A Multi-Ingredient Formula Ameliorates Exercise-Induced Fatigue by Changing Metabolic Pathways and Increasing Antioxidant Capacity in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
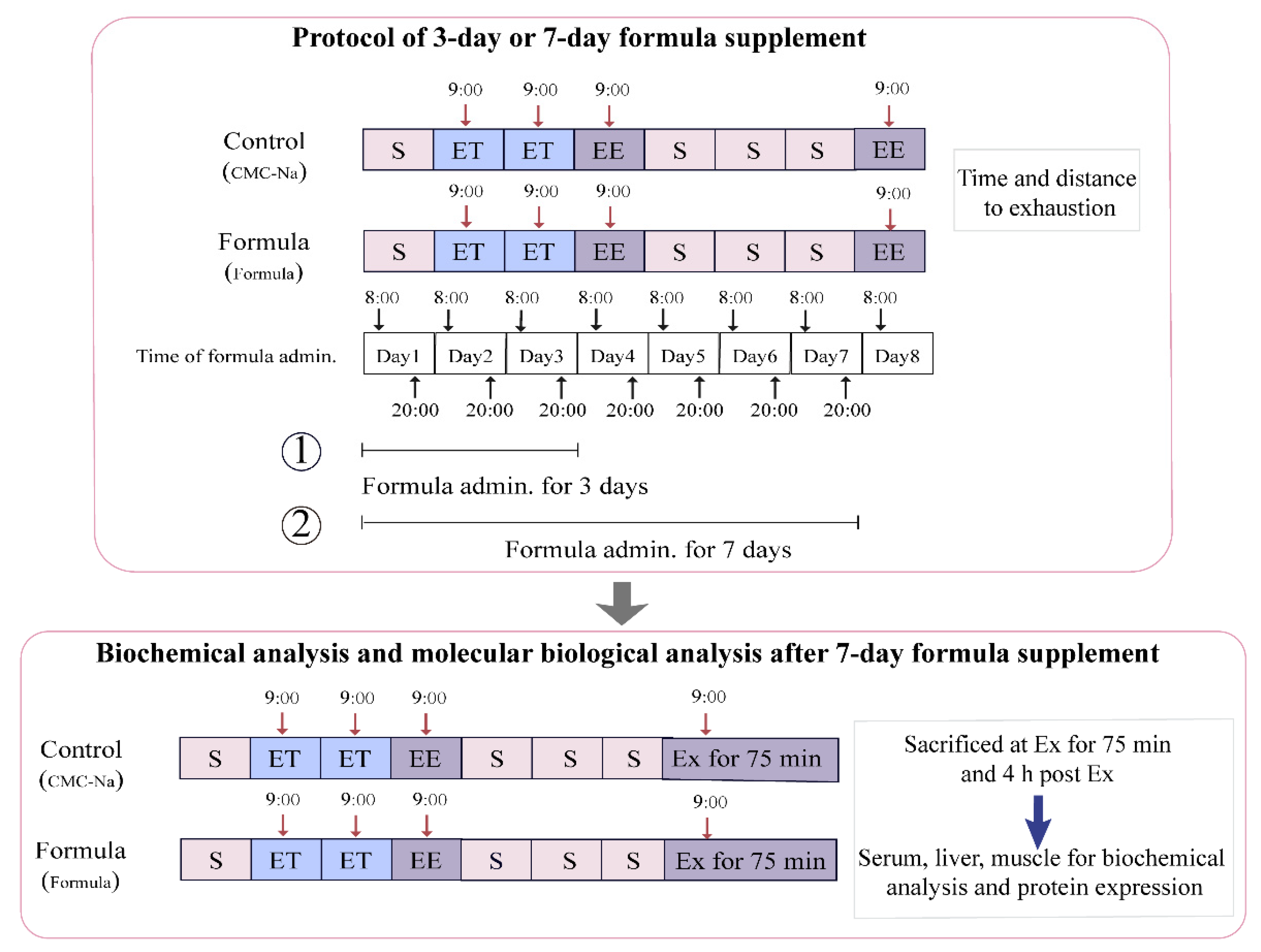
2.2. Animals and Treatments
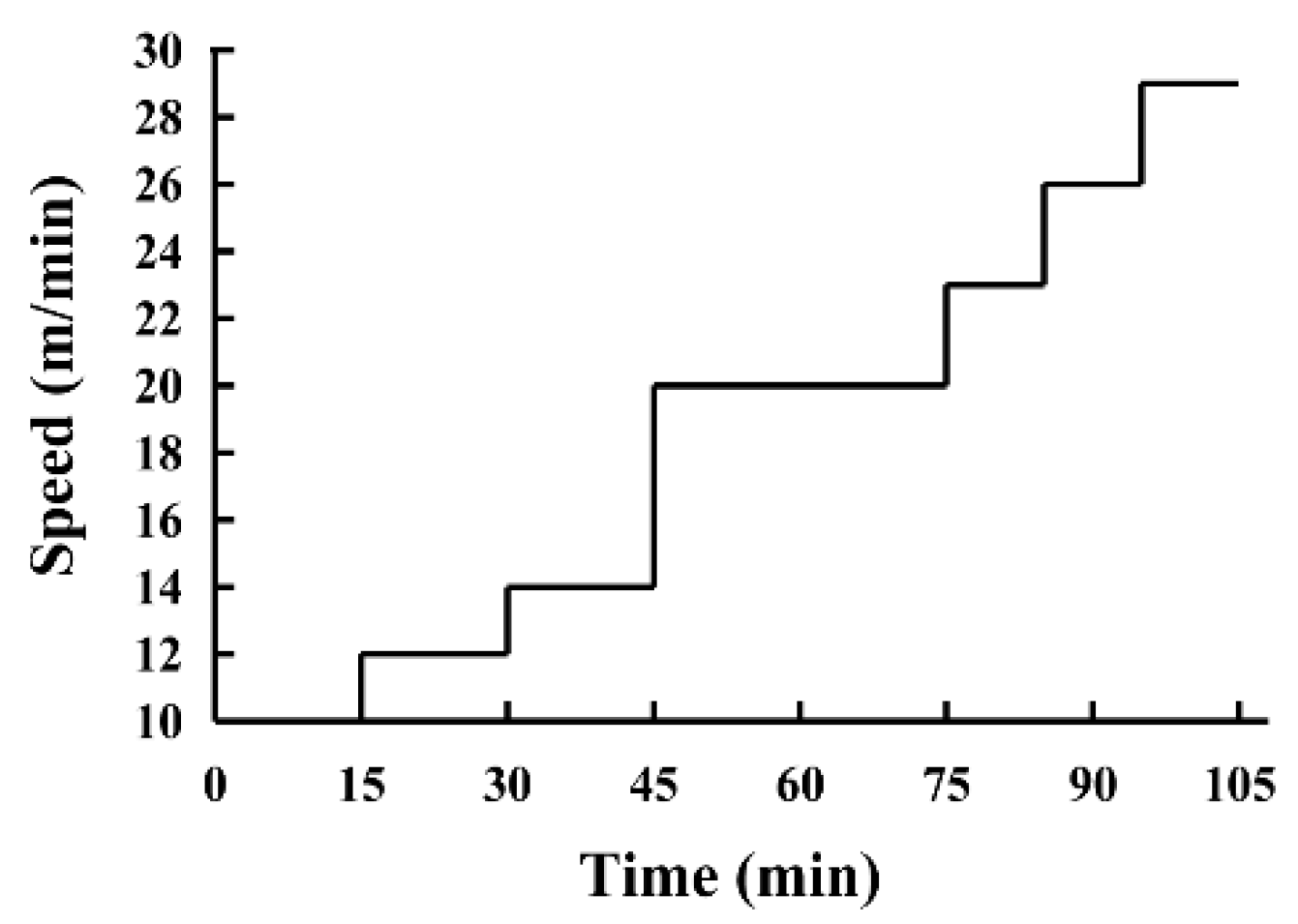
2.3. Exhaustive Treadmill Test
2.4. Sample Collection
2.5. Biochemical Serum and Tissue Analysis
2.6. Western Blot Analysis
2.7. Immunofluorescence of the Muscle Paraffin Sections for GLUT4 Analysis
2.8. Statistical Analysis
3. Results
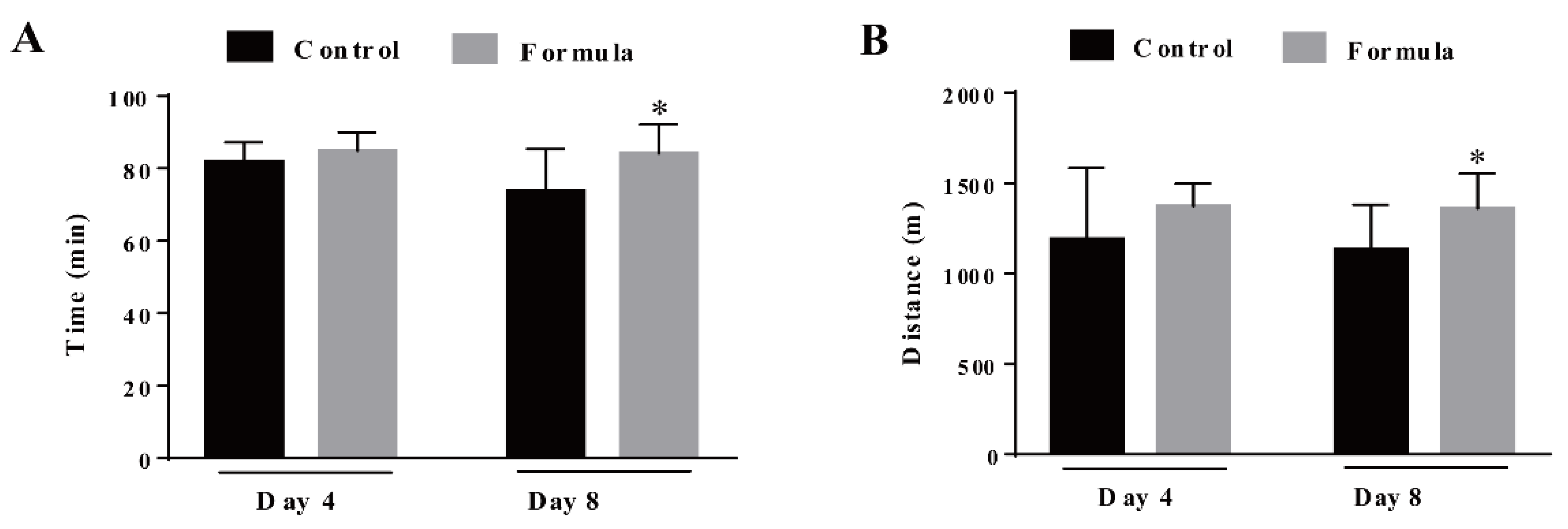
3.1. The Formula Supplement Prolongs the Time and Running Distance Required to Reach Exhaustion during the Exhaustive Treadmill Test
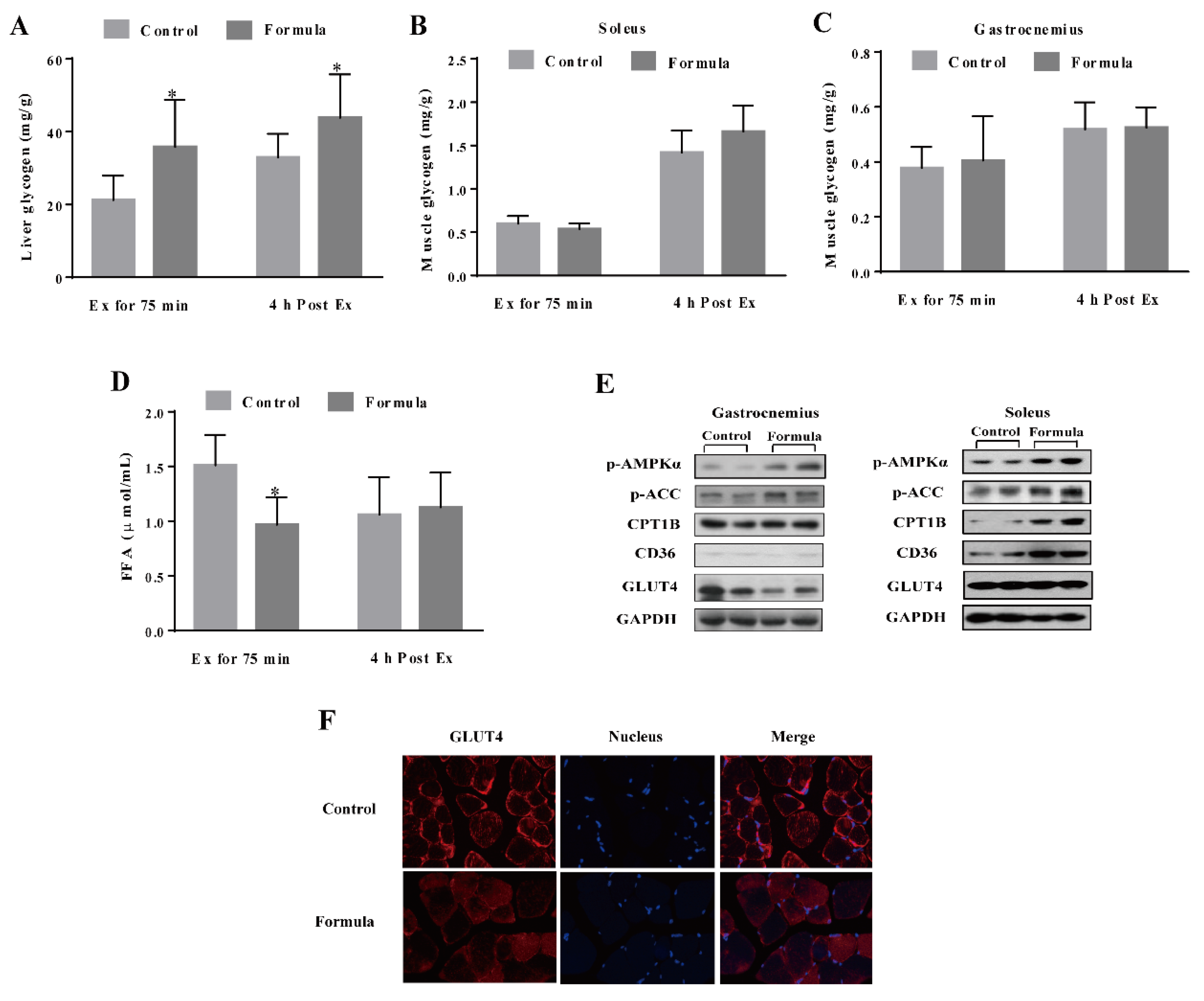
3.2. The Formula Supplement Induces Glucose and Lipid Metabolism Changes in Response to Acute Exercise
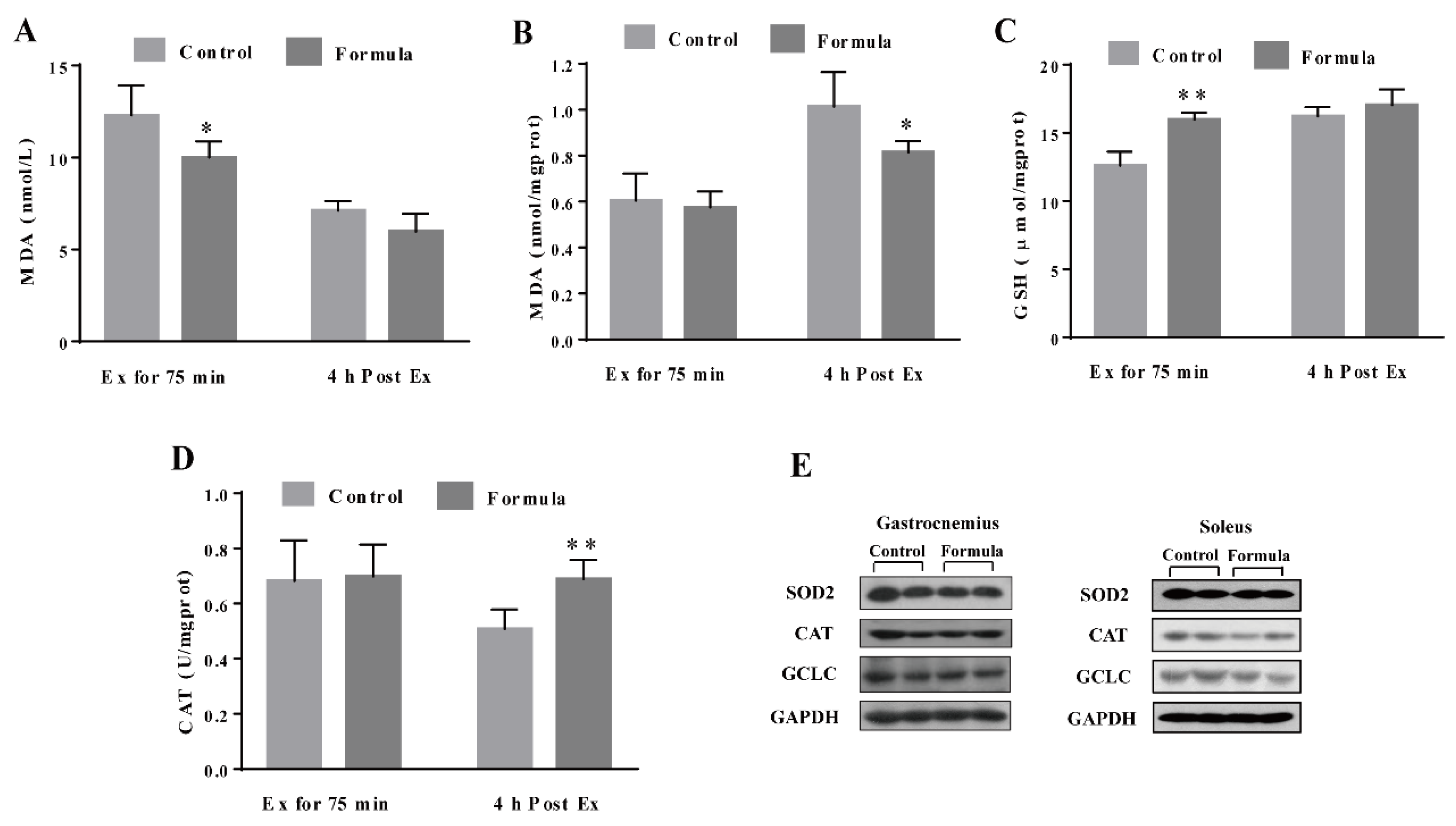
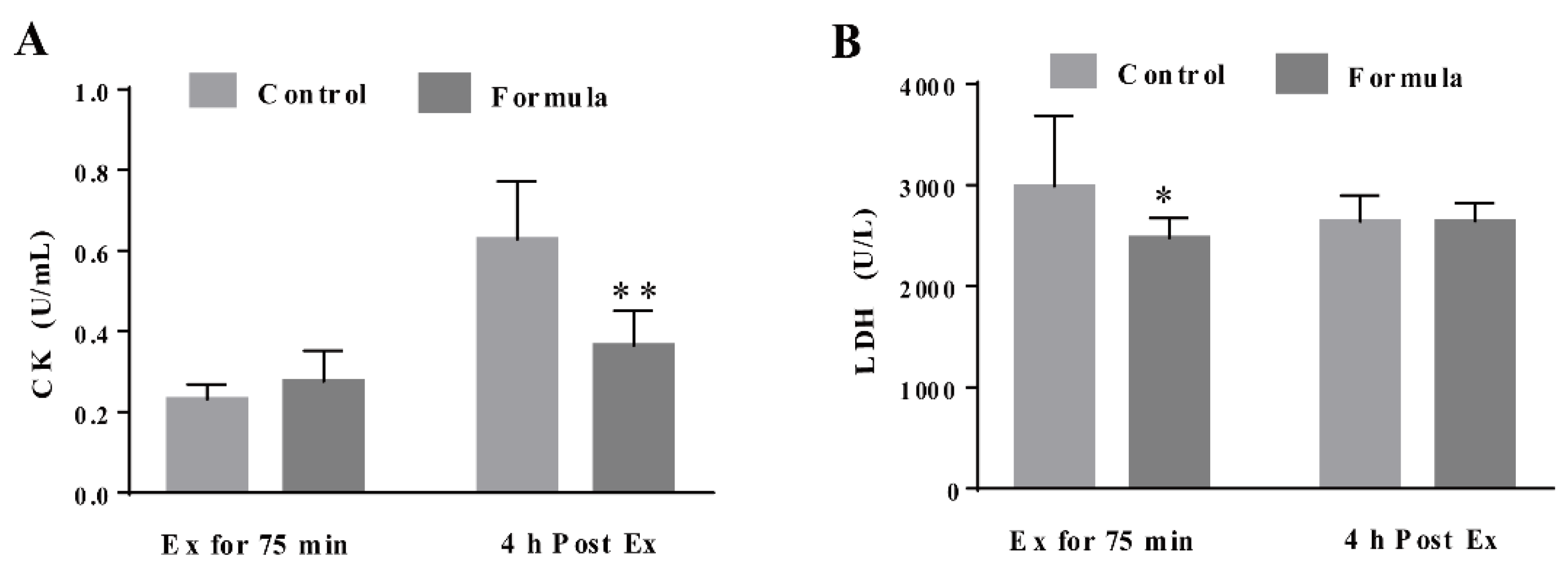
3.3. The Formula Supplement Ameliorates Exercise-Induced Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lambert, E.V.; Noakes, T.D.; Hampson, D.B.; Gibson, A.S.C.; Lambert, M.I. Exercise and fatigue-control mechanisms. Int. Sport Med. J. 2001, 2, 1–14. [Google Scholar]
- Finaud, J.; Lac, G.; Filaire, E. Oxidative stress relationship with exercise and training review. Sports Med. 2006, 36, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Falavigna, G.; de Araújo Junior, J.A.; Rogero, M.M.; de Oliveira Pires, I.S.; Pedrosa, R.G.; Junior, E.M.; de Castro, I.A.; Tirapegui, J. Effects of diets supplemented with branched-chain amino acids on the performance and fatigue mechanisms of rats submitted to prolonged physical exercise. Nutrients 2012, 4, 1767–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamad Shalan, N.A.A.; Mustapha, N.M.; Mohamed, S. Morinda citrifolia leaf enhanced performance by improving angiogenesis, mitochondrial biogenesis, antioxidant, anti-inflammatory & stress responses. Food Chem. 2016, 212, 443–452. [Google Scholar] [PubMed]
- Oh, S.; Komine, S.; Warabi, E.; Akiyama, K.; Ishii, A.; Ishige, K.; Mizokami, Y.; Kuga, K.; Horie, M.; Miwa, Y.; et al. Nuclear factor (erythroid derived 2)-like 2 activation increases exercise endurance capacity via redox modulation in skeletal muscles. Sci. Rep. 2017, 7, 12902–12912. [Google Scholar] [PubMed] [Green Version]
- Mensah, F.; Bansal, A.S.; Ford, B.; Cambridge, G. Chronic fatigue syndrome and the immune system: Where are we now? Neurophysiol. Clin. 2017, 47, 131–138. [Google Scholar]
- Britannica, T.E.O.E. Fatigue, in physiology. In Columbia Electronic Encyclopedia, 6th ed.; Columbia University: New York, NY, USA, 2019; p. 1. [Google Scholar]
- Ma, X.; Chen, H.; Cao, L.; Zhao, S.; Zhao, C.; Yin, S.; Hu, H. Mechanisms of physical fatigue and its applications in nutritional interventions. J. Agric. Food Chem. 2021, 69, 6755–6768. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, Y.; Iwasawa, K.; Yamaguchi, M. Acute supplementation of valine reduces fatigue during swimming exercise in rats. Biosci. Biotechnol. Biochem. 2018, 82, 856–861. [Google Scholar]
- Artioli, G.G.; Gualano, B.; Smith, A.; Stout, J.; Lancha, A.H., Jr. The role of β-alanine supplementation on muscle carnosine and exercise performance. Med. Sci. Sports Exerc. 2010, 42, 1162–1173. [Google Scholar] [CrossRef]
- Danaher, J.; Gerber, T.; Wellard, R.M.; Stathis, C.G. The effect of β-alanine and NaHCO3 co-ingestion on buffering capacity and exercise performance with high-intensity exercise in healthy males. Eur. J. Appl. Physiol. 2014, 114, 1715–1724. [Google Scholar] [CrossRef] [Green Version]
- Gualano, B.; Novaes, R.B.; Artioli, G.G.; Freire, T.O.; Coelho, D.F.; Scagliusi, F.B.; Rogeri, P.S.; Roschel, H.; Ugrinowitsch, C.; Lancha, A.H., Jr. Effects of creatine supplementation on glucose tolerance and insulin sensitivity in sedentary healthy males undergoing aerobic training. Amino Acids 2008, 34, 245–250. [Google Scholar] [CrossRef]
- Yarizadh, H.; Shab-Bidar, S.; Zamani, B.; Vanani, A.N.; Baharlooi, H.; Djafarian, K. The effect of l-carnitine supplementation on exercise-induced muscle damage: A systematic review and meta-analysis of randomized clinical trials. J. Am. Coll. Nutr. 2020, 39, 457–468. [Google Scholar] [CrossRef]
- Seiler, S.E.; Koves, T.R.; Gooding, J.R.; Wong, K.E.; Stevens, R.D.; Ilkayeva, O.R.; Wittmann, A.H.; DeBalsi, K.L.; Davies, M.N.; Lindeboom, L.; et al. Carnitine acetyltransferase mitigates metabolic inertia and muscle fatigue during exercise. Cell Metab. 2015, 22, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D. Effects of the dietary flavonoid quercetin upon performance and health. Curr. Sports Med. Rep. 2009, 8, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Patrizio, F.; Ditroilo, M.; Felici, F.; Duranti, G.; De Vito, G.; Sabatini, S.; Sacchetti, M.; Bazzucchi, I. The acute effect of quercetin on muscle performance following a single resistance training session. Eur. J. Appl. Physiol. 2018, 118, 1021–1031. [Google Scholar] [CrossRef]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Davis, B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1071–R1077. [Google Scholar] [CrossRef] [Green Version]
- Cholewa, J.M.; Guimaraes-Ferreira, L.; Zanchi, N.E. Effects of betaine on performance and body composition: A review of recent findings and potential mechanisms. Amino Acids 2014, 46, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Farney, T.M.; Trepanowski, J.F.; McCarthy, C.G.; Canale, R.E. Effect of betaine supplementation on plasma nitrate/nitrite in exercise-trained men. J. Int. Soc. Sport Nutr. 2011, 8, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Yan, M.; Ye, L.; Yin, S.; Lu, X.; Liu, X.; Lu, S.; Cui, J.; Fan, L.; Kaplowitz, N.; Hu, H. Glycycoumarin protects mice against acetaminophen-induced liver injury predominantly via activating sustained autophagy. Br. J. Pharmacol. 2018, 175, 3747–3757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjrer, M. Hepatic glucose production during exercise. Adv. Exp. Med. Biol. 1998, 441, 117–127. [Google Scholar]
- Wilmore, J.H.; Costill, D.L.; Kenney, W.L. Physiology of Sport and Exercise; Human Kinetics, Inc.: Champaign, IL, USA, 2015; p. 648. [Google Scholar]
- Gonzalez, J.T.; Fuchs, C.J.; Betts, J.A.; van Loon, L.J.C. Liver glycogen metabolism during and after prolonged endurance-type exercise. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E543–E553. [Google Scholar] [CrossRef]
- Fritzen, A.M.; Lundsgaard, A.M.; Jeppesen, J.; Christiansen, M.L.; Bienso, R.; Dyck, J.R.; Pilegaard, H.; Kiens, B. 5’-AMP activated protein kinase alpha2 controls substrate metabolism during post-exercise recovery via regulation of pyruvate dehydrogenase kinase 4. J. Physiol. 2015, 593, 4765–4780. [Google Scholar] [CrossRef] [Green Version]
- Coburn, C.T.; Hajri, T.; Ibrahimi, A.; Abumrad, N.A. Role of CD36 in membrane transport and utilization of long-chain fatty acids by different tissues. J. Mol. Neurosci. 2001, 16, 117–121. [Google Scholar] [CrossRef]
- Van Weeghel, M.; Abdurrachim, D.; Nederlof, R.; Argmann, C.A.; Houtkooper, R.H.; Hagen, J.; Nabben, M.; Denis, S.; Ciapaite, J.; Kolwicz, S.C.; et al. Increased cardiac fatty acid oxidation in a mouse model with decreased malonyl-CoA sensitivity of CPT1B. Cardiovasc. Res. 2018, 114, 1324–1334. [Google Scholar] [CrossRef]
- Stephens, F.B.; Constantin-Teodosiu, D.; Greenhaff, P.L. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J. Physiol. 2007, 581, 431–444. [Google Scholar] [CrossRef]
- Chen, X.; Liang, D.; Huang, Z.; Jia, G.; Zhao, H.; Liu, G. Anti-fatigue effect of quercetin on enhancing muscle function and antioxidant capacity. J. Food Biochem. 2021, 45, e13968. [Google Scholar] [CrossRef]
- Fukaya, M.; Sato, Y.; Kondo, S.; Adachi, S.I.; Yoshizawa, F.; Sato, Y. Quercetin enhances fatty acid beta-oxidation by inducing lipophagy in AML12 hepatocytes. Heliyon 2021, 7, e7324. [Google Scholar] [CrossRef] [PubMed]
- Bach, A. Carnitine biosynthesis in mammals. Reprod. Nutr. Dev. 1982, 22, 583–596. [Google Scholar] [CrossRef]
- Just, P.; Charawi, S.; Denis, R.G.P.; Savall, M.; Traore, M.; Foretz, M.; Bastu, S.; Magassa, S.; Senni, N.; Sohier, P.; et al. Lkb1 suppresses amino acid-driven gluconeogenesis in the liver. Nat. Commun. 2020, 11, 6127–6142. [Google Scholar] [CrossRef]
- Mason, S.A.; Morrison, D.; McConell, G.K.; Wadley, G.D. Muscle redox signalling pathways in exercise. Role of antioxidants. Free Radic. Biol. Med. 2016, 98, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Bazzucchi, I.; Patrizio, F.; Ceci, R.; Duranti, G.; Sgrò, P.; Sabatini, S.; Sabatini, S.; Di, L.L.; Sacchetti, M.; Felici, F. The effects of quercetin supplementation on eccentric exercise-induced muscle damage. Nutrients 2019, 11, 205. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.A.; Oh, S.H.; Lee, G.H.; Hoa, P.T.; Jin, S.W.; Chung, Y.C.; Lee, Y.C.; Jeong, H.G. Platycodon grandiflorum-derived saponin attenuates the eccentric exercise-induced muscle damage. Food Chem. Toxicol. 2018, 112, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.Y.; Ambigaipalan, P.; Shahidi, F. Preparation of quercetin esters and their antioxidant activity. J. Agric. Food Chem. 2019, 67, 10653–10659. [Google Scholar] [CrossRef] [PubMed]
- Blancquaert, L.; Everaert, I.; Missinne, M.; Baguet, A.; Stegen, S.; Volkaert, A.; Petrovic, M.; Vervaet, C.; Achten, E.; De, M.M.; et al. Effects of histidine and β-alanine supplementation on human muscle carnosine storage. Med. Sci. Sports Exerc. 2017, 49, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Jargin, S.V. Use of carnosine for oxidative stress reduction in different pathologies. Oxidative Med. Cell. Longev. 2016, 2016, 2939087. [Google Scholar] [CrossRef] [PubMed]
- Sung, D.J.; Kim, S.; Kim, J.; An, H.S.; So, W.Y. Role of l-carnitine in sports performance: Focus on ergogenic aid and antioxidant. Sci. Sports 2016, 31, 177–188. [Google Scholar] [CrossRef]
| Ingredients | Functional Characteristics | References |
|---|---|---|
| BCAAs (Valine, isoleucine and leucine) | Reduces the formation of 5-hydroxytryptamine, delays central fatigue, protects muscle damage, energy substrates for long-term endurance exercise inhibits muscle lactate production, reduces serum creatine level, promotes adipose decomposition (isoleucine), and stimulates muscle protein synthesis (leucine). | [4,10] |
| β-alanine | Involved in carnosine synthesis and enhances the total buffering ability of the skeletal muscles. | [11,12] |
| Creatine | Rapidly supplies energy in a short time, improves glucose uptake, increases muscle glycogen accumulation, acts as an H+ buffer, and promotes aerobic metabolism. | [13] |
| L-carnitine | Promotes fatty acids oxidation, enhances the antioxidant effect, improves glucose tolerance, reduces the blood lactate level, improves maximum oxygen consumption by the body, and alleviates muscle injury. | [14,15] |
| Quercetin | Enhances the antioxidant effect, promotes mitochondrial synthesis, reduces protein or amino acid consumption, increases fat mobilization, protects mitochondrial functionality, and improves energy metabolism. | [16,17,18] |
| Betaine | Increases the creatine synthesis, improves the nitric oxide level in the blood, increases blood flow, stimulates lipid decomposition, inhibits adipogenesis, stimulates the release of autocrine/endocrine IGF-1 and insulin receptor signaling pathways, stimulates growth hormone secretion, and increases protein synthesis. | [19,20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Ma, X.; Cao, L.; Zhao, S.; Zhao, C.; Yin, S.; Hu, H. A Multi-Ingredient Formula Ameliorates Exercise-Induced Fatigue by Changing Metabolic Pathways and Increasing Antioxidant Capacity in Mice. Foods 2021, 10, 3120. https://doi.org/10.3390/foods10123120
Chen H, Ma X, Cao L, Zhao S, Zhao C, Yin S, Hu H. A Multi-Ingredient Formula Ameliorates Exercise-Induced Fatigue by Changing Metabolic Pathways and Increasing Antioxidant Capacity in Mice. Foods. 2021; 10(12):3120. https://doi.org/10.3390/foods10123120
Chicago/Turabian StyleChen, Hui, Xuan Ma, Lixing Cao, Shuang Zhao, Chong Zhao, Shutao Yin, and Hongbo Hu. 2021. "A Multi-Ingredient Formula Ameliorates Exercise-Induced Fatigue by Changing Metabolic Pathways and Increasing Antioxidant Capacity in Mice" Foods 10, no. 12: 3120. https://doi.org/10.3390/foods10123120
APA StyleChen, H., Ma, X., Cao, L., Zhao, S., Zhao, C., Yin, S., & Hu, H. (2021). A Multi-Ingredient Formula Ameliorates Exercise-Induced Fatigue by Changing Metabolic Pathways and Increasing Antioxidant Capacity in Mice. Foods, 10(12), 3120. https://doi.org/10.3390/foods10123120







