Abstract
In the present study, the toxicity and physiological disorders of the essential oil isolated from Artemisia annua flowers were assessed against one of the main insect pests of mulberry, Glyphodes pyloalis Walker, announcing one of the safe and effective alternatives to synthetic pesticides. The LC50 (lethal concentration to kill 50% of tested insects) values of the oral and fumigant bioassays of A. annua essential oil were 1.204 % W/V and 3.343 μL/L air, respectively. The A. annua essential oil, rich in camphor, artemisia ketone, β-selinene, pinocarvone, 1,8-cineole, and α-pinene, caused a significant reduction in digestive and detoxifying enzyme activity of G. pyloalis larvae. The contents of protein, glucose, and triglyceride were also reduced in the treated larvae by oral and fumigant treatments. The immune system in treated larvae was weakened after both oral and fumigation applications compared to the control groups. Histological studies on the midgut and ovaries showed that A. annua essential oil caused an obvious change in the distribution of the principal cells of tissues and reduction in yolk spheres in oocytes. Therefore, it is suggested that the essential oil from A. annua flowers, with wide-range bio-effects on G. pyloalis, be used as an available, safe, effective insecticide in the protection of mulberry.
1. Introduction
The mulberry (Morus sp. (Rosales: Moraceae)) leaves are used for rearing silkworm (Bombyx mori L. (Lepidoptera: Bombycidae)). The importance of lesser mulberry pyralid Glyphodes pyloalis Walker (Lepidoptera: Pyralidae)) is from the larvae damaging mulberry leaves and the transmission of plant pathogenic agents [1]. The extensive use of synthetic chemical pesticides has led to many concerns about the safety of humans, beneficial insects, and the environment [2,3]. Thus, management of insect pest through eco-friendly and biodegradable agents is critical in sericulture.
The essential oils obtained from several parts of plants, including leaves, flowers, fruits, twigs, bark, seeds, wood, rhizomes, and roots, are made as secondary metabolites in the plant and possess diverse chemical compositions [4]. The effectiveness of essential oils as a more sustainable pest management tool has been noted previously [5,6,7]. It can easily be inferred from their biodegradable nature and safety compared to many of the synthetic insecticides. Since they have multiple target sites in insects, their application is less likely to result in resistance in comparison with synthetic insecticides [8]. It was indicated that plant-derived essential oils may have several effects, including ovicidal, ovipositional deterrents, feeding deterrents, growth retardants, and inhibition in detoxification enzymes [9,10,11].
The annual wormwood, Artemisia annua L. (Asterales: Asteraceae), native to temperate Asia, has been naturalized in many countries [12]. The A. annua has traditionally been used to treat certain diseases of humans, including asthma, fever, malaria, skin diseases, jaundice, circulatory disorders, and hemorrhoids [13]. Although our previous findings of the essential oil or extracts in the vegetative stage of A. annua showed the high potential of this medicinal plant species on insect pest control [14,15,16,17,18], the insecticidal effects of its floral essential oil were evaluated against G. pyloalis in the present study.
The evaluation of lethal (acute) and sublethal (chronic) effects of essential oil extracted from A. annua flowers on G. pyloalis was the main objective of the current study, recommending a biorational and available agent as a possible replacement for synthetic insecticides. Fumigant toxicity is considered to be a non-residual treatment in which no residue will commonly remain for future contaminants. In oral toxicity, the pest is eliminated by swallowing infested food, and it is a suitable method for controlling leaf-eating pests. Therefore, fumigant and oral toxicity and the effect on some key enzymes and biochemical compounds, immunology, digestive system in the larvae, and the ovary of emerged adults of insects, along with the chemical analysis of the essential oil, were evaluated.
2. Materials and Methods
2.1. Insects’ Rearing
The larvae of G. pyloalis were handpicked from a mulberry orchard within the University of Guilan campus, Rasht (37.2682° N, 49.5891° E), Iran. The larvae were maintained on fresh leaves of ‘Shin Ichinoise’ mulberry variety in disposable transparent containers (high-density polyethylene plastic containers, 10 × 20 × 5 cm) in a rearing room set at 25 ± 1 °C, 75 ± 5% RH (Relative Humidity), and 16:8 L:D (Light:Dark). The emerging adults were reserved in glass jars (18 × 7 × 5 cm), in which fresh leaves were positioned for egg laying, and 10% honey-soaked cotton wool was provided for feeding.
2.2. Essential Oil
2.2.1. Extraction of the Essential Oil
The mature and immature flowers of A. annua (autumn 2018) were collected on the University of Guilan campus. Samples were dried on a table out of direct sunlight for about a week until sufficiently dry to form a powder when ground. The dried flowers were made into a fine powder by a grinder (354, Moulinex, Normandy, France), and a solution was made with distilled water (50 g/750 mL). The solution was let to stand in the dark at laboratory room temperature for 24 h to maximum essential oil extraction. The mixture was distilled to extract the essential oil using a Clevenger apparatus (J3230, Sina glass, Tehran, Iran). The distillation process was run for two hours and the obtained essential oil was dried over anhydrous sodium sulfate. The obtained essential oil was stored in dark glass vials at 4 °C in a refrigerator until used.
2.2.2. Determination of Essential Oil Composition
The essential oil was analyzed through gas chromatography (Agilent Technologies 7890B) coupled with a mass spectrometer (Agilent Technologies 5977A), which was armed with an HP-5MS ((5%-phenyl)-methylpolysiloxane) capillary column with a 30-m length, 0.25-mm width, and an internal thickness of 0.25 µm. Helium gas at a 1 mL/min flow rate was used, while the column temperature started from 50 and reached to 280 °C at a rate of 5 °C/min. A 10% A. annua essential oil solution in methanol (v/v) was prepared, and 1 µL of solution was injected. Spectra were obtained in the electron impact mode with 70 eV of ionization energy. The scan range was between 30–600 m/z. The identification of components was performed by comparing mass spectral fragmentation patterns and retention indices with those described in the databases [19,20].
2.3. Insecticidal Activity
2.3.1. Oral Toxicity
Initial tests were conducted to assist in selecting the appropriate range of concentrations. Bioassays were carried out on 4th instar larvae, which were deprived of nutrition for 4 h before the onset of experiments. The essential oil concentrations of 0.5, 0.7, 1, 1.4 and 2% (W/V) in acetone as solvent (Merck, Darmstadt, Germany) were selected. For bioassays, mulberry leaf disks (8 cm in diameter) were immersed in desired concentrations for 10 s and then air-dried at room temperature for 30 min. Ten 4th instar G. pyloalis were placed on each disk. The mortality was documented after 24 h. Control groups were placed on disks treated with acetone. The control and treated groups were replicated four times.
2.3.2. Fumigant Activity
In order to carry out fumigation bioassays, two transparent polyethylene plastic containers (Pharman polymer company, Rasht, Iran) were used. A 250-mL container was used to place 10 4th instar larvae of mulberry pyralid. They were provided with fresh mulberry leaf disks, and the container top was covered with fine cotton fabric for aeration. The container was then placed inside a 1000-mL container. The desired amount of pure essential oil was poured onto filter papers (Whatman No. 1) cut to 2 cm in diameter using a micro applicator. It was then placed in the corner of the larger container, and its lid tightly sealed using Parafilm. The concentrations of 2, 3, 4, 5 and 6 µL/L air were used for this bioassay based on the initial tests. The controls were treated in the same way without any treatments of the filter papers. All tests were replicated four times.
2.4. Digestive Enzymes’ Assays
In order to evaluate digestive enzymes activity, the larvae that were treated with LC50, LC30, and LC10 (Lethal Concentration to kill 50, 30, and 10% of insects, respectively) dosages of essential oil obtained from oral and fumigant bioassays and the controls were dissected in ringer’s solution (9% v/v NaCl and isotonic) 24 h after treatment and their digestive systems (only midguts) were dissected out. Five midguts for each treatment and control were first homogenized in 500 µL of universal buffer (50 mM sodium phosphate-borate at pH 7.1) in a tissue homogenizer (DWK885300-0001-1EA, Merk, Darmstadt, Germany). The supernatant was then kept at −20 °C until analyzed.
2.4.1. The α-Amylase Activity
The reagent dinitrosalicylic acid (DNS, Sigma, St. Louis, MI, USA) in 1% soluble starch was used to estimate α-amylase activity according to the method of Bernfeld (1955) [21]. Briefly, 20 µL of the enzyme was poured into 40 μL of soluble starch and 100 μL of universal buffer (pH 7). The mixture was incubated for 30 min at 35 °C, and DNS (100 μL) was then added to stop the reaction. The absorbance was read at 540 nm in an ELISA reader (Awareness, Temecula, CA, USA).
2.4.2. Protease Assay
The protease activity was assessed by addition of 200 μL of casein solution casein (1%) to 100 μL of enzyme and 100 μL universal buffer (pH 7). Then, the obtained mixture was incubated at 37 °C for 60 min [22]. The mixture was centrifuged at 8000× g within 15 min and the absorbance was read at 440 nm.
2.4.3. Lipase Estimation
The method of Tsujita et al. (1989) [23] was adopted to estimate lipase. Concisely, 10 μL enzyme, 18 μL p-nitrophenyl butyrate (50 mM), and 172 μL universal buffer (pH 7) were mixed and incubated at 37 °C for 30 min. The absorbance was recorded at 405 nm in the ELISA reader.
2.4.4. The α- and β-Glucosidase Estimation
Here, we used Triton X-100 in order to hydrolyze glucosidases (α- and β-) for 20 h at 40 °C in a ratio of 10 mg of Triton X-100/mg protein. Then, we incubated 75 mL p-nitrophenyl-α-d-glucopyranoside (pNaG, 5 mM), p-nitrophenyl-β-d-glucopyranoside (pNbG, 5 mM), 125 mL universal buffer (made of 2%Mol MES (2-(N-morpholino)ethanesulfonic acid), glycine, and succinate, 100 mM, pH 5.0), and 50 mL enzyme solution. In order to stop the reaction, 2 mL of sodium carbonate (1 M) was used and the absorbance was read at 450 nm [24].
2.5. Detoxifying Enzymes’ Assays
Quantitative analyses of biochemical constituents were carried out on insects remaining after treatments with LC10, LC30, and LC50 and controls. To quantify the whole body protein, the method of Bradford (1976) [25], using the kit (GDA01A, Biochem Co., Tehran, Iran), was incorporated, while glucose and triglyceride were measured by Siegert (1987) [26] method and the triglyceride diagnostic kit, respectively (Pars Azmoon Co., Tehran, Iran). Key enzymes including esterase (general esterases with α- and β-naphthyl acetate substrates), glutathione S-transferase (GST), and phenol oxidase (PO) were assessed by the method described by van Asperen (1962) [27], Habing et al. (1974) [28], and Parkinson and Weaver (1999) [29], respectively.
2.6. Hematological Study
The amount of various circulating blood cells in mm−3 of larval lesser mulberry pyralid treated with sublethal doses of A. annua oil and in controls were assessed. The hemolymph was drawn from one of the larval prolegs, cutting by a fine scissor, using a capillary glass tube (10 µL for each treatment). Then, the blood was diluted five times with a solution of anticoagulant (0.017 M EDTA, 0.186 M NaCl, 0.098 M NaOH, and 0.041 M citric acid at pH 4.5). An improved Neubauer hemocytometer (mlabs, HBG, Giessen, Germany) [30] was used to assess the total cells using the formula of Jones (1962) [31]. A drop of hemolymph was collected from cut proleg of treated and control larvae. A smear was formed and stained with diluted Giemsa (Merck, Darmstadt, Germany) in distilled water (1:9) for 25 min, then just dipped in a saturated solution of lithium carbonate, and, finally, washed with distilled water. Permanent slides were prepared in Canada balsam (Merck Darmstadt, Germany). The percentage profile of different cells was done after identification and counting of 200 cells per slide [32].
Immunity Responses
Initially the treated or control larvae were made immobile by keeping them on ice cubes for five minutes. Then, they were surface sterilized and injected with 1 × 104 spores/mL in 0.01% Tween-80 of Beauveria bassiana (IRAN403C isolate) or latex beads (1:10 dilution for each suspension and Tween-80, respectively) on the second abdominal sternum using a 10-µL Hamilton syringe. The treated larvae were then transferred to glass jars and were given fresh leaves of mulberry. The control larvae were injected with 1 µL of distilled water comprising 0.01% of Tween-80 only. The hemolymph was collected 24 h post-injection from each larva, and the number of nodules formed was scored in a hemocytometer [33]. The counting was repeated four times for each group.
2.7. Histological Studies of Larvae Midgut and Adults’ Ovary
The larvae midguts were separated from the whole dissected gut in insect ringer and were immediately fixed in aqueous Buine solution for 24 h [10]. Also, the ovary of adults (2 days old), emerging from either treated or control larvae, were separated and fixed. The tissues were processed for embedding in paraffin after being dehydrated in grades of ethanol alcohol and also cleaned by xylene. The fixed tissues were then cut by 5-μM thickness through a rotary microtome (Model 2030; Leica, Wetzlar, Germany). The hematoxylin and eosin were used for staining and then permanent slides were thus prepared, observed, and photographed under a light microscope (M1000 light microscope; Leica, Wetzlar, Germany) armed with an EOS 600D digital camera (Canon, Tokyo, Japan).
2.8. Statistical Analysis
LC values were determined using the Polo-Plus software (2002) [34]. All the data were analyzed by ANOVA (SAS Institute, Cary, Cary, NC, USA, 1997) [35], and the comparison of means was performed using Tukey’s multiple comparison test (p < 0.05).
3. Results
3.1. A. annua Essential Oil Analysis
The chemical composition of extracted A. annua essential oil is presented in Table 1. We identified 55 compounds in flowers of this plant, which represent 93.0% of the total composition. Camphor (13.1%), artemisia ketone (11.8%), β-selinene (10.7%), pinocarvone (7.4%), 1,8-cineole (6.8%), and α-pinene (5.9%) were considered as the major compounds detected, all of which are terpenes. However, other groups such as ester and phenylpropene were also recognized (Table 1).

Table 1.
Chemical composition of the of Artemisia annua floral essential oil.
3.2. Insecticidal Activity
Based on oral and fumigant bioassays, A. annua essential oil was toxic to 4th instar larva of G. pyloalis 24 h post treatments. Probit analysis revealed that the LC50 values were 1.204 % W/V and 3.343 μL/L air for oral and fumigant toxicity, respectively. The mortality of tested larvae was augmented with increasing concentration (Table 2). Besides LC50, the LC10 and LC30 values were used to evaluate sublethal bio-activities, including effects on energy reserves, digestive and detoxifying enzymes activity, and hematological and immunity responses and histological study of midgut and ovary of larvae (Table 2).

Table 2.
Probit analysis of the oral and fumigant toxicity of Artemisia annua floral essential oil on 4th instar larva of Glyphodes pyloalis.
3.3. Energy Reserves
The essential oil of A. annua flowers on the energy reserves of G. pyloalis larvae is shown in Table 3. As can be seen, for all macromolecules, increasing dose of essential oil decreased the concentrations of protein, glucose, and triglycerides. For example, doubling the essential oil concentration (LC10 to LC50) reduced glucose by 29% in oral tests, while a 1.7-fold increase in fumigant concentration resulted in a 32% drop in glucose levels. The protein was also affected but the decrease in protein with increasing essential oil levels was insufficient to detect given background variability.

Table 3.
Effect of Artemisia annua flowers’ essential oil on macromolecules in 4th instar larvae of Glyphodes pyloalis.
3.4. Digestive and Detoxifying Enzymes
The effects of A. annua floral essential oil on digestive enzymes’ activity of G. pyloalis larvae was manifested by a decrease in protease, α-glucosidase, β-glucosidase, α-amylase, and lipase contents. The difference was significant between the LC50 versus the control in both oral and fumigant applications while other concentrations of the essential oil produced intermediate responses (Table 4).

Table 4.
Effect of Artemisia annua floral essential oil on digestive enzyme activities in 4th instar larvae of Glyphodes pyloalis.
The effect of essential oil of A. annua flowers on the activity of esterase and glutathione S-transferase (GST) of G. pyloalis larvae is shown in the Table 5. Glutathione S-transferase and esterase contents were reduced significantly when LC50 was applied in both oral and fumigation methods compared to the controls (Table 5).

Table 5.
Effect of the different concentrations of Artemisia annua flowers’ essential oil on the activity of glutathione S-transferase (GST) and esterase in 4th instar larvae of Glyphodes pyloalis.
3.5. Hematological Study and Immunity Responses
The essential oil affected the immune system, which included cellular quantity and quality, phenol oxidase activity, and the immune responses after B. bassiana and latex beads’ injection (Figure 1, Figure 2, Figure 3 and Figure 4). Total hemocyte counts (THC), plasmatocytes and granular cells, nodule formation, and phenol oxidase activity was recorded the lowest in LC50 both in oral and fumigation assays, respectively.
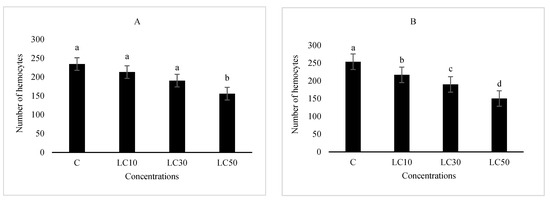
Figure 1.
The effect of Artemisia annua floral essential oil on total hemocyte counts (THC) of Glyphodes pyloalis larvae treated with oral (A) and fumigant (B) assays. Bars with different letters above them indicate significant differences between means at p < 0.05, Tukey’s test. Number of hemocytes ×104.
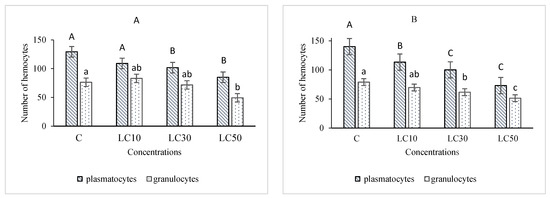
Figure 2.
The effect of Artemisia annua floral essential oil on the plasmatocytes and granular cells of Glyphodes pyloalis larvae treated with oral (A) and fumigant (B) assays. Bars with different letters indicate significant differences among means of each hemocyte at p < 0.05, Tukey’s test. The number of hemocytes ×104.
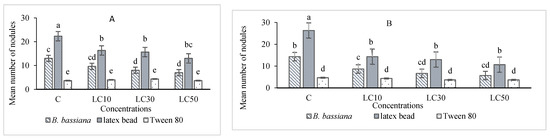
Figure 3.
Effects of Artemisia annua floral essential oil on the nodule formation of Glyphodes pyloalis larvae treated with oral (A) and fumigant assays (B) and inoculated with Beauveria bassiana spores or latex beads. Bars with different letters indicate significant differences between means at p < 0.05. Tukey’s test. The number of hemocytes ×104.
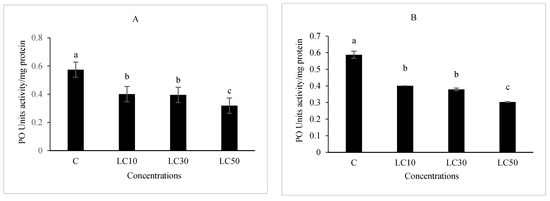
Figure 4.
The effect of Artemisia annua floral essential oil on phenol oxidase (PO) activity of Glyphodes pyloalis larvae treated with oral (A) and fumigant (B) assays. Bars with different letters above them indicate significant differences between means at p < 0.05, Tukey’s test. The number of hemocytes ×104.
3.6. Histological Studies
The histological texture of larval midgut upon treatment with A. annua essential oil revealed significant differences with the controls, the most significant of which was the elongation and separation of epithelial cells losing the compactness (Figure 5). The most significant changes in ovarian structure was thinning of epithelial cells around each follicle compared with that of control. Also, the significant reduction in cytoplasm was seen after vacuolization in yolk spheres of the oocytes (Figure 6).
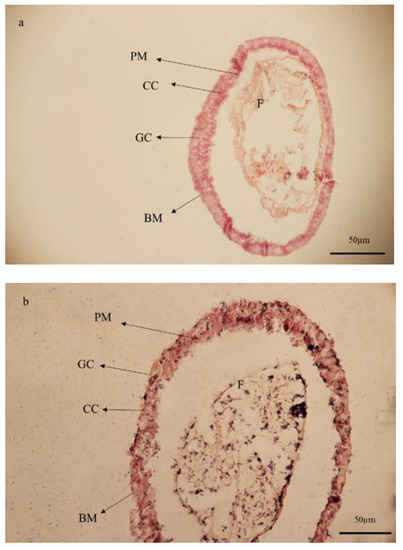
Figure 5.
Light microscopy of the larval midgut of Glyphodes pyloalis in control (a) and after oral treatment with Artemisia annua floral essential oil (b). Normal texture of all cell types (a) was contrasted to changes in size and texture in treated larvae (b). In the midgut of insects treated with essential oil from A. annua the cohesion of the columnar epithelial layer was damaged. (BM) basement membrane, (CC) columnar cell, (GC) goblet cell, and (PM) peritrophic membrane.
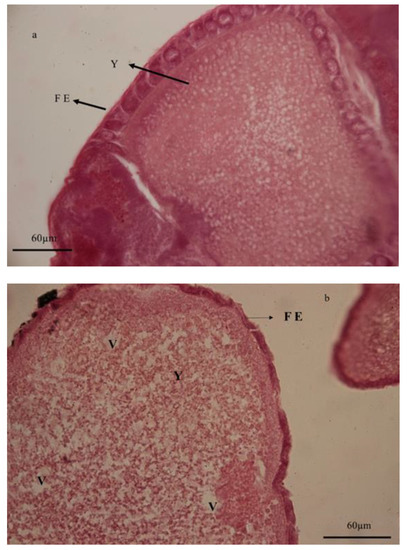
Figure 6.
Histology of ovaries in adults of Glyphodes pyloalis emerging from untreated (a) and treated larvae by Artemisia annua floral essential oil (b). In treatments of the ovarian sheath significant changes and yolk granules were reduced under the influence of vacuolization in cytoplasm compared to the control. (FE) follicular epithelium, (V) vacuole, and (Y) yolk granules.
4. Discussion
The chemical composition of A. annua essential oil in the vegetative stage was investigated in the previous studies [15,36,37,38,39], in which terpenes such as 1,8-cineole, camphor, and artemisia ketone were introduced as major constituents. Although 1,8-cineole (6.8%), camphor (13.1%), and artemisia ketone (11.8%) were also identified as main compounds in the essential oil extracted from A. annua flowers, some other terpenes such as β-selinene (10.7%), pinocarvone (7.4%), and α-pinene (5.9%) had high amounts. However, a range of minor constituents, including compounds from ester and phenylpropene groups, were also recognized. Such differences can be caused by exogenous and endogenous factors, including geographic location, harvesting time, and the growth stage of plants [40]. The chemical composition of each essential oil has a significant impact on its insecticidal activity. For example, the promising insecticidal effects of terpenes like camphor and 1,8-cineole identified and extracted from essential oils were reported [41,42].
Our study clearly showed decreased enzymatic activity in G. pyloalis larvae related to ingestion of A. annua essential oil-treated mulberry leaves. Our findings support earlier findings where disruption in insects’ physiology and their inability to digest food was reported [43,44]. Reduction in α-amylase, protease, and α- and β-glucosidase, and disruptions on immunology and digestive system in the larvae and the ovary of emerged adults of G. pyloalis were described in our results. Such activities are common for botanical insecticides against several insect pests [45,46,47]. Also, there were further supports for the interference or even deformation of midgut cells, which were responsible for the production of key enzymes in insects [15,48].
Protein plays a key role in digestion, metabolism, and also energy conversion. Klowden (2007) [49] believes that reduction in the insect’s protein content after applying biopesticides may stem from the reduction of growth hormone level. We observed a reduction in protein content and also retardation in growth; however, growth hormone level was not worked out. Lipids are other important macromolecules that help the insect reserve energy from feeding. They play a key role in insects’ intermediary metabolism and, therefore, they are essential in insect physiology [49]. Significant reduction in the triglyceride content of G. pyloalis larvae treated with A. annua essential oil was observed in the present study. There are several reasons for reducing insect lipid content after treatments by toxins, alteration in lipid synthesis patterns, and hormonal dysfunction to control its metabolism [49]. Glucose as a key carbohydrate (monosaccharide) was also decreased following treatment with A. annua essential oil. This reduction could be related to reduced feeding following treatment, since the essential oil acts as a deterrent [2]. Any disruption causing reducing resources at larval stages could affect insects’ survival and reproduction in their later generations. A reduction in protein, lipid, and glucose contents may have adverse effects on the reproductive parameters such as egg production, fertility, and fecundity [50].
Detoxifying enzymes, including esterases and glutathione S-transferases, are involved in reducing the impacts of exogenous compounds [51]. In the current study, the activity of detoxifying enzymes, including esterases and glutathione S-transferases, was reduced by essential oil of A. annua flowers. Certainly, the reduced activity of these enzymes is related to their production halt somewhere in the process of production [15].
Insect cellular immunity is considered as the main system challenging natural enemies entering the insect body [52]. The immunocytes provide the insect ability to combat invading organisms by several means including phagocytosis, nodulation, and encapsulation [53]. So, the reduced immunocytes, as shown for G. pyloalis larvae treated with A. annua essential oil in the present study, could cause larvae to become susceptible to any invasion [54,55]. The reduced number of hemocytes is mostly due to cytotoxic effect of the botanicals used [56]. We do believe this toxic effect of botanicals to be more reliable as a reasoning for the reduction of immunocytes [57,58,59].
Phenol oxidase system is considered as the key component in the immune system of insect and a bridge in the gap between cellular and humeral insect immunity. Its action is critically required in the last stage of cellular defense in order to form melanization, a process that terminates the action and kills the pathogenic agent. Phenol oxidase inhibition, documented for G. pyloalis larvae treated with A. annua essential oil in the present study, probably helps to make the insects susceptible to pathogenic agents if they have not received the toxic concentration [45,58,60].
The insect midgut principal cells are the main cells taking the role of producing the enzymes needed for digestion and then absorbing the nutrients. Therefore, any damages to these cells will lower the activities in digestive enzymes already reported by other researchers [15,31,61]. The elongation and separation of midgut epithelial cells of G. pyloalis larvae treated by A. annua essential oil were observed in the present study.
Inhibiting insect reproduction has long been the subject of many studies. In lepidopterans, obtaining all nutrients at larval stages is necessary for reproductive development [62]. So, if larval nutrition is disrupted by any means, it will be reflected in adult reproductive function. Our previous findings and the current study display the changes in morphology and histology of emerging adults [15,31]. Our study showed the essential oil of A. annua brought about subtle changes in ovarian tissue, such as disruption of follicular cells. As the insect tries to compromise to reduce nutrients in detoxification processes, follicles’ cells deplete its content into the oocytes, which then disrupts the cell texture [63].
5. Conclusions
Plant-derived allelochemicals are beneficial agents in controlling pests. As we know, the plant kingdom mainly depends on secondary metabolites to defend against herbivores. With this knowledge in mind, scientists exploit the use of secondary plant chemicals for pest control. One of the main reasons for this increased demand is that the plant-originated chemicals are comparatively safer for humans and the environment. Our study’s results clearly document that the essential oil of A. annua flowers is toxic to larval mulberry pyralid and disrupt its various physiological systems in a way that the insect can hardly get resistance to it. Consequently, this wild-growing plant in Iran can be considered an efficient natural source capable of controlling insect pests. To apply the research results, it is recommended to evaluate the possible side effects of essential oil on mulberry and the biological control agents in future research. Regarding the insect pest’s resistance, identifying specific modes of action of essential oil active components and their overlapping with other insecticides should also be assessed.
Author Contributions
Conceptualization, M.O., J.J.S., and A.E.; methodology, M.O. and J.J.S.; formal analysis, M.O., J.J.S., A.E., and W.N.S.; investigation, M.O.; writing—original draft preparation, M.O., J.J.S., A.E. and P.K.; writing—review and editing, M.O., J.J.S., A.E., P.K. and W.N.S.; supervision, J.J.S. and A.E.; funding acquisition, P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available upon request from the authors.
Acknowledgments
This research was financially supported by the University of Guilan, Rasht, Iran, and was partially supported by Chiang Mai University, Thailand, which is greatly appreciated. W.N.S. participated in this work as part of the activities of the Aromatic Plant Research Center (APRC, https://aromaticplant.org/).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khosravi, R.; Sendi, J.J. Biology and demography of Glyphodes pyloalis Walker (Lepidoptera: Pyralidae) on mulberry. J. Asia-Pacific Èntomol. 2010, 13, 273–276. [Google Scholar] [CrossRef]
- Isman, M.B. Plant essential oils for pest and disease management. Crop. Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Dachbrodt-Saaydeh, S.; Kudsk, P.; Messéan, A. Toward a reduced reliance on conventional pesticides in european agriculture. Plant. Dis. 2016, 100, 10–24. [Google Scholar] [CrossRef] [PubMed]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B.; Grieneisen, M.L. Botanical insecticide research: Many publications, limited useful data. Trends Plant. Sci. 2014, 19, 140–145. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Ziaee, M.; Palla, F. Essential oils extracted from different species of the Lamiaceae plant family as prospective bioagents against several detrimental pests. Molecules 2020, 25, 1556. [Google Scholar] [CrossRef]
- Basaid, K.; Chebli, B.; Mayad, E.H.; Furze, J.N.; Bouharroud, R.; Krier, F.; Barakate, M.; Paulitz, T. Biological activities of essential oils and lipopeptides applied to control plant pests and diseases: A review. Int. J. Pest. Manag. 2020, 1–23. [Google Scholar] [CrossRef]
- Campos, E.V.; Proença, P.L.; Oliveira, J.L.; Bakshi, M.; Abhilash, P.; Fraceto, L.F. Use of botanical insecticides for sustainable agriculture: Future perspectives. Ecol. Indic. 2019, 105, 483–495. [Google Scholar] [CrossRef]
- Nathan, S.S.; Choi, M.-Y.; Paik, C.-H.; Seo, H.-Y. Food consumption, utilization, and detoxification enzyme activity of the rice leaffolder larvae after treatment with Dysoxylum triterpenes. Pestic. Biochem. Physiol. 2007, 88, 260–267. [Google Scholar] [CrossRef]
- Lazarevic, J.; Jevremović, S.; Kostić, I.; Kostić, M.; Vuleta, A.; Jovanović, S.M.; Jovanović, D. Šešlija Toxic, oviposition deterrent and oxidative stress effects of Thymus vulgaris essential oil against Acanthoscelides obtectus. Insects 2020, 11, 563. [Google Scholar] [CrossRef]
- Afraze, Z.; Sendi, J.J.; Karimi-Malati, A.; Zibaee, A. Methanolic extract of winter cherry causes morpho-histological and immunological ailments in mulberry pyralid Glyphodes pyloalis. Front. Physiol. 2020, 11, 908. [Google Scholar] [CrossRef] [PubMed]
- Konovalov, D.; Khamilonov, A.A. Biologically active compounds of Artemisia annua essential oil. Pharm. Pharmacol. 2016, 4, 4–33. [Google Scholar] [CrossRef][Green Version]
- Xiao, L.; Tan, H.; Zhang, L. Artemisia annua glandular secretory trichomes: The biofactory of antimalarial agent artemisinin. Sci. Bull. 2016, 61, 26–36. [Google Scholar] [CrossRef]
- Shekari, M.; Sendi, J.J.; Etebari, K.; Zibaee, A.; Shadparvar, A. Effects of Artemisia annua L. (Asteracea) on nutritional physiology and enzyme activities of elm leaf beetle, Xanthogaleruca luteola Mull. (Coleoptera: Chrysomellidae). Pestic. Biochem. Physiol. 2008, 91, 66–74. [Google Scholar] [CrossRef]
- Hasheminia, S.M.; Sendi, J.J.; Jahromi, K.T.; Moharramipour, S. The effects of Artemisia annua L. and Achillea millefolium L. crude leaf extracts on the toxicity, development, feeding efficiency and chemical activities of small cabbage Pieris rapae L. (Lepidoptera: Pieridae). Pestic. Biochem. Physiol. 2011, 99, 244–249. [Google Scholar] [CrossRef]
- Zibaee, A. Botanical Insecticides and Their Effects on Insect Biochemistry and Immunity. In Pesticides in the Modern World—Pests Control and Pesticides Exposure and Toxicity Assessment; IntechOpen: London, UK, 2011. [Google Scholar]
- Mojarab-Mahboubkar, M.; Sendi, J.J.; Aliakbar, A. Effect of Artemisia annua L. essential oil on toxicity, enzyme activities, and energy reserves of cotton bollworm Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). J. Plant. Prot. Res. 2015, 55, 371–377. [Google Scholar] [CrossRef]
- Oftadeh, M.; Sendi, J.J.; Ebadollahi, A. Toxicity and deleterious effects of Artemisia annua essential oil extracts on mulberry pyralid (Glyphodes pyloalis). Pestic. Biochem. Physiol. 2020, 170, 104702. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas. Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- NIST. NIST17; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2017. [Google Scholar]
- Bernfeld, P. Amylases alpha and beta. Methods Enzymol. 1955, 1, 140–146. [Google Scholar]
- García-Carreño, F.L.; Haard, N.F. Characterization of proteinase classes in Langostilla (Pleuroncodes planipes) and Crayfish (Pacifastacus astacus) extracts. J. Food Biochem. 1993, 17, 97–113. [Google Scholar] [CrossRef]
- Tsujita, T.; Ninomiya, H.; Okuda, H. P-nitrophenyl 865. buyrate hydrolyzing activity of Hormone-senditive lipase 866 from bovine adipose tissue. J. Lipid. Res. 1989, 30, 867–997. [Google Scholar] [CrossRef]
- Ferreira, C.; Terra, W.R. Physical and kinetic properties of a plasma-membrane-bound β-d-glucosidase (cellobiase) from midgut cells of an insect (Rhynchosciara americana larva). Biochem. J. 1983, 213, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-Dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Siegert, K.J. Carbohydrate metabolism in Manduca sexta during late larval development. J. Insect Physiol. 1987, 33, 421–427. [Google Scholar] [CrossRef]
- Van Asperen, K. A study of housefly esterases by means of a sensitive colorimetric method. J. Insect Physiol. 1962, 8, 401–416. [Google Scholar] [CrossRef]
- Habing, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar]
- Parkinson, N.M.; Weaver, R.J. Noxious Components of Venom from the Pupa-Specific Parasitoid Pimpla hypochondriaca. J. Invertebr. Pathol. 1999, 73, 74–83. [Google Scholar] [CrossRef]
- El-Aziz, N.M.A.; Awad, H.H. Changes in the haemocytes of Agrotis ipsilon larvae (Lepidoptera: Noctuidae) in relation to dimilin and Bacillus thuringiensis infections. Micron 2010, 41, 203–209. [Google Scholar] [CrossRef]
- Jones, J.C. Current concepts concerning insect hemocytes. Am. Zoöl. 1962, 2, 209–246. [Google Scholar] [CrossRef]
- Arnold, J.W.; Hinks, C.F. Haemopoiesis in Lepidoptera. I. The multiplication of circulating haemocytes. Can. J. Zool. 1976, 54, 1003–1012. [Google Scholar] [CrossRef]
- Seyedtalebi, F.S.; Safavi, S.; Talaei-Hasanloui, A.R.; Bandani, A.R. Quantitative comparison for some immune responses among Eurygaster integriceps, Ephestia kuehniella and Zophobas morio against the entomopathogenic fungus Beuveria bassiana. Invert. Surviv. J. 2017, 14, 174–181. [Google Scholar] [CrossRef]
- LeOra Software. Polo Plus, a User’s Guide to Probit or Logit Analysis; LeOra Software: Berkeley, CA, USA, 2002. [Google Scholar]
- SAS Institute. SAS/STAT User’s Guide for Personal Computers; SAS Institute: Cary, NC, USA, 1997. [Google Scholar]
- Mojarab-Mahboubkar, M.; Sendi, J.J. Chemical composition, insecticidal and physiological effect of methanol extract of sweet wormwood (Artemisia annua L.) on Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Toxin Rev. 2016, 35, 106–115. [Google Scholar] [CrossRef]
- Bedini, S.; Flamini, G.; Cosci, F.; Ascrizzi, R.; Echeverria, M.C.; Guidi, L.; Landi, M.; Benelli, G.; Conti, B. Artemisia spp. essential oils against the disease-carrying blowfly Calliphora vomitoria. Parasit. Vectors 2017, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Singh, P. The Genus Artemisia: A 2012–2017 Literature review on chemical composition, antimicrobial, insecticidal and antioxidant activities of essential oils. Medicines 2017, 4, 68. [Google Scholar] [CrossRef]
- Nigam, M.; Atanassova, M.; Mishra, A.P.; Pezzani, R.; Devkota, H.P.; Plygun, S.; Salehi, B.; Setzer, W.N.; Sharifi-Rad, J. Bio-active compounds and health benefits of Artemisia species. Nat Prod. Commun 2019. [Google Scholar] [CrossRef]
- Isman, M.B. Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem. Rev. 2020, 19, 235–241. [Google Scholar] [CrossRef]
- Kumar, P.; Mishra, S.; Malik, A.; Satya, S. Repellent, larvicidal and pupicidal properties of essential oils and their formulations against the housefly, Musca domestica. Med. Veter Èntomol. 2011, 25, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Filomeno, C.A.; Barbosa, L.C.; Teixeira, R.R.; Pinheiro, A.L.; Farias, E.D.S.; Ferreira, J.S.; Picanço, M.C. Chemical diversity of essential oils of Myrtaceae species and their insecticidal activity against Rhyzopertha dominica. Crop. Prot. 2020, 137, 105309. [Google Scholar] [CrossRef]
- Shannag, H.K.; Capinera, J.L.; Freihat, N.M. Effects of neem-based insecticides on consumption and utilization of food in larvae of Spodoptera eridania (Lepidoptera: Noctuidae). J. Insect Sci. 2015, 15, 152. [Google Scholar] [CrossRef]
- Zou, C.; Wang, Y.; Zou, H.; Ding, N.; Geng, N.; Cao, C.; Zhang, G. Sanguinarine in Chelidonium majus induced antifeeding and larval lethality by suppressing food intake and digestive enzymes in Lymantria dispar. Pestic. Biochem. Physiol. 2019, 153, 9–16. [Google Scholar] [CrossRef]
- Zibaee, A.; Bandani, A.R. A study on the toxicity of the medicinal plant, Artemisia annua L. (Astracea) extracts the Sunn pest, Eurygaster integriceps Puton (Heteroptera: Scutelleridae). J. Plant. Prot. Res. 2010, 50, 48–54. [Google Scholar] [CrossRef]
- Bezzar-Bendjazia, R.; Kilani-Morakchi, S.; Maroua, F.; Aribi, N. Azadirachtin induced larval avoidance and antifeeding by disruption of food intake and digestive enzymes in Drosophila melanogaster (Diptera: Drosophilidae). Pestic. Biochem. Physiol. 2017, 143, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Magierowicz, K.; Górska-Drabik, E.; Sempruch, C. The effect of Tanacetum vulgare essential oil and its main components on some ecological and physiological parameters of Acrobasis advenella (Zinck.) (Lepidoptera: Pyralidae). Pestic. Biochem. Physiol. 2020, 162, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Sharma, A.; Dagar, V.S.; Kumar, S. Effects of β-sitosterol on growth, development and midgut enzymes of Helicoverpa armigera Hübner. Arch. Biol. Sci. 2020, 72, 271–278. [Google Scholar] [CrossRef]
- Klowden, M.J. Physiological Systems in Insects, 2nd ed.; Academic Press: Cambridge, MA, USA, 2007. [Google Scholar]
- Wu, M.-Y.; Ying, Y.-Y.; Zhang, S.-S.; Li, X.-G.; Yan, W.-H.; Yao, Y.-C.; Shah, S.; Wu, G.; Yang, F.-L. Effects of diallyl trisulfide, an active substance from garlic essential oil, on energy metabolism in male moth Sitotroga cerealella (Olivier). Insects 2020, 11, 270. [Google Scholar] [CrossRef]
- Nattudurai, G.; Baskar, K.; Paulraj, M.G.; Islam, V.I.H.; Ignacimuthu, S.; Duraipandiyan, V. Toxic effect of Atalantia monophylla essential oil on Callosobruchus maculatus and Sitophilus oryzae. Environ. Sci. Pollut. Res. 2016, 24, 1619–1629. [Google Scholar] [CrossRef]
- Perazzolo, L.M.; Gargioni, R.; Ogliari, P.; Barracco, M.A. Evaluation of some hemato-immunological parameters in the shrimp Farfantepenaeus paulensis submitted to environmental and physiological stress. Aquac. 2002, 214, 19–33. [Google Scholar] [CrossRef]
- Kraaijeveld, A.R.; Limentani, E.C.; Godfray, H.C.J. Basis of the trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. In Proceedings of the Royal Society B: Biological Sciences; The Royal Society: London, UK, 2001; Volume 268, pp. 259–261. [Google Scholar]
- Ghasemi, V.; Yazdi, A.K.; Tavallaie, F.Z.; Sendi, J.J. Effect of essential oils from Callistemon viminalis and Ferula gummosaon toxicity and on the hemocyte profile of Ephestia kuehniella (Lep.: Pyralidae). Arch. Phytopathol. Plant. Prot. 2014, 47, 268–278. [Google Scholar] [CrossRef]
- Sadeghi, R.; Eshrati, M.R.; Mortazavian, S.M.M.; Jamshidnia, A. The Effects of the essential oils isolated from four ecotypes of cumin (Cuminum cyminum L.) on the blood cells of the pink stem borer, Sesamia cretica Ledere (Lepidoptera: Noctuidae). J. Kans. Èntomol. Soc. 2019, 92, 390–399. [Google Scholar] [CrossRef]
- Clark, K.D.; Strand, M.R. Hemolymph melanization in the silkmoth bombyx mori involves formation of a high molecular mass complex that metabolizes tyrosine. J. Biol. Chem. 2013, 288, 14476–14487. [Google Scholar] [CrossRef]
- Khanikor, B.; Bora, D. Effect of plant based essential oil on immune response of silkworm, Antheraea assama Westwood (Lepidoptera: Saturniidae). Int. J. Ind. Èntomol. 2012, 25, 139–146. [Google Scholar] [CrossRef][Green Version]
- Ghoneim, K. Disturbed hematological and immunological parameters of insects by botanicals as an effective approach of pest control: A review of recent progress. South Asian. J. Exp. Biol. 2018, 1, 112–144. [Google Scholar]
- Rahimi, V.; Hajizadeh, J.; Zibaee, A.; Sendi, J.J. Changes in immune responses of Helicoverpa armigera Hübner followed by feeding on Knotgrass, Polygonum persicaria agglutinin. Arch. Insect Biochem. Physiol. 2019, 101, e21543. [Google Scholar] [CrossRef] [PubMed]
- Cerenius, L.; Lee, B.L.; Söderhäll, K. The proPO-system: Pros and cons for its role in invertebrate immunity. Trends Immunol. 2008, 29, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Murfadunnisa, S.; Vasantha-Srinivasan, P.; Ganesan, R.; Senthil-Nathan, S.; Kim, T.-J.; Ponsankar, A.; Kumar, S.D.; Chandramohan, D.; Krutmuang, P. Larvicidal and enzyme inhibition of essential oil from Spheranthus amaranthroids (Burm.) against lepidopteran pest Spodoptera litura (Fab.) and their impact on non-target earthworms. Biocatal. Agric. Biotechnol. 2019, 21. [Google Scholar] [CrossRef]
- Riddiford, L.M. How does juvenile hormone control insect metamorphosis and reproduction? Gen. Comp. Endocrinol. 2012, 179, 477–484. [Google Scholar] [CrossRef]
- Reis, T.C.; Soares, M.A.; Dos Santos, J.B.; Dos Santos, C.A.; Serrão, J.E.; Zanuncio, J.C.; Ferreira, E.A. Atrazine and nicosulfuron affect the reproductive fitness of the predator Podisus nigrispinus (Hemiptera: Pentatomidae). Anais da Academia Brasileira de Ciências 2018, 90, 3625–3633. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).