Protein Enrichment of Wheat Bread with Microalgae: Microchloropsis gaditana, Tetraselmis chui and Chlorella vulgaris
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Cultivation of Microalgae
2.1.2. Taxonomic Identification
2.1.3. Cell Wall Disruption of Microalgae by Bead Milling
2.1.4. Drying of Cell-Wall Disrupted Microalgae Biomass
2.2. Soxhlet Treatment with Ethanol
2.3. Compositional Analysis
2.4. Assessment of Protein Quality
2.5. In Vitro Protein Digestibility
2.6. Dough Rheology
2.7. Baking
2.8. Colour Measurement
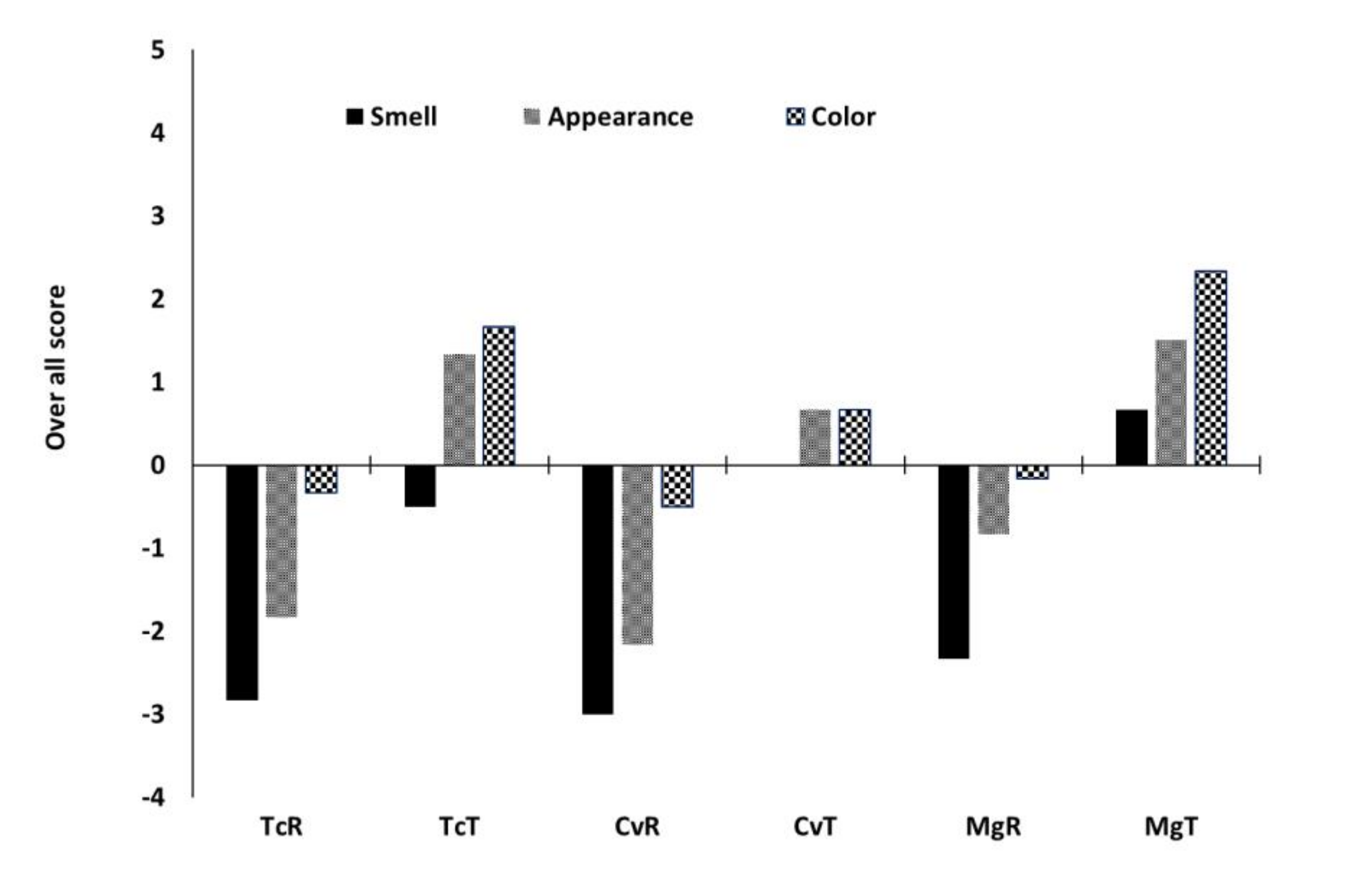
2.9. Preliminary Sensory Assessment
2.10. Statistical Analysis
3. Results and Discussion
3.1. Nutrient Composition of Microalgae Samples
3.2. Amino Acid Composition and Assessment of Protein Quality in the Microalgae
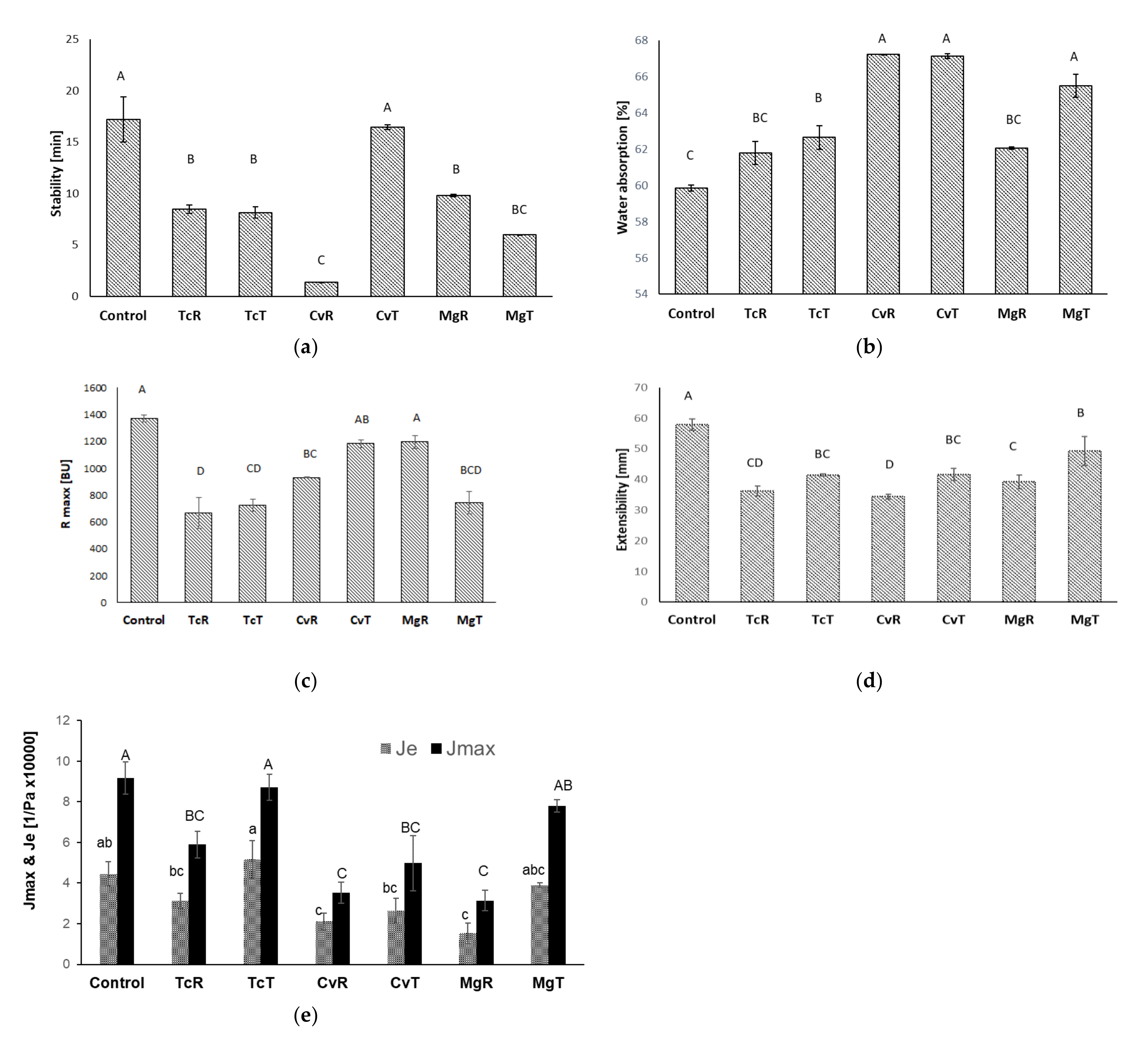
3.3. Rheological Properties of Microalgae Substituted Wheat Doughs
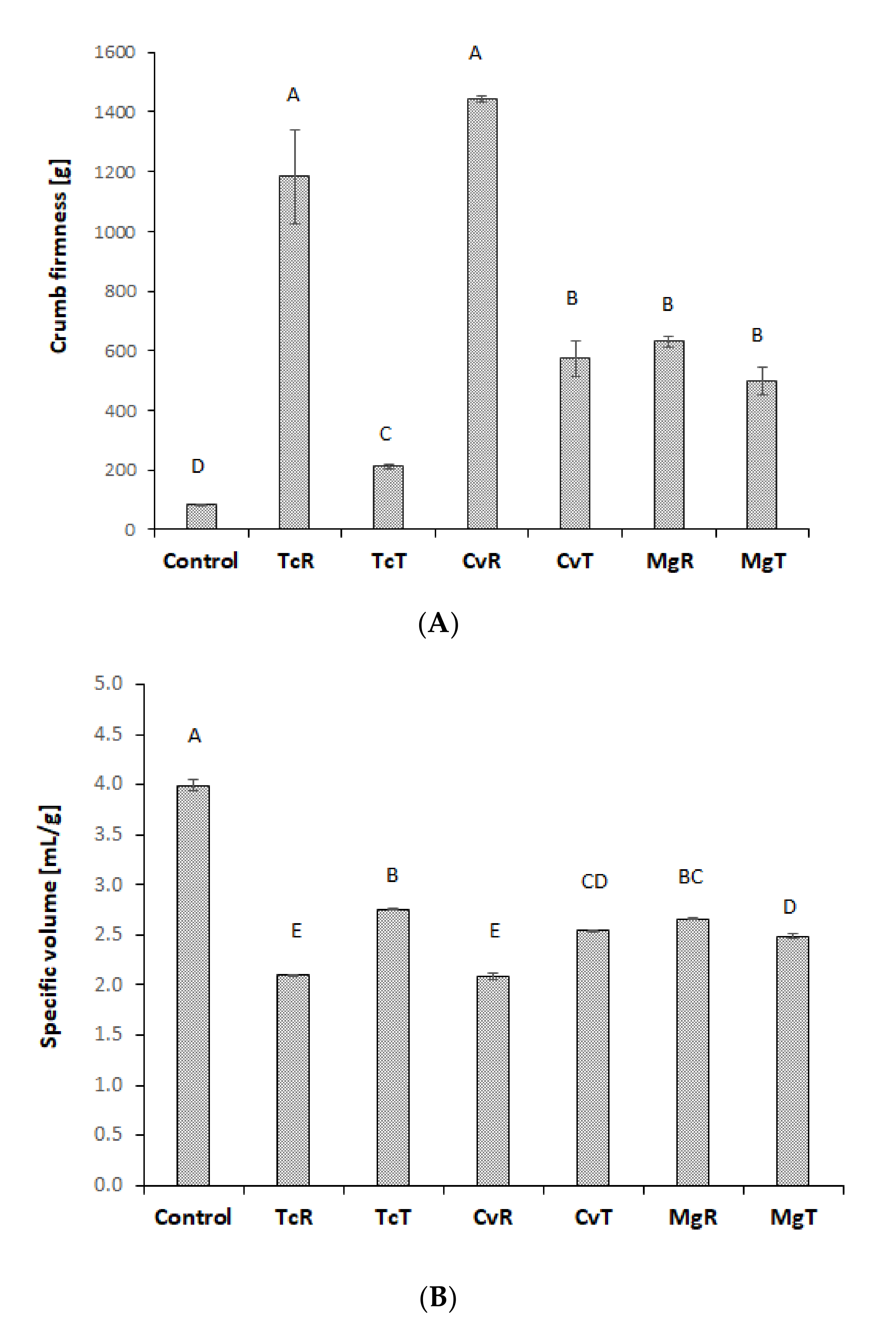
3.4. Bread Quality
3.5. Amino Acid Composition and Assessment of Protein Quality in the Microalgae
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Hafsa, Y.A.; Amel, D.; Samia, S.; Sidahmed, S. Evaluation of nutritional and sensory properties of bread enriched with Spirulina. Ann. Food Sci. Technol. 2014, 15, 270–275. [Google Scholar]
- Ak, B.; Avşaroğlu, E.; Isiko, O.; Özyurt, G.; Kafkas, E.; Etyemez, M.; Uslu, L. Nutritional and Physicochemical Characteristics of Bread Enriched with Microalgae Spirulina platensis. Int. J. Eng. Res. Appl. 2016, 6, 30–38. [Google Scholar]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J. Appl. Phycol. 2015, 27, 1109–1119. [Google Scholar] [CrossRef]
- Oser, B.L. Method for integrating essential amino acid content in the nutritional evaluation of protein. J. Am. Diet. Assoc. 1951, 27, 396–402. [Google Scholar] [CrossRef]
- Kent, M.; Welladsen, H.M.; Mangott, A.; Li, Y. Nutritional Evaluation of Australian Microalgae as Potential Human Health Supplements. PLoS ONE 2015, 10, e0118985. [Google Scholar] [CrossRef] [PubMed]
- Sanjari, S.; Sarhadi, H.; Shahdadi, F. Investigating the Effect of Spirulina Platensis Microalgae on Textural and Sensory Properties of Baguette Bread. Available online: https://www.semanticscholar.org/paper/Investigating-the-Effect-of-Spirulina-Platensis-on-Sanjari-Sarhadi/633eb5e61fff809cb45b2e695545f5dc686cfe44 (accessed on 2 October 2020).
- Graça, C.; Fradinho, P.; Sousa, I.; Raymundo, A. Impact of Chlorella vulgaris on the rheology of wheat flour dough and bread texture. LWT 2018, 89, 466–474. [Google Scholar] [CrossRef]
- Lafarga, T. Effect of microalgal biomass incorporation into foods: Nutritional and sensorial attributes of the end products. Algal Res. 2019, 41, 101566. [Google Scholar] [CrossRef]
- Nunes, M.C.; Fernandes, I.; Vasco, I.; Sousa, I.; Raymundo, A. Tetraselmis chuii as a Sustainable and Healthy Ingredient to Produce Gluten-Free Bread: Impact on Structure, Colour and Bioactivity. Foods 2020, 9, 579. [Google Scholar] [CrossRef]
- García-Segovia, P.; Pagán-Moreno, M.J.; Lara, I.; Martínez-Monzó, J. Effect of microalgae incorporation on physicochemical and textural properties in wheat bread formulation. J. Food Sci. Technol. Int. 2017, 23, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Khemiri, S.; Khelifi, N.; Nunes, M.C.; Ferreira, A.; Gouveia, L.; Smaali, I.; Raymundo, A. Microalgae biomass as an additional ingredient of gluten-free bread: Dough rheology, texture quality and nutritional properties. Algal Res. 2020, 50, 101998. [Google Scholar] [CrossRef]
- Qazi, W.M.; Ballance, S.; Uhlen, A.K.; Kousoulaki, K.; Haugen, J.-E.; Rieder, A. Protein enrichment of wheat bread with the marine green microalgae Tetraselmis chuii—Impact on dough rheology and bread quality. LWT 2021, 143, 111115. [Google Scholar] [CrossRef]
- Finney, K.F.; Pomeranz, Y.; Bruinsma, B.L. Use of algae Dunaliella as a protein supplement in bread. Cereal Chem. 1984, 61, 402–406. [Google Scholar]
- Ahmed, J.; Thomas, L. Effect of xanthan and guar gum on the pasting, stickiness and extensional properties of brown wheat flour/β-glucan composite doughs. LWT 2018, 87, 443–449. [Google Scholar] [CrossRef]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Zanella, L.; Vianello, F. Microalgae of the genus Nannochloropsis: Chemical composition and functional implications for human nutrition. J. Funct. Foods 2020, 68, 103919. [Google Scholar] [CrossRef]
- Saleh, A.M.; EL-Fouly, M.M.; Shaheen, A.B.; EL-Malky, W.; Khorshid, A.M. Blaugrüne Algen bei der Brotherstellung. Getreide Mehl. Brot. 1987, 41, 19–21. [Google Scholar]
- Harris, E.H. Chlamydomonas in the Laboratory. In The Chlamydomonas Sourcebook, 2nd ed.; Elsevier: San Diago, CA, USA, 2009. [Google Scholar] [CrossRef]
- Kliphuis, A.M.J.; de Winter, L.; Vejrazka, C.; Martens, D.E.; Janssen, M.; Wijffels, R.H. Photosynthetic efficiency of Chlorella sorokiniana in a turbulently mixed short light-path photobioreactor. Biotechnol Prog. 2010, 26, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Khaw, Y.S.; Khong, N.M.H.; Shaharuddin, N.A.; Yusoff, F.M. A simple 18S rDNA approach for the identification of cultured eukaryotic microalgae with an emphasis on primers. J. Microbiol. Methods 2020, 172, 105890. [Google Scholar] [CrossRef] [PubMed]
- Kokkali, M.E.; Kleinegris, B.O.; Kleinegris, D.M.M.; de Vree, J.H.; Kousoulaki, K. Bead Type and Agitator Configuration Effects in Bead Milling of Phaeodactylum Tricornutum and Nannochloropsis sp. on Biomass Disintegration Efficiency and Nutrient Release and Stability; AlgaEurope: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Cohen, S.A.; Michaud, D.P. Synthesis of a Fluorescent Derivatizing Reagent, 6-Aminoquinolyl-N-Hydroxysuccinimidyl Carbamate, and Its Application for the Analysis of Hydrolysate Amino Acids via High-Performance Liquid Chromatography. Anal. Biochem. 1993, 211, 279–287. [Google Scholar] [CrossRef]
- Rieder, A.; Afseth, N.K.; Böcker, U.; Knutsen, S.H.; Kirkhus, B.; Mæhre, H.K.; Ballance, S.; Wubshet, S.G. Improved estimation of in vitro protein digestibility of different foods using size exclusion chromatography. Food Chem. 2021, 358, 129830. [Google Scholar] [CrossRef]
- Kirsten, W.J. Automatic methods for the simultaneous determination of carbon, hydrogen, nitrogen, and sulfur, and for sulfur alone in organic and inorganic materials. Anal. Chem. 1979, 51, 1173–1179. [Google Scholar] [CrossRef]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting Nitrogen into Protein—Beyond 6.25 and Jones’ Factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef]
- Brekken, B. Kvantitativ Bestemmelse av Frie Aminosyrer; SSF-Rapport: Stockholm, Sweden, 1989; A-142, 13 s. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- FAO. Dietary Protein Quality Evaluation in Human Nutrition; 2011; pp. 25–29. Available online: http://www.fao.org/ag/humannutrition/35978-02317b979a686a57aa4593304ffc17f06.pdf (accessed on 10 November 2020).
- Hennig, U.; Hackl, W.; Priepke, A.; Tuchscherer, A.; Souffrant, W.; Metges, C.; Palta, J.; Berger, J. In Pigs the True Ileal Digestibility of Essential Amino Acids of Lupin and Wheat are Equal but the Induced Ileal Basal Endogenous Losses are Different. Available online: https://www.semanticscholar.org/paper/In-pigs-the-true-ileal-digestibility-of-essential-Hennig-Hackl/fb660390bf2270b6e71b22a83e2fb3fc7280a6a0 (accessed on 2 October 2020).
- Devi, S.; Varkey, A.; Sheshshayee, M.S.; Preston, T.; Kurpad, A.V. Measurement of protein digestibility in humans by a dual-tracer method. Am. J. Clin. Nutr. 2018, 107, 984–991. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Wubshet, S.G.; Måge, I.; Böcker, U.; Lindberg, D.; Knutsen, S.H.; Rieder, A.; Rodriguez, D.A.; Afseth, N.K. FTIR as a rapid tool for monitoring molecular weight distribution during enzymatic protein hydrolysis of food processing by-products. Anal. Methods 2017, 9, 4247–4254. [Google Scholar] [CrossRef]
- Coultate, T.P. Food: The Chemistry of Its Components, 4th ed.; Royal Society of Chemistry: Cambridge, UK, 2002. [Google Scholar]
- Taş, A.; Umit, N.; Alkan, R.; Boynak, A.; Yeral, S. Approximation of Protein Quality (DIAAS) of Vegetarian Dishes Served in Restaurants. Int. J. Obes. Nutr. Sci. 2019, 1, 1–5. [Google Scholar] [CrossRef][Green Version]
- Tavano, O.L.; Neves, V.A.; da Silva Júnior, S.I. In vitro versus in vivo protein digestibility techniques for calculating PDCAAS (protein digestibility-corrected amino acid score) applied to chickpea fractions. Food Res. Int. (Ott. Ont.) 2016, 89, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Lazo-Velez, M.A.; Chuck-Hernandez, C.; Mata-Ramarez, D.; Serna-Saldivar Sergio, O. Functional Effects of Soybean Concentrates Obtained from Sprouted Seeds Enriched in Selenium in Wheat Breadmaking. Cereal Chem. 2017, 94, 740–745. [Google Scholar] [CrossRef]
- Rieder, A.; Holtekjølen, A.K.; Sahlstrøm, S.; Moldestad, A. Effect of barley and oat flour types and sourdoughs on dough rheology and bread quality of composite wheat bread. J. Cereal Sci. 2012, 55, 44–52. [Google Scholar] [CrossRef]
- Skendi, A.; Papageorgiou, M.; Biliaderis, C.G. Effect of barley β-glucan molecular size and level on wheat dough rheological properties. J. Food Eng. 2009, 91, 594–601. [Google Scholar] [CrossRef]
- Mohammed, I.; Ahmed, A.R.; Senge, B. Dough rheology and bread quality of wheat–chickpea flour blends. Ind. Crops Prod. 2012, 36, 196–202. [Google Scholar] [CrossRef]
- Belton, P.S. Mini Review: On the Elasticity of Wheat Gluten. J. Cereal Sci. 1999, 29, 103–107. [Google Scholar] [CrossRef]
- Jekle, M.; Becker, T. Wheat dough microstructure: The relation between visual structure and mechanical behavior. Crit. Rev. Food Sci. Nutr. 2015, 55, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Kontogiorgos, V.; Shah, P.; Bills, P. Influence of supramolecular forces on the linear viscoelasticity of gluten. Rheol. Acta 2016, 55, 187–195. [Google Scholar] [CrossRef][Green Version]
- Meerts, M.; Cardinaels, R.; Oosterlinck, F.; Courtin, C.M.; Moldenaers, P. The Interplay between the Main Flour Constituents in the Rheological Behaviour of Wheat Flour Dough. Food Bioprocess Technol. 2017, 10, 249–265. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Van Bockstaele, F.; De Leyn, I.; Eeckhout, M.; Dewettinck, K. Rheological Properties of Wheat Flour Dough and the Relationship with Bread Volume. I. Creep-Recovery Measurements. Cereal Chem. 2008, 85, 753–761. [Google Scholar] [CrossRef]
- Wang, F.C.; Sun, X.S. Creep-Recovery of Wheat Flour Doughs and Relationship to Other Physical Dough Tests and Breadmaking Performance. Cereal Chem. 2002, 79, 567–571. [Google Scholar] [CrossRef]
- Da Silva Vaz, B.; Botelho Moreira, J.; de Morais, M.G.; Vieira Costa, J.A. Microalgae as a new source of bioactive compounds in food supplements. Curr. Opin. Food Sci. 2016, 7, 73–77. [Google Scholar] [CrossRef]
- Vilar, E.G.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Volatile compounds of six species of edible seaweed: A review. Algal Res. 2020, 45, 101740. [Google Scholar] [CrossRef]

| Wheat Flour | TcR | TcT | CvR | CvT | MgR | MgT | |
|---|---|---|---|---|---|---|---|
| Protein | 13.5 ± 0.3 | 42.1 ± 0.1 | 59.5 ± 0.2 | 47.8 ± 1.1 | 58.8 ± 0.3 | 43.3 ± 1.5 | 61.7 ± 2.8 |
| Free amino acids | n.a. | 5.4 | 2.9 | 3.3 | 0.8 | 3.7 | 0.4 |
| Fat | 2.3 + | 13.8 | 0.3 | 15.7 | 0.6 | 21.6 | 0.4 |
| Dietary fibre | 3.8 + | 8.9 ± 0.8 | 15.1 ± 2.2 | 13.8 ± 0.5 | 19.0 ± 0.6 | 12.2 ± 0.6 | 21.8 ± 0.8 |
| Ash | 0.6–0.75 + | 16.0 ± 0.1 | 16.7 ± 0.1 | 6.7 ± 0.6 | 8.2 ± 0.0 | 7.0 ± 1.1 | 7.4 ± 0.1 |
| L* | 92.3 ± 0.1 | 20.9 ± 1.3 | 51.6 ± 3.4 | 12.9 ± 1.0 | 48.3 ± 3.6 | 22.7 ± 0.6 | 58.0 ± 0.9 |
| a* | 0.48 ± 0.1 | −10.1 ± 1.0 | −5.6 ± 0.2 | −1.3 ± 0.4 | 6.6 ± 0.5 | −4.4 ± 0.1 | 3.6 ± 0.3 |
| b* | 10.8 ± 0.3 | 15.4 ± 1.9 | 17.6 ± 0.7 | 17.0 ± 0.6 | 23.6 ± 0.9 | 10.2 ± 0.4 | 29.3 ± 0.4 |
| ∆E (compared to wheat flour) | 72.2 | 41.6 | 79.6 | 46.4 | 70.1 | 39.2 |
| Amino Acid 1 (g/100 g Protein) | Wheat Flour | TcR | TcT | CvR | CvT | MgR | MgT | Egg 2 |
|---|---|---|---|---|---|---|---|---|
| Alanine | 2.8 | 8.6 | 7.1 | 9.5 | 8.1 | 6.7 | 7.2 | - |
| Arginine | 3.5 | 4.5 | 5.1 | 6.3 | 6.9 | 5.7 | 6.5 | 6.2 |
| Aspartic acid | 3.9 | 11.9 | 12.5 | 8.9 | 9.6 | 8.6 | 9.6 | 11.0 |
| Cysteine | 2.6 | 2.0 | 1.2 | 1.1 | 0.8 | 1.0 | 1.0 | 2.3 |
| Glutamic acid | 33.8 | 12.8 | 13.5 | 10.5 | 11.5 | 10.7 | 12.0 | 12.6 |
| Glycine | 3.5 | 6.4 | 6.6 | 6.5 | 6.2 | 5.9 | 6.3 | 4.2 |
| Histidine | 2.0 | 1.7 | 1.7 | 1.8 | 1.7 | 1.9 | 2.0 | 2.4 |
| Isoleucine | 3.1 | 4.0 | 4.4 | 4.0 | 4.4 | 4.1 | 4.9 | 6.6 |
| Leucine | 6.7 | 8.5 | 8.9 | 9.4 | 10.2 | 8.6 | 9.9 | 8.8 |
| Lysine | 2.4 | 6.2 | 4.9 | 5.7 | 4.5 | 6.3 | 5.3 | 5.3 |
| Methionine | 1.9 | 2.4 | 2.5 | 1.9 | 2.2 | 2.0 | 2.3 | 3.2 |
| Phenylalanine | 5.1 | 5.4 | 5.9 | 5.3 | 5.7 | 4.6 | 5.3 | 5.8 |
| Proline | 12.2 | 5.4 | 4.4 | 8.9 | 5.2 | 14.2 | 5.7 | 4.2 |
| Serine | 5.1 | 4.6 | 4.8 | 4.7 | 4.7 | 4.2 | 4.8 | 6.9 |
| Threonine | 2.7 | 4.6 | 4.9 | 4.7 | 5.0 | 4.7 | 5.2 | 4.0 |
| Tryptophan | 1.9 | 1.8 | 2.1 | 1.9 | 2.3 | 1.9 | 2.3 | 1.7 |
| Tyrosine | 2.9 | 3.4 | 3.3 | 3.4 | 3.6 | 3.3 | 3.6 | 4.2 |
| Valine | 3.8 | 5.9 | 6.1 | 6.0 | 6.4 | 5.3 | 6.0 | 7.2 |
| EAAI | 0.74 | 0.89 | 0.91 | 0.90 | 0.93 | 0.88 | 0.97 | 1.00 |
| DIAAS (%) 3 | 45 (Lys) | 54 (Val) | 78 (Lys) | 69 (Met) | 73 (Lys) | 73 (Met) | 84 (Met) |
| In Vitro Protein Digestibility (%) | TcR | TcT | CvR | CvT | MgR | MgT |
|---|---|---|---|---|---|---|
| Dissolved protein | 57.5 ± 2.7 b | 33.7 ± 1.5 c | 68.5 ± 1.3 a | 52.9 ± 5.2 b | 68.5 ± 3.7 a | 53.6 ± 0.2 b |
| Small peptides | 90.3 ± 0.4 a | 87.2 ± 1.3 b | 89.4 ± 0.2 a | 82.3 ± 0.3 c | 90.8 ± 0.2 a | 87.4 ± 0.4 b |
| In vitro protein digestibility as DSEC | 51.9 ± 2.7 b | 29.4 ± 1.5 d | 61.2 ± 1.0 a | 43.5 ± 4.2 c | 62.1 ± 3.5 a | 46.8 ± 0.3 bc |
| Control | TcR | TcT | CvR | CvT | MgR | MgT | |
|---|---|---|---|---|---|---|---|
| L* | 74.1 ± 3.1 a | 24.3 ± 1.0 f | 45.0 ± 0.5 c | 25.3 ± 0.5 f | 38.3 ± 1.4 d | 33.0 ± 0.5 e | 55.6 ± 1.2 b |
| a* | 0.4 ± 0.3 d | −1.0 ± 0.5 e | −2.7 ± 0.0 f | 2.0 ± 0.1 c | 2.5 ± 0.9 b | 1.9 ± 0.3 c | 5.7 ± 0.2 a |
| b* | 14.9 ± 0.7 a | −2.5 ± 0.4 e | 15.4 ± 1.2 c | −1.5 ± 0.3 e | 11.4 ± 1.5 d | 8.4 ± 0.6 d | 22.9 ± 0.3 b |
| ∆E | 52.6 | 29.1 | 51.4 | 35.9 | 42.5 | 20.7 |
| Amino Acid 1 (g/100 g Protein) | Control | TcR | TcT | CvR | CvT | MgR | MgT | Egg 2 |
|---|---|---|---|---|---|---|---|---|
| Alanine | 3.4 | 5.1 | 4.9 | 4.9 | 4.8 | 4.0 | 4.5 | - |
| Arginine | 3.2 | 3.3 | 3.6 | 4.2 | 4.7 | 4.2 | 4.6 | 6.2 |
| Aspartic acid | 3.6 | 5.9 | 6.7 | 5.6 | 6.4 | 5.2 | 6.1 | 11 |
| Cysteine | 2.3 | 2.0 | 2.0 | 1.9 | 1.8 | 1.9 | 1.7 | 2.3 |
| Glutamic acid | 34.2 | 28.1 | 26.8 | 27.8 | 27.1 | 29.1 | 27.1 | 12.6 |
| Glycine | 3.8 | 4.6 | 5.0 | 4.4 | 4.6 | 4.2 | 4.6 | 4.2 |
| Histidine | 2.1 | 2.1 | 2.0 | 2.0 | 1.9 | 1.9 | 2.0 | 2.4 |
| Isoleucine | 3.7 | 4.0 | 4.2 | 3.7 | 3.8 | 3.7 | 4.0 | 6.6 |
| Leucine | 6.7 | 7.5 | 7.8 | 7.5 | 8.0 | 7.3 | 8.2 | 8.8 |
| Lysine | 2.4 | 3.4 | 3.5 | 3.3 | 3.0 | 3.3 | 3.6 | 5.3 |
| Methionine | 1.4 | 1.7 | 1.7 | 1.9 | 2.0 | 1.7 | 2.0 | 3.2 |
| Phenylalanine | 5.3 | 5.5 | 5.6 | 5.5 | 5.3 | 5.2 | 5.6 | 5.8 |
| Proline | 12.6 | 10.0 | 9.5 | 10.5 | 9.1 | 12.1 | 8.7 | 4.2 |
| Serine | 4.7 | 4.9 | 4.9 | 4.5 | 4.5 | 4.3 | 4.5 | 6.9 |
| Threonine | 2.4 | 3.3 | 3.4 | 3.1 | 3.4 | 3.3 | 3.6 | 4.0 |
| Tryptophan | 1.7 | 1.4 | 1.6 | 1.6 | 1.7 | 1.6 | 1.6 | 1.7 |
| Tyrosine | 2.1 | 2.2 | 1.8 | 2.8 | 3.0 | 2.4 | 2.7 | 4.2 |
| Valine | 4.3 | 4.9 | 5.1 | 4.9 | 5.1 | 4.6 | 4.9 | 7.2 |
| Protein (N × 5.7) g/100 g freeze-dried bread | 13.14 ± 0.05 | 16.69 ± 0.05 | 18.31 ± 0.13 | 17.78 ± 0.03 | 19.16 ± 0.25 | 16.69 ± 0.1 | 19.06 ± 0.03 | |
| Protein (g/50 g bread) | 4.5 ± 0.02 | 5.0 ± 0.01 | 5.7 ± 0.04 | 5.1 ± 0.01 | 6.0 ± 0.08 | 5.2 ± 0.03 | 6.2 ± 0.01 | |
| % increase in protein rel. to control | 10.5 | 25.3 | 13.2 | 32.1 | 14.4 | 35.6 | ||
| Moisture content (%) | 36.7 ± 0.4 | 41.2 ± 2.1 | 40.0 ± 1.4 | 43.2 ± 1.1 | 39.8 ± 1.5 | 41.9 ± 0.2 | 40.7 ± 1.3 | |
| EAAI | 0.67 | 0.73 | 0.73 | 0.72 | 0.74 | 0.71 | 0.77 | 1.0 |
| DIAAS (%) 3 | 46 | 65 | 60 | 63 | 57 | 66 | 63 |
| In Vitro Protein Digestibility (%) | Control | TcR | TcT | CvR | CvT | MgR | MgT |
|---|---|---|---|---|---|---|---|
| Dissolved protein | 83.2 ± 1.4 a | 67.4 ± 1.8 c | 59.3 ± 1.4 d | 74.2 ± 1.2 b | 67.6 ± 1.7 c | 70.1 ± 1.6 bc | 61.7 ± 0.4 d |
| Small peptides | 82.7 ± 0.8 d | 86.9 ± 0.1 ab | 86.2 ± 0.1 a | 85.5 ± 0.1 bc | 84.8 ± 0.5 c | 86.0 ± 0.3 ab | 86.2 ± 0.3 ab |
| In vitro protein digestibility as DSEC | 68.5 ± 0.6 a | 56.1 ± 2.8 c | 50.5 ± 0.6 d | 63.4 ± 1 b | 57.3 ± 1.2 c | 60.3 ± 1.3 bc | 53.2 ± 0.7 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qazi, W.M.; Ballance, S.; Kousoulaki, K.; Uhlen, A.K.; Kleinegris, D.M.M.; Skjånes, K.; Rieder, A. Protein Enrichment of Wheat Bread with Microalgae: Microchloropsis gaditana, Tetraselmis chui and Chlorella vulgaris. Foods 2021, 10, 3078. https://doi.org/10.3390/foods10123078
Qazi WM, Ballance S, Kousoulaki K, Uhlen AK, Kleinegris DMM, Skjånes K, Rieder A. Protein Enrichment of Wheat Bread with Microalgae: Microchloropsis gaditana, Tetraselmis chui and Chlorella vulgaris. Foods. 2021; 10(12):3078. https://doi.org/10.3390/foods10123078
Chicago/Turabian StyleQazi, Waqas Muhammad, Simon Ballance, Katerina Kousoulaki, Anne Kjersti Uhlen, Dorinde M. M. Kleinegris, Kari Skjånes, and Anne Rieder. 2021. "Protein Enrichment of Wheat Bread with Microalgae: Microchloropsis gaditana, Tetraselmis chui and Chlorella vulgaris" Foods 10, no. 12: 3078. https://doi.org/10.3390/foods10123078
APA StyleQazi, W. M., Ballance, S., Kousoulaki, K., Uhlen, A. K., Kleinegris, D. M. M., Skjånes, K., & Rieder, A. (2021). Protein Enrichment of Wheat Bread with Microalgae: Microchloropsis gaditana, Tetraselmis chui and Chlorella vulgaris. Foods, 10(12), 3078. https://doi.org/10.3390/foods10123078






