotechnol. 2019, 29, 409–418. [Google Scholar] [CrossRef] [PubMed]
- An, Y.H.; Lee, I.S.; Kim, H.S. Quality characteristics of sikhye with varied levels of sweet pumpkin during storage. Korean J. Food Cookery Sci. 2011, 27, 803–814. [Google Scholar] [CrossRef][Green Version]
- Lin, Y.; Wang, T.; Lee, M.; Su, N. Biologically active components and nutraceuticals in the Monascus-fermented rice: A review. Appl. Microbiol. Biotechnol. 2008, 77, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.Y.; Lee, E.J.; Kim, M.L. Storage properties and sensory characteristics of sikhe added Ulmus pumila L. extract. Korean J. Food Preserv. 2012, 19, 12–18. [Google Scholar] [CrossRef]
- Yang, S.J.; Kim, K.T.; Kim, T.Y.; Paik, H.D. Probiotic properties and antioxidant activities of Pediococcus pentosaceus SC28 and Levilactobacillus brevis KU15151 in fermented black gamju. Foods 2020, 9, 1154. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Lee, D.; Lee, M.; Kwon, H.O.; Kim, H.; Park, J. The effects of Curcuma longa L., purple sweet potato and mixtures of the two on immunomodulation in C57BL/6J mice infected with LP-BM5 murine leukemia retrovirus. J. Med. Food 2018, 21, 689–700. [Google Scholar] [CrossRef]
- Lee, I.H.; Lee, S.H.; Lee, I.S.; Park, Y.K.; Chung, D.K.; Choue, R.W. Effects of probiotic extracts of kimchi on immune function in NC/Nga mice. Korean J. Food Sci. Technol. 2008, 40, 82–87. [Google Scholar]
- Ryu, D.S.; Kim, S.H.; Lee, D.S. Immunomodulating activity of Salicornia herbacea extract. Korean J. Microbiol. Biotechnol. 2008, 36, 135–141. [Google Scholar]
- Lei, H.Y.; Chang, C.P. Lectin of concanavalin A as an anti-hepatoma therapeutic agent. J. Biomed. Sci. 2009, 16, 1–12. [Google Scholar] [CrossRef]
- O’Garra, A.; Chang, R.; Go, N.; Hastings, R.; Haughton, G.; Howard, M. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur. J. Immunol. 1992, 22, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Sweet, M.J.; Hume, D.A. Endotoxin signal transduction in macrophages. J. Leukoc. Biol. 1996, 60, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Parham, P. The Immune System, 4th ed.; Garland Science: New York, NY, USA, 2015; p. 62. [Google Scholar]
- Shin, J.; Kim, O.K.; Kim, S.; Bae, D.; Lee, J.; Park, J.; Jun, W. Immunomodulatory effect of a Salvia plebesia R. aqueous extract in forced swimming exercise-induced mice. Nutrients 2020, 12, 2260. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C. Exercise immunology: Practical applications. Int. J. Sports Med. 1997, 18, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.K.; Park, H.G.; Jeong, J.H.; Lee, W.L. Effects of moderate exercise training on splenocyte inflammatory cytokine production in high fat diet induced obese mice. Korean J. Life Sci. 2011, 21, 1176–1182. [Google Scholar] [CrossRef]
- Connolly, P.H.; Caiozzo, V.J.; Zaldivar, F.; Nemet, D.; Larson, J.; Hung, S.P.; Heck, J.D.; Hatfield, G.W.; Dan, M.; Cooper, D.M. Effects of exercise on gene expression in human peripheral blood mononuclear cells. J. Appl. Physiol. 2004, 97, 1461–1469. [Google Scholar] [CrossRef]
- Jeong, J.H.; Jang, S.; Jung, B.J.; Jang, K.S.; Kim, B.G.; Chung, D.K.; Kim, H. Differential immune-stimulatory effects of LTAs from different lactic acid bacteria via MAPK signaling pathway in RAW 264.7 cells. Immunobiology 2015, 220, 460–466. [Google Scholar] [CrossRef]
- Cher, D.J.; Mosmann, T.R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J. Immunol. 1987, 138, 3688–3694. [Google Scholar]
- Teh, H.S.; Kisielow, P.; Scott, B.; Kishi, H.; Uematsu, Y.; Blüthmann, H.; von Boehmer, H. Thymic major histocompatibility complex antigens and the αβ T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature 1988, 335, 229–233. [Google Scholar] [CrossRef]
- Powrie, F.; Coffman, R.L. Cytokine regulation of T-cell function: Potential for therapeutic intervention. Trends Pharmacol. Sci. 1993, 14, 164–168. [Google Scholar] [CrossRef]
- Fernández-Ortega, C.; Dubed, M.; Ramos, Y.; Navea, L.; Álvarez, G.; Lobaina, L.; López, L.; Casillas, D.; Rodríguez, L. Non-induced leukocyte extract reduces HIV replication and TNF secretion. Biochem. Biophys. Res. Commun. 2004, 325, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, J.; Qian, J.; Cheng, X.; Wu, H.; Li, L.; Qian, C.; Su, J.; Wu, D.; Burns, B.; et al. Target genes involved in corticosterone-induced PC12 cell viability and neurite disorders: A potential molecular mechanism of major depressive disorder. Psychiatry Res. 2016, 235, 206–208. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Pyne, D.B. Exercise, training, and neutrophil function. Exerc. Immunol. Rev. 1997, 3, 96–116. [Google Scholar] [PubMed]
- Song, I.B.; Han, H.J.; Kwon, J. Immune-enhancing effects of gamma-irradiated sericin. Food Sci. Biotechnol. 2020, 29, 969–976. [Google Scholar] [CrossRef]
- Plackett, T.P.; Boehmer, E.D.; Faunce, D.E.; Kovacs, E.J. Aging and innate immune cells. J. Leukoc. Biol. 2004, 76, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Elmadfa, I.; Klein, P.; Meyer, A.L. Immune-stimulating effects of lactic acid bacteria in vivo and in vitro. Proc. Nutr. Soc. 2010, 69, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Wichers, H. Immunomodulation by food: Promising concept for mitigating allergic disease? Anal. Bioanal. Chem. 2009, 395, 37–45. [Google Scholar] [CrossRef]
- Monobe, M.; Ema, K.; Kato, F.; Maeda-Yamamoto, M. Immunostimulating activity of a crude polysaccharide derived from green tea (Camellia sinensis) extract. J. Agric. Food Chem. 2008, 56, 1423–1427. [Google Scholar] [CrossRef]
- Duffy, L.C.; Zielezny, M.A.; Riepenhoff-Talty, M.; Dryja, D.; Sayahtaheri-Altaie, S.; Griffiths, E.; Ruffin, D.; Barrett, H.; Rossman, J.; Ogra, P.L. Effectiveness of Bifidobacterium bifidum in mediating the clinical course of murine rotavirus diarrhea. Pediatr. Res. 1994, 35, 690–695. [Google Scholar] [CrossRef]
- Duffy, L.C.; Zielezny, M.A.; Riepenhoff-Talty, M.; Dryja, D.; Sayahtaheri-Altaie, S.; Griffiths, E.; Ruffin, D.; Barrett, H.; Ogra, P.L. Reduction of virus shedding by B. bifidum in experimentally induced MRV infection. Dig. Dis. Sci. 1994, 39, 2334–2340. [Google Scholar] [CrossRef]
- Offit, P.A. Host factors associated with protection against rotavirus disease: The skies are clearing. J. Infec. Dis. 1996, 174, S59–S64. [Google Scholar] [CrossRef] [PubMed]
- Shu, Q.; Lin, H.; Rutherfurd, K.J.; Fenwick, S.G.; Prasad, J.; Gopal, P.K.; Gill, H.S. Dietary Bifidobacterium lactis (HN019) enhances resistance to oral Salmonella typhimurium infection in mice. Microbiol. Immunol. 2000, 44, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Yang, S.J.; Paik, H.D. Probiotic properties of novel probiotic Levilactobacillus brevis KU15147 isolated from radish kimchi and its antioxidant and immune-enhancing activities. Food Sci. Biotechnol. 2021. [Google Scholar] [CrossRef]

| Gene Name | Description | Primer Sequences | |
|---|---|---|---|
| IL-4 | Interleukin-4 | Forward: | ARCATCGGCATTTTGAACGA |
| Reverse: | AAGCCCGAAAGAGTCTCTGC | ||
| IL-6 | Interleukin-6 | Forward: | CCTTCCTACCCCAATTTCCA |
| Reverse: | CGCACTAGGTTTGCCGAGTA | ||
| IL-10 | Interleukin-10 | Forward: | TAAGGCTGGCCACACTTGAG |
| Reverse: | AGTTTTCAGGGATGAAGCGG | ||
| GAPDH | Glyceraldehyde 3-Phosphate dehydrogenase | Forward: | CGCTCTCTGCTCCTCCTGTTC |
| Reverse: | CGCCCAATACGACCAAATCCG | ||
| Items | NC 1 | PC | SR | FSR |
|---|---|---|---|---|
| Food intake (g/day) | 3.13 ± 0.00 2,ns | 3.50 ± 0.00 | 2.98 ± 0.00 | 3.14 ± 0.00 |
| Weight gain (g/day) | 0.05 ± 0.01 | 0.05 ± 0.02 | 0.05 ± 0.05 | 0.07 ± 0.05 |
| FER 3 | 1.52 ± 0.38 ns | 1.53 ± 0.51 | 1.80 ± 0.35 | 2.11 ± 1.54 |
| Liver (g) | 0.82 ± 0.06 ns | 0.82 ± 0.05 | 0.85 ± 0.06 | 0.85 ± 0.4 |
| Kidney (g) | 0.28 ± 0.01 | 0.29 ± 0.01 | 0.28 ± 0.01 | 0.28 ± 0.01 |
| Spleen (g) | 0.06 ± 0.00 | 0.07 ± 0.01 | 0.06 ± 0.00 | 0.07 ± 0.00 |
| Heart (g) | 0.11 ± 0.01 | 0.11 ± 0.00 | 0.10 ± 0.01 | 0.11 ± 0.00 |
| Treatments 1 | IL-4 | IL-6 | IL-10 |
|---|---|---|---|
| NC | 1.00 ± 0.02 b,2 | 1.00 ± 0.17 b | 1.00 ± 0.28 b |
| PC | 0.52 ± 0.02 a | 0.52 ± 0.01 a | 0.18 ± 0.04 a |
| SR | 0.89 ± 0.17 ab | 0.54 ± 0.03 a | 0.13 ± 0.14 a |
| FSR | 0.79 ± 0.15 ab | 0.75 ± 0.13 ab | 0.16 ± 0.12 a |
| Treatemnts 1 | IgA (μg/mL) | IgE (ng/mL) | IgG (μg/mL) |
|---|---|---|---|
| NC | 52.3 ± 5.1 2,ns | 64.4 ± 2.2 ns | 433.5 ± 13.6 a |
| PC | 69.4 ± 7.9 | 75.7 ± 4.5 | 803.3 ± 83.4 c |
| SR | 66.9 ± 7.3 | 69.6 ± 6.4 | 412.2 ± 44.8 a |
| FSR | 62.5 ± 4.9 | 65.9 ± 2.1 | 502.6 ± 25.8 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, E.; Kim, K.-T.; Choi, M.; Lee, Y.; Paik, H.-D. In Vivo Evaluation of Immune-Enhancing Activity of Red Gamju Fermented by Probiotic Levilactobacillus brevis KU15154 in Mice. Foods 2021, 10, 253. https://doi.org/10.3390/foods10020253
Park E, Kim K-T, Choi M, Lee Y, Paik H-D. In Vivo Evaluation of Immune-Enhancing Activity of Red Gamju Fermented by Probiotic Levilactobacillus brevis KU15154 in Mice. Foods. 2021; 10(2):253. https://doi.org/10.3390/foods10020253
Chicago/Turabian StylePark, Eunju, Kee-Tae Kim, Mijoo Choi, Yunjung Lee, and Hyun-Dong Paik. 2021. "In Vivo Evaluation of Immune-Enhancing Activity of Red Gamju Fermented by Probiotic Levilactobacillus brevis KU15154 in Mice" Foods 10, no. 2: 253. https://doi.org/10.3390/foods10020253
APA StylePark, E., Kim, K.-T., Choi, M., Lee, Y., & Paik, H.-D. (2021). In Vivo Evaluation of Immune-Enhancing Activity of Red Gamju Fermented by Probiotic Levilactobacillus brevis KU15154 in Mice. Foods, 10(2), 253. https://doi.org/10.3390/foods10020253