The Influence of Weather on the Occurrence of Aflatoxin B1 in Harvested Maize from Kenya and Tanzania
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area and Participants
2.2. Collection of Maize Samples
2.3. Preparation of Maize Samples
2.4. Chemicals and Reagents
2.5. Mycotoxin Extraction
2.6. Aflatoxin Detection and Quantification by LC-MS/MS Method
2.7. Weather Data
2.8. Data Analysis
3. Results
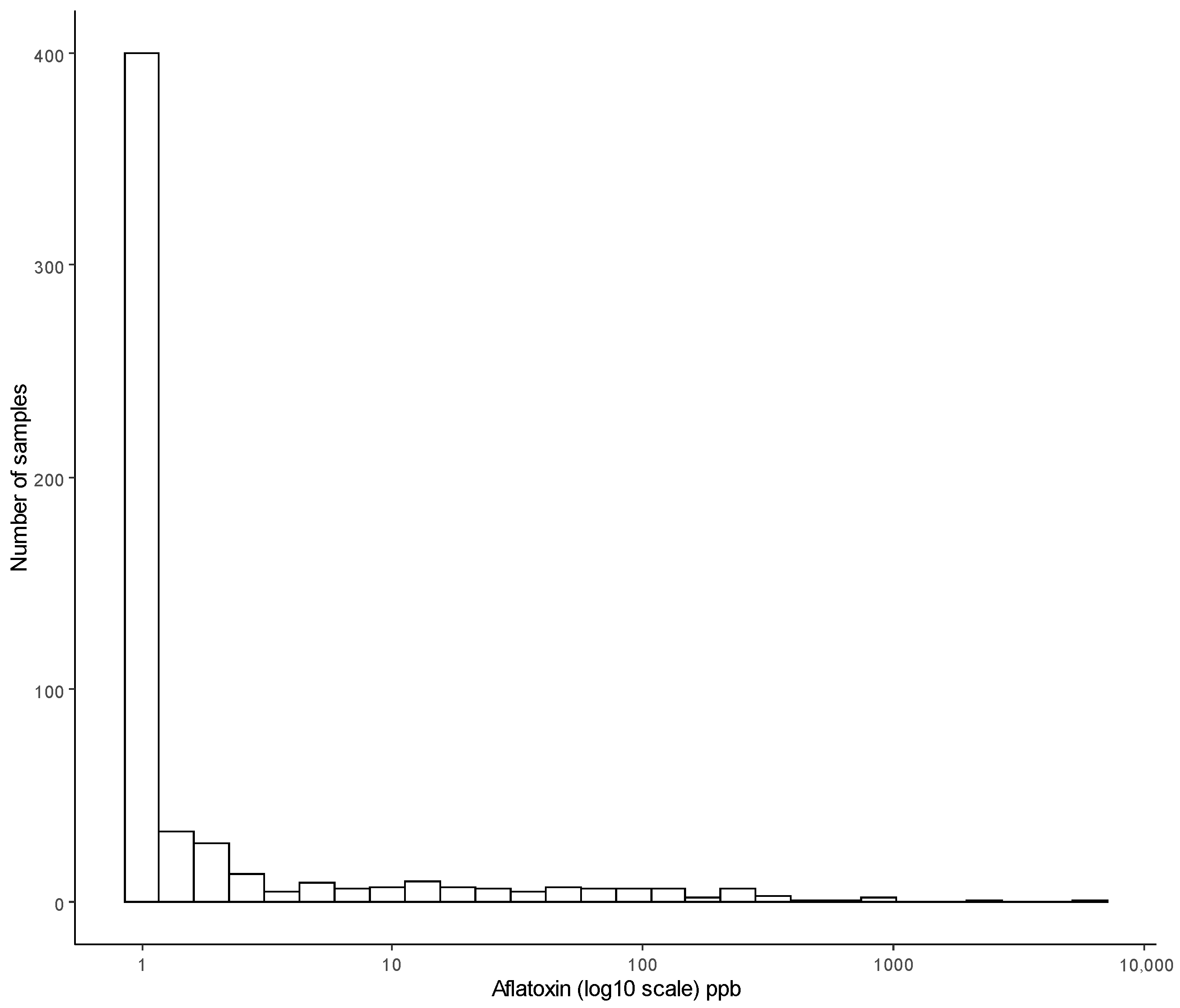
3.1. Incidence and Concentration of Aflatoxin B1
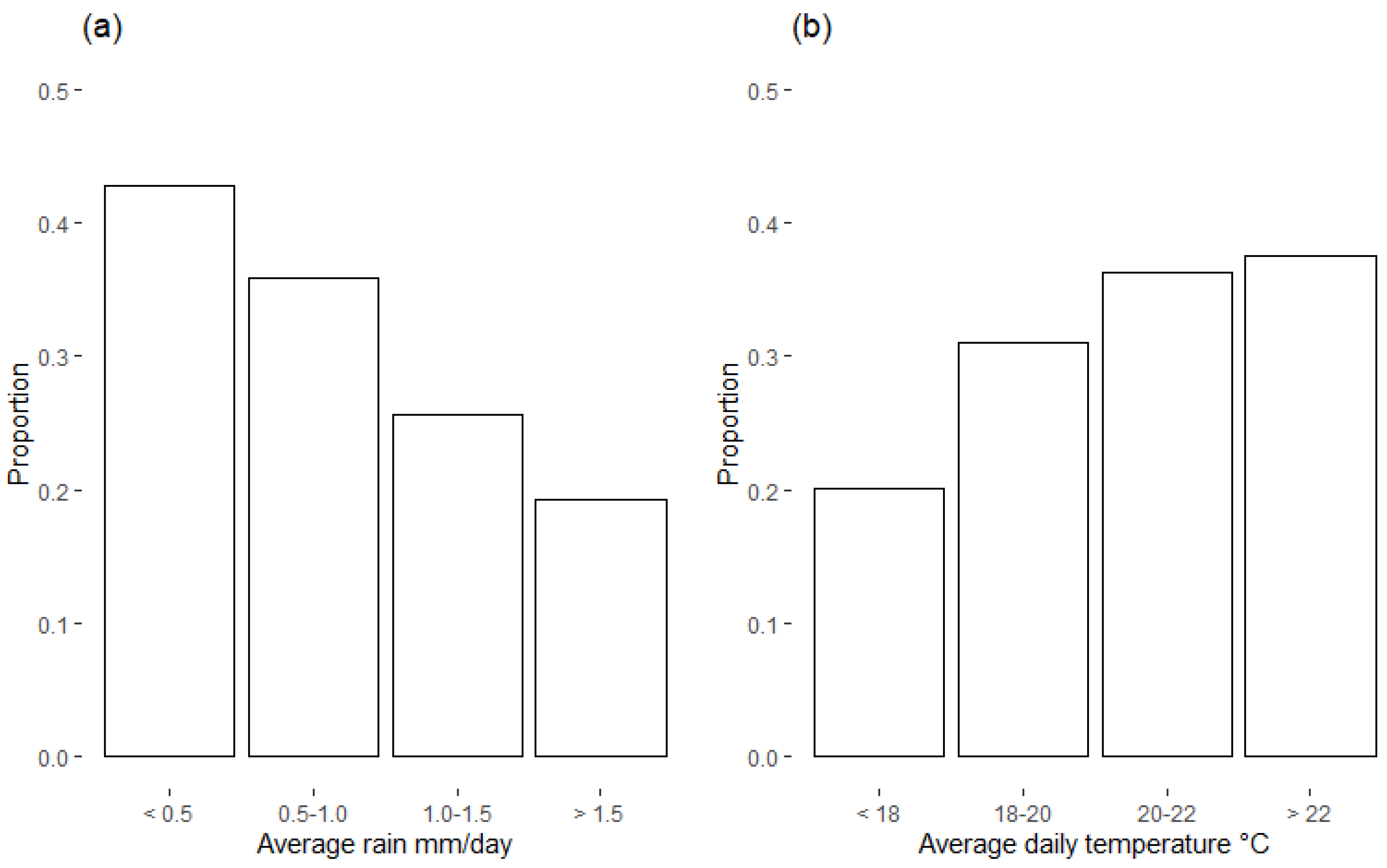
3.2. Weather Observations during Crop Growth
3.3. Statistical Models for Incidence of Aflatoxin
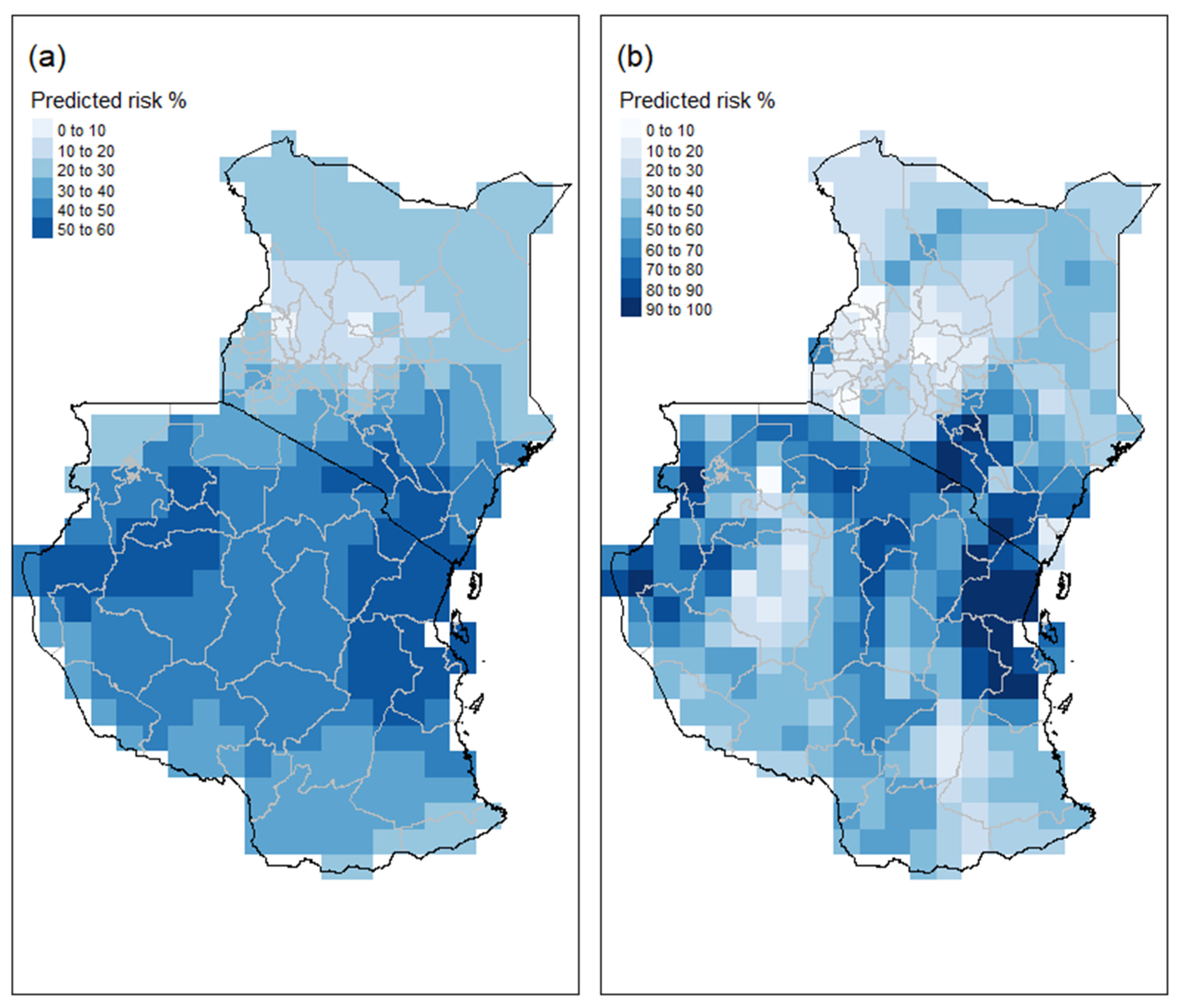
3.4. Estimated Probability of Aflatoxin in the 2013 Season
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Azziz-Baumgartner, E.; Lindblade, K.; Gieseker, K.; Rogers, H.S.; Kieszak, S.; Njapau, H.; Schleicher, R.; McCoy, L.F.; Misore, A.; DeCock, K.; et al. Case-control study of an acute aflatoxicosis outbreak, Kenya, 2004. Environ. Health Perspect. 2005, 113, 1779–1783. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public health impacts of foodborne mycotoxins. Annu. Rev. Food Sci. Technol. 2014, 5, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Daily-News TFDA Tests Find Higher Aflatoxin Levels in Cereals, Maize Flour. 2016. Available online: http://www.dailynews.co.tz/index.php/home-news/52281-tfda-tests-find-higher-aflatoxin-levels-in-cereals-maize-flour (accessed on 15 January 2021).
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, M. Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. 1994. Available online: https://www.citethisforme.com/topic-ideas/biology/Aromatic%20components%20of%20essential%20oil-107921489 (accessed on 15 January 2021).
- World Health Organisation (WHO). Mycotoxins in African Foods: Implications to Food Safety and Health. Available online: www.afro.who.int/des (accessed on 15 January 2021).
- Ngindu, A.; Kenya, P.; Ocheng, D.; Omondi, T.; Ngare, W.; Gatei, D.; Johnson, B.; Ngira, J.; Nandwa, H.; Jansen, A.; et al. Outbreak of acute hepatitis caused by aflatoxin poisoning in Kenya. Lancet 1982, 319, 1346–1348. [Google Scholar] [CrossRef]
- Probst, C.; Njapau, H.; Cotty, P.J. Outbreak of an acute aflatoxicosis in Kenya in 2004: Identification of the causal agent. Appl. Environ. Microbiol. 2007, 73, 2762–2764. [Google Scholar] [CrossRef]
- Mutegi, C.; Wagacha, M.; Kimani, J.; Otieno, G.; Wanyama, R.; Hell, K.; Christie, M.E. Incidence of aflatoxin in peanuts (Arachis hypogaea linnaeus) from markets in western, Nyanza and Nairobi provinces of Kenya and related market traits. J. Stored Prod. Res. 2013, 52, 118–127. [Google Scholar] [CrossRef]
- Shirima, C.P.; Kimanya, M.E.; Kinabo, J.L.; Routledge, M.N.; Srey, C.; Wild, C.P.; Gong, Y.Y. Dietary exposure to aflatoxin and fumonisin among Tanzanian children as determined using biomarkers of exposure. Mol. Nutr. Food Res. 2013. [Google Scholar] [CrossRef]
- Shephard, G.S. Aflatoxin and food safety: Recent African perspectives. J. Toxicol. Toxin Rev. 2003, 22, 267–286. [Google Scholar] [CrossRef]
- Ogwang, B.A.; Chen, H.; Li, X.; Gao, C. The influence of topography on East African October to December climate: Sensitivity experiments with RegCM4. Adv. Meteorol. 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Lima, N. How will climate change affect mycotoxins in food? Food Res. Int. 2010, 43, 1902–1914. [Google Scholar] [CrossRef]
- Bernhoft, A.; Torp, M.; Clasen, P.-E.; Løes, A.-K.; Kristoffersen, A.B. Influence of agronomic and climatic factors on fusarium infestation and mycotoxin contamination of cereals in Norway. Food Addit. Contam. Part A 2012, 29, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Magan, N.; Aldred, D. Post-harvest control strategies: Minimizing mycotoxins in the food chain. Int. J. Food Microbiol. 2007, 119, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I.; Miscamble, B.F. Water relations of Aspergillus flavus and closely related species. J. Food Prot. 1995, 58, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P.; Leggieri, M.C.; Rossi, V.; Giorni, P. AFLA-maize, a mechanistic model for Aspergillus flavus infection and aflatoxin B1 contamination in maize. Comput. Electron. Agric. 2013, 94, 38–46. [Google Scholar] [CrossRef]
- Smith, L.M.; Stasiewicz, R.; Hestrin, L.; Mutiga, S.; Nelson, R. Examining environmental drivers of spatial variability in aflatoxin accumulation in Kenyan maize: Potential utility in risk drivers prediction models. Afr. J. Food Agric. Nutr. Dev. 2016, 16. [Google Scholar] [CrossRef]
- Sulyok, M.; Berthiller, F.; Krska, R.; Schuhmacher, R. Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Commun. Mass Spectrom. 2006, 20, 2649–2659. [Google Scholar] [CrossRef]
- Sparks, A.H.; Hengl, T.; Nelson, A. GSODR: Global summary daily weather data in R. J. Open Source Softw. 2017, 2, 177. [Google Scholar] [CrossRef]
- Pebesma, E.J. Multivariable geostatistics in S: The gstat package. Comput. Geosci. 2004, 30, 683–691. [Google Scholar] [CrossRef]
- Gräler, B.; Pebesma, E.; Heuvelink, G. Spatio-temporal interpolation using gstat. RFID J. 2016, 8, 204–218. [Google Scholar] [CrossRef]
- Aitkin, M.; Francis, B.; Hinde, J.; Darnell, R. Statistical Modelling in R; Oxford Statistical Series; Oxford University Press: Oxford, UK, 2009; ISBN 978-0-19-921914-8. [Google Scholar]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future köppen-geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Wing, J.; Kuhn, M. Contributions from Steve Weston, Andre Williams, Chris Keefer, Allan Engelhardt, Tony Cooper, Zachary Mayer; et al. Caret: Classification and Regression Training. 2016. Available online: https://ui.adsabs.harvard.edu/abs/2015ascl.soft05003K/abstract (accessed on 15 January 2021).
- Kimanya, M.E.; Meulenaer, B.D.; Tiisekwa, B.; Ndomondo-Sigonda, M.; Devlieghere, F.; Camp, J.V.; Kolsteren, P. Co-occurrence of fumonisins with aflatoxins in home-stored maize for human consumption in rural villages of Tanzania. Food Addit. Contam. Part A 2008, 25, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Manjula, K.; Hell, K.; Fandohan, P.; Abass, A.; Bandyopadhyay, R. Aflatoxin and fumonisin contamination of cassava products and maize grain from markets in Tanzania and Republic of the Congo. Toxin Rev. 2009, 28, 63–69. [Google Scholar] [CrossRef]
- Kajuna, F.F.; Temba, B.A.; Mosha, R.D. Surveillance of aflatoxin B1 contamination in chicken commercial feeds in Morogoro, Tanzania. Livest. Res. Rural Dev. 2013, 25, 51. [Google Scholar]
- Mutiga, S.K.; Hoffmann, V.; Harvey, J.W.; Milgroom, M.G.; Nelson, R.J. Assessment of aflatoxin and fumonisin contamination of maize in western Kenya. Phytopathology 2015, 105, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.; Onsongo, M.; Njapau, H.; Schurz-Rogers, H.; Luber, G.; Kieszak, S.; Nyamongo, J.; Backer, L.; Dahiye, A.M.; Misore, A.; et al. Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and central Kenya. Environ. Health Perspect. 2005, 113, 1763–1767. [Google Scholar] [CrossRef] [PubMed]
- Hiza, P. Epidemiological study of carcinoma of liver in Dodoma region in Tanzania. J. Natl. Med Assoc. 1979, 71, 585. [Google Scholar]
- Mutiga, S.K.; Were, V.; Hoffmann, V.; Harvey, J.W.; Milgroom, M.G.; Nelson, R.J. Extent and drivers of mycotoxin contamination: Inferences from a survey of Kenyan maize mills. Phytopathology 2014, 104, 1221–1231. [Google Scholar] [CrossRef]
- Diao, E.; Dong, H.; Hou, H.; Zhang, Z.; Ji, N.; Ma, W. Factors influencing aflatoxin contamination in before and after harvest peanuts: A review. J. Food Res. 2014, 4, 148. [Google Scholar] [CrossRef]
- Yard, E.E.; Daniel, J.H.; Lewis, L.S.; Rybak, M.E.; Paliakov, E.M.; Kim, A.A.; Montgomery, J.M.; Bunnell, R.; Abudo, M.U.; Akhwale, W.; et al. Human aflatoxin exposure in Kenya, 2007: A cross-sectional study. Food Addit. Contam. Part A 2013, 30, 1322–1331. [Google Scholar] [CrossRef]
- Mutegi, C.K.; Ngugi, H.K.; Hendriks, S.L.; Jones, R.B. Prevalence and factors associated with aflatoxin contamination of peanuts from western Kenya. Int. J. Food Microbiol. 2009, 130, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Kos, J.; Mastilović, J.; Hajnal, E.J.; Šarić, B. Natural occurrence of aflatoxins in maize harvested in Serbia during 2009–2012. Food Control 2013, 34, 31–34. [Google Scholar] [CrossRef]
- Medina, A.; Rodriguez, A.; Magan, N. Effect of climate change on Aspergillus flavus and aflatoxin B1 production. Front. Microbiol. 2014, 5, 348. [Google Scholar] [CrossRef] [PubMed]
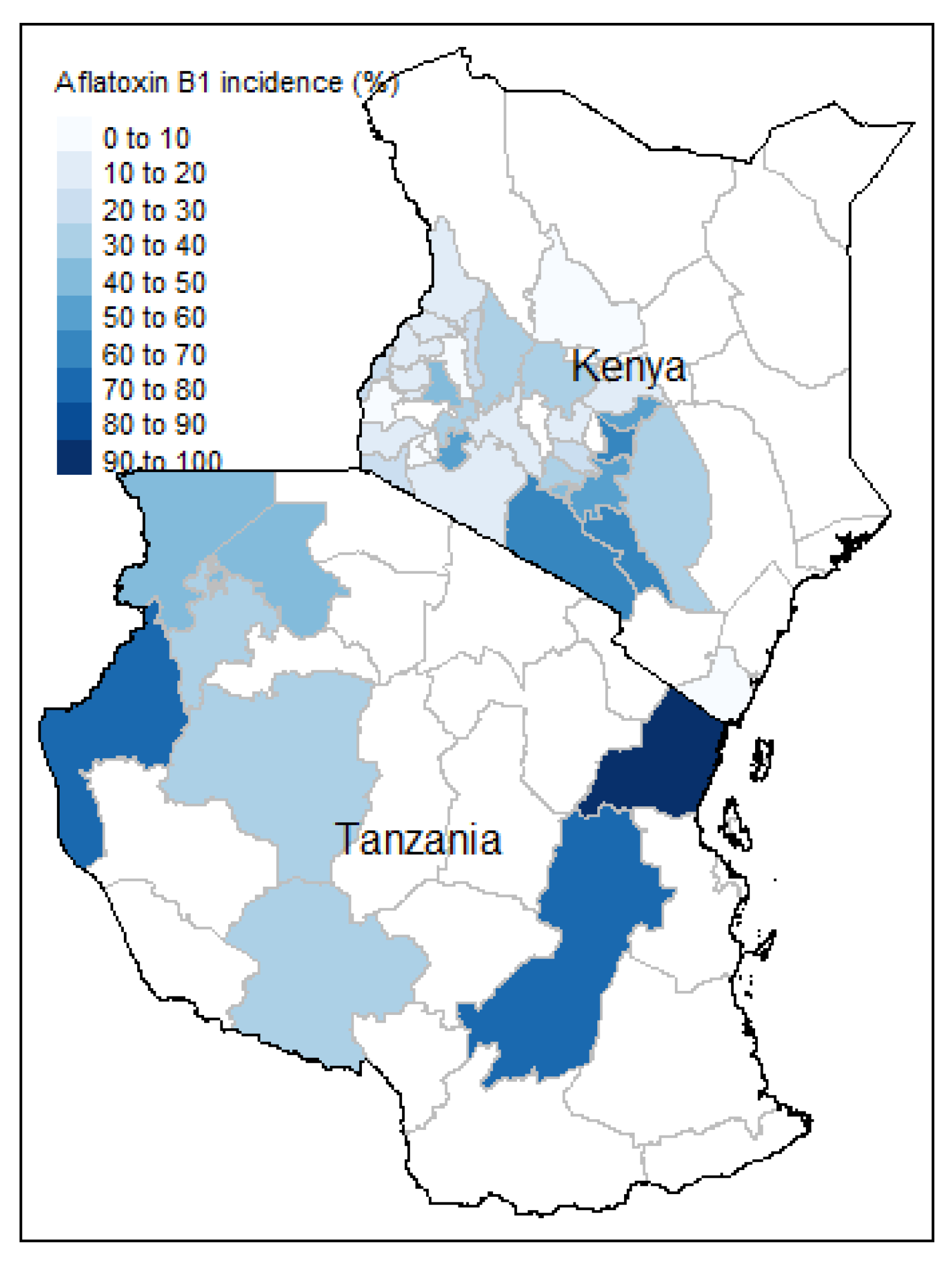
| Number of Samples | 571 |
|---|---|
| Incidence | 29.4% |
| Median level of aflatoxin of samples with detectable level of aflatoxin i.e., greater than LOD (0.2) | 4.775 µg/kg |
| Maximum level of aflatoxin | 6074 µg/kg |
| Number of counties surveyed in Kenya | 38 |
| Number of regions surveyed in Tanzania | 12 |
| Measures | LR | CART | SVM | RF |
|---|---|---|---|---|
| Average Accuracy % | 64.9 | 64.1 | 70.2 | 80.0 |
| Sensitivity % | 57.5 | 56.4 | 60.8 | 82.9 |
| Specificity % | 72.4 | 71.8 | 80.0 | 76.2 |
| Pos Pred Value % | 67.5 | 66.7 | 74.8 | 77.2 |
| Neg Pred Value % | 63.0 | 62.2 | 67.0 | 81.6 |
| Parameters | Odds Ratio | 2.5% | 97.5% |
|---|---|---|---|
| Intercept | 1.579 | 0.819 | 3.062 |
| Early rain | 0.556 | 0.441 | 0.695 |
| Late temp | 1.574 | 1.298 | 1.920 |
| Variable | Relative Importance |
|---|---|
| Early rain | 100.0 |
| Early RH | 77.9 |
| Late rain | 29.6 |
| Late temp | 26.8 |
| Early temp | 8.5 |
| Late RH | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Temba, B.A.; Darnell, R.E.; Gichangi, A.; Lwezaura, D.; Pardey, P.G.; Harvey, J.J.; Karanja, J.; Massomo, S.M.S.; Ota, N.; Wainaina, J.M.; et al. The Influence of Weather on the Occurrence of Aflatoxin B1 in Harvested Maize from Kenya and Tanzania. Foods 2021, 10, 216. https://doi.org/10.3390/foods10020216
Temba BA, Darnell RE, Gichangi A, Lwezaura D, Pardey PG, Harvey JJ, Karanja J, Massomo SMS, Ota N, Wainaina JM, et al. The Influence of Weather on the Occurrence of Aflatoxin B1 in Harvested Maize from Kenya and Tanzania. Foods. 2021; 10(2):216. https://doi.org/10.3390/foods10020216
Chicago/Turabian StyleTemba, Benigni A., Ross E. Darnell, Anne Gichangi, Deogratias Lwezaura, Philip G. Pardey, Jagger J. Harvey, James Karanja, Said M. S. Massomo, Noboru Ota, James M. Wainaina, and et al. 2021. "The Influence of Weather on the Occurrence of Aflatoxin B1 in Harvested Maize from Kenya and Tanzania" Foods 10, no. 2: 216. https://doi.org/10.3390/foods10020216
APA StyleTemba, B. A., Darnell, R. E., Gichangi, A., Lwezaura, D., Pardey, P. G., Harvey, J. J., Karanja, J., Massomo, S. M. S., Ota, N., Wainaina, J. M., Fletcher, M. T., & Kriticos, D. J. (2021). The Influence of Weather on the Occurrence of Aflatoxin B1 in Harvested Maize from Kenya and Tanzania. Foods, 10(2), 216. https://doi.org/10.3390/foods10020216











