Effect of Copper and Titanium-Exchanged Montmorillonite Nanostructures on the Packaging Performance of Chitosan/Poly-Vinyl-Alcohol-Based Active Packaging Nanocomposite Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Preparation of CuMt and TiMt Ion-Exchanged Nanoclays
2.1.2. Preparation of CS/PVOH/CuMt and CS/PVOH/TiMt Active Nanocomposite Films
2.2. XRD Analysis
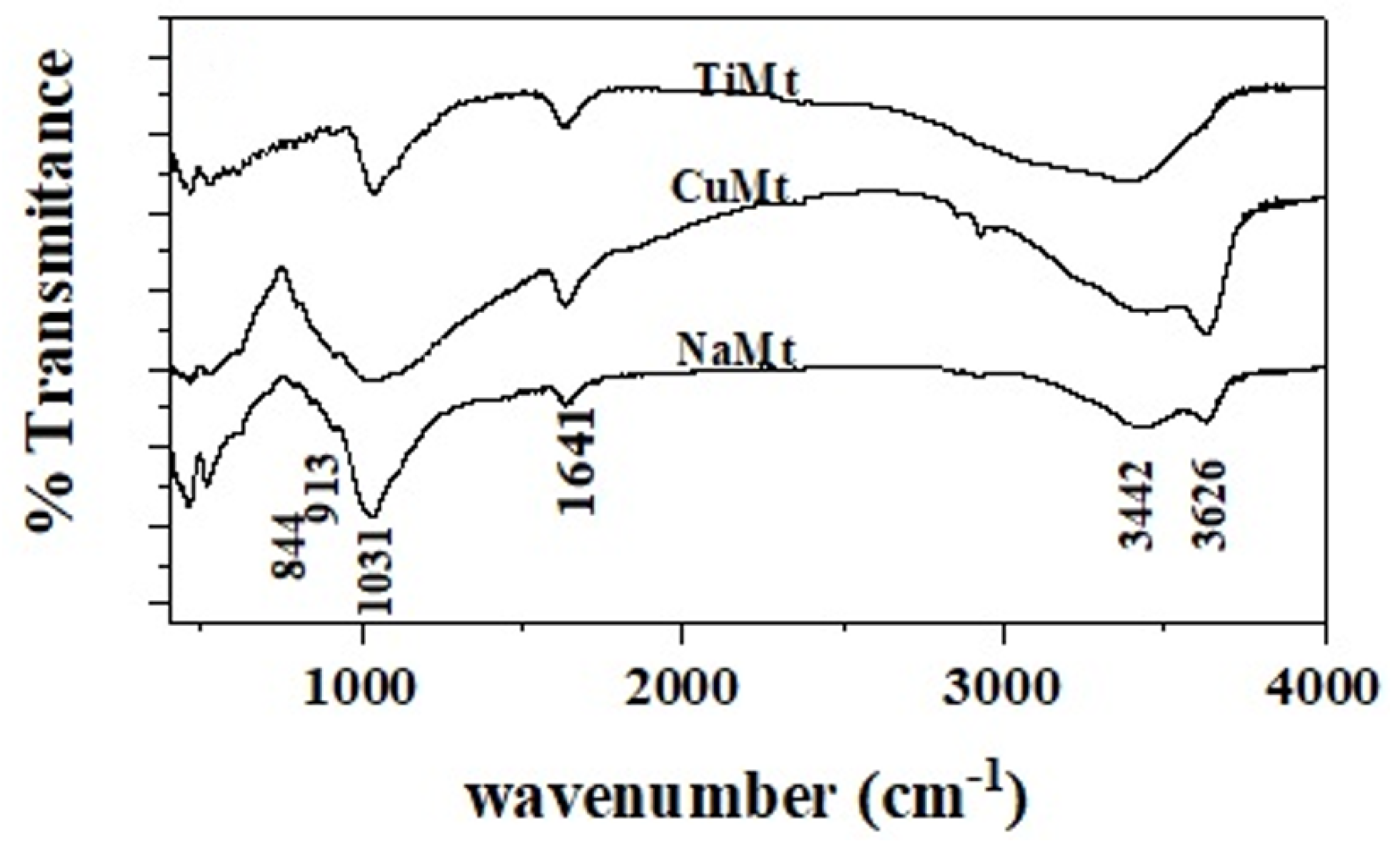
2.3. FTIR Spectrometry
2.4. Tensile Properties
2.5. Water Sorption
2.6. Water-Vapor Diffusivity
2.7. Oxygen Permeability
2.8. Antimicrobial Activity Tests
3. Results
3.1. XRD
3.2. FTIR
3.3. Tensile Properties
3.4. Water Sorption
3.5. Barrier Properties
3.6. Antimicrobial Activity
3.7. Statistical Analysis of the Experimental Data
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dainelli, D.; Gontard, N.; Spyropoulos, D.; Zondervan-van den Beuken, E.; Tobback, P. Active and intelligent food packaging: Legal aspects and safety concerns. Trends Food Sci. Technol. 2008, 19, S103–S112. [Google Scholar] [CrossRef]
- Soltani Firouz, M.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Food Res. Int. 2021, 141, 110113. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, A.P.A.; Conte Junior, C.A. Green strategies for active food packagings: A systematic review on active properties of graphene-based nanomaterials and biodegradable polymers. Trends Food Sci. Technol. 2020, 103, 130–143. [Google Scholar] [CrossRef]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Rhim, J.-W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Chawla, R.; Sivakumar, S.; Kaur, H. Antimicrobial edible films in food packaging: Current scenario and recent nanotechnological advancements- a review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100024. [Google Scholar] [CrossRef]
- Azlin-Hasim, S.; Cruz-Romero, M.C.; Cummins, E.; Kerry, J.P.; Morris, M.A. The potential use of a layer-by-layer strategy to develop LDPE antimicrobial films coated with silver nanoparticles for packaging applications. J. Colloid Interface Sci. 2016, 461, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Khodaei, D.; Lacroix, M. Effect of chitosan/essential oils/silver nanoparticles composite films packaging and gamma irradiation on shelf life of strawberries. Food Hydrocoll. 2021, 117, 106750. [Google Scholar] [CrossRef]
- Wang, W.; Yu, Z.; Alsammarraie, F.K.; Kong, F.; Lin, M.; Mustapha, A. Properties and antimicrobial activity of polyvinyl alcohol-modified bacterial nanocellulose packaging films incorporated with silver nanoparticles. Food Hydrocoll. 2020, 100, 105411. [Google Scholar] [CrossRef]
- Yin, M.; Lin, X.; Ren, T.; Li, Z.; Ren, X.; Huang, T.-S. Cytocompatible quaternized carboxymethyl chitosan/poly(vinyl alcohol) blend film loaded copper for antibacterial application. Int. J. Biol. Macromol. 2018, 120, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Mousazadeh, S.; Ehsani, A.; Moghaddas Kia, E.; Ghasempour, Z. Zinc oxide nanoparticles and periodate oxidation in developing pH-sensitive packaging film based on modified gelatin. Food Packag. Shelf Life 2021, 28, 100654. [Google Scholar] [CrossRef]
- Kaewklin, P.; Siripatrawan, U.; Suwanagul, A.; Lee, Y.S. Active packaging from chitosan-titanium dioxide nanocomposite film for prolonging storage life of tomato fruit. Int. J. Biol. Macromol. 2018, 112, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Siripatrawan, U.; Kaewklin, P. Fabrication and characterization of chitosan-titanium dioxide nanocomposite film as ethylene scavenging and antimicrobial active food packaging. Food Hydrocoll. 2018, 84, 125–134. [Google Scholar] [CrossRef]
- Busolo, M.A.; Lagaron, J.M. Oxygen scavenging polyolefin nanocomposite films containing an iron modified kaolinite of interest in active food packaging applications. Innov. Food Sci. Emerg. Technol. 2012, 16, 211–217. [Google Scholar] [CrossRef]
- Bagchi, B.; Kar, S.; Dey, S.K.; Bhandary, S.; Roy, D.; Mukhopadhyay, T.K.; Das, S.; Nandy, P. In situ synthesis and antibacterial activity of copper nanoparticle loaded natural montmorillonite clay based on contact inhibition and ion release. Colloids Surf B Biointerfaces 2013, 108, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.H.; Xu, Z.R.; Xia, M.S. Antibacterial effect of Cu2+-exchanged montmorillonite on aeromonas hydrophila and discussion on its mechanism. Vet. Microbiol. 2005, 109, 83–88. [Google Scholar] [CrossRef] [PubMed]
- He, H.P.; Guo, J.G.; Xie, X.D.; Peng, J.L. Location and migration of cations in Cu2+-adsorbed montmorillonite. Environ. Int. 2001, 26, 347–352. [Google Scholar] [CrossRef]
- Malachová, K.; Praus, P.; Rybková, Z.; Kozák, O. Antibacterial and antifungal activities of silver, copper and zinc montmorillonites. Appl. Clay Sci. 2011, 53, 642–645. [Google Scholar] [CrossRef]
- Mitsudome, T.; Matsuno, T.; Sueoka, S.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Titanium cation-exchanged montmorillonite as an active heterogeneous catalyst for the beckmann rearrangement under mild reaction conditions. Tetrahedron Lett. 2012, 53, 5211–5214. [Google Scholar] [CrossRef]
- Kawabata, T.; Kato, M.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Highly efficient deprotection of acetals by titanium cation-exchanged montmorillonite as a strong solid acid catalyst. Chem. Lett. 2003, 32, 648–649. [Google Scholar] [CrossRef]
- Sterte, J. Synthesis and properties of titanium oxide cross-linked montmorillonite. Clays Clay Miner. 1986, 34, 658–664. [Google Scholar] [CrossRef]
- Bruna, J.E.; Galotto, M.J.; Guarda, A.; Rodríguez, F. A novel polymer based on MtCu2+/cellulose acetate with antimicrobial activity. Carbohydr Polym 2014, 102, 317–323. [Google Scholar] [CrossRef]
- Martucci, J.F.; Ruseckaite, R.A. Antibacterial activity of gelatin/copper (II)-exchanged montmorillonite films. Food Hydrocoll. 2017, 64, 70–77. [Google Scholar] [CrossRef][Green Version]
- Bruna, J.E.; Peñaloza, A.; Guarda, A.; Rodríguez, F.; Galotto, M.J. Development of MtCu2+/LDPE nanocomposites with antimicrobial activity for potential use in food packaging. Appl. Clay Sci. 2012, 58, 79–87. [Google Scholar] [CrossRef]
- Effects of Exchange Titanium Cations on the Pore Structure and Adsorption Characteristics of Montmorillonite | Semantic Scholar. Available online: https://www.semanticscholar.org/paper/effects-of-exchange-titanium-cations-on-the-pore-of-Huang-Lee/7abbbfcd11812309ee9bc2442b6adcaf1321f6d9 (accessed on 27 August 2021).
- Giannakas, A.; Grigoriadi, K.; Leontiou, A.; Barkoula, N.-M.; Ladavos, A. Preparation, characterization, mechanical and barrier properties investigation of chitosan–clay nanocomposites. Carbohydr. Polym. 2014, 108, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Giannakas, A.; Vlacha, M.; Salmas, C.; Leontiou, A.; Katapodis, P.; Stamatis, H.; Barkoula, N.-M.; Ladavos, A. Preparation, characterization, mechanical, barrier and antimicrobial properties of chitosan/PVOH/clay nanocomposites. Carbohydr. Polym. 2016, 140, 408–415. [Google Scholar] [CrossRef]
- Giannakas, A.; Patsaoura, A.; Barkoula, N.-M.; Ladavos, A. A novel solution blending method for using olive oil and corn oil as plasticizers in chitosan based organoclay nanocomposites. Carbohydr. Polym. 2017, 157, 550–557. [Google Scholar] [CrossRef]
- Vlacha, M.; Giannakas, A.; Katapodis, P.; Stamatis, H.; Ladavos, A.; Barkoula, N.-M. On the efficiency of oleic acid as plasticizer of chitosan/clay nanocomposites and its role on thermo-mechanical, barrier and antimicrobial properties—Comparison with glycerol. Food Hydrocoll. 2016, 57, 10–19. [Google Scholar] [CrossRef]
- Grigoriadi, K.; Giannakas, A.; Ladavos, A.K.; Barkoula, N.-M. Interplay between processing and performance in chitosan-based clay nanocomposite films. Polym. Bull. 2015, 72. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Karydis-Messinis, A.; Moschovas, D.; Kollia, E.; Tsigkou, V.; Proestos, C.; Avgeropoulos, A.; Zafeiropoulos, N.E. Nanoclay and polystyrene type efficiency on the development of polystyrene/montmorillonite/oregano oil antioxidant active packaging nanocomposite films. Appl. Sci. 2021, 11, 9364. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Leontiou, A.; Baikousi, M.; Moschovas, D.; Asimakopoulos, G.; Zafeiropoulos, N.E.; Avgeropoulos, A. Synthesis of a novel chitosan/basil oil blend and development of novel low density poly ethylene/chitosan/basil oil active packaging films following a melt-extrusion process for enhancing chicken breast fillets shelf-life. Molecules 2021, 26, 1585. [Google Scholar] [CrossRef] [PubMed]
- Salmas, C.E.; Giannakas, A.E.; Baikousi, M.; Leontiou, A.; Siasou, Z.; Karakassides, M.A. Development of poly(L-lactic acid)/chitosan/basil oil active packaging films via a melt-extrusion process using novel chitosan/basil oil blends. Processes 2021, 9, 88. [Google Scholar] [CrossRef]
- Salmas, C.; Giannakas, A.; Katapodis, P.; Leontiou, A.; Moschovas, D.; Karydis-Messinis, A. Development of ZnO/Na-montmorillonite hybrid nanostructures used for PVOH/ZnO/Na-montmorillonite active packaging films preparation via a melt-extrusion process. Nanomaterials 2020, 10, 1079. [Google Scholar] [CrossRef] [PubMed]
- Giannakas, A.; Giannakas, A.; Ladavos, A. Preparation and characterization of polystyrene/organolaponite nanocomposites. Polym. Plast. Technol. Eng. 2012, 51, 1411–1415. [Google Scholar] [CrossRef]
- Giannakas, A.; Salmas, C.; Leontiou, A.; Tsimogiannis, D.; Oreopoulou, A.; Braouhli, J. Novel LDPE/chitosan rosemary and melissa extract nanostructured active packaging films. Nanomaterials 2019, 9, 1105. [Google Scholar] [CrossRef]
- Giannakas, A.; Stathopoulou, P.; Tsiamis, G.; Salmas, C. The effect of different preparation methods on the development of chitosan/thyme oil/montmorillonite nanocomposite active packaging films. J. Food Process. Preserv. 2019, 44, e14327. [Google Scholar] [CrossRef]
- Edible Food Packaging: Materials and Processing Technologies—1st Edition. Available online: https://www.routledge.com/edible-food-packaging-materials-and-processing-technologies/cerqueira-pereira-ramos-teixeira-vicente/p/book/9781482234169 (accessed on 11 November 2021).
- Units of Gas Permeability Constants—Yasuda—1975—Journal of Applied Polymer Science—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/app.1975.070190915 (accessed on 11 November 2021).
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Mekhzoum, M.E.M.; Benzeid, H.; Qaiss, A.E.K.; Essassi, E.M.; Bouhfid, R. Copper(I) confined in interlayer space of montmorillonite: A highly efficient and recyclable catalyst for click reaction. Catal. Lett. 2016, 146, 136–143. [Google Scholar] [CrossRef]
- Gartner, C.; López, B.L.; Sierra, L.; Graf, R.; Spiess, H.W.; Gaborieau, M. Interplay between structure and dynamics in chitosan films investigated with solid-state NMR, dynamic mechanical analysis, and X-ray diffraction. Biomacromolecules 2011, 12, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Naveen Kumar, H.M.P.; Prabhakar, M.N.; Venkata Prasad, C.; Madhusudhan Rao, K.; Ashok Kumar Reddy, T.V.; Chowdoji Rao, K.; Subha, M.C.S. Compatibility studies of chitosan/PVA blend in 2% aqueous acetic acid solution at 30 °C. Carbohydr. Polym. 2010, 82, 251–255. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Jin, C.; Zhang, R.; Li, L.; Li, X.; Jiang, S. Sodium lactate loaded chitosan-polyvinyl alcohol/montmorillonite composite film towards active food packaging. Innov. Food Sci. Emerg. Technol. 2017, 42, 101–108. [Google Scholar] [CrossRef]
- Ming-li, C.; Ying-bo, Z.; Yong-fu, Y. Preparation and microstructure of Al-pillared interlayered montmorillonite. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2002, 17, 13–16. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Liu, S.; Wu, Y.; Zhou, Q.; Zhang, Y.; Zheng, X.; Han, Y.; Xie, C.; Liu, N. Adsorption of deoxynivalenol by pillared montmorillonite. Food Chem. 2021, 343, 128391. [Google Scholar] [CrossRef]
- Srinivasa, P.C.; Ramesh, M.N.; Kumar, K.R.; Tharanathan, R.N. Properties and sorption studies of chitosan-polyvinyl alcohol blend films. Carbohydr. Polym. 2003, 53, 431–438. [Google Scholar] [CrossRef]
- Costa-Júnior, E.S.; Barbosa-Stancioli, E.F.; Mansur, A.A.P.; Vasconcelos, W.L.; Mansur, H.S. Preparation and characterization of chitosan/poly(vinyl alcohol) chemically crosslinked blends for biomedical applications. Carbohydr. Polym. 2009, 76, 472–481. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Leontiou, A.A. Montmorillonite composite materials and food packaging. In Composites Materials for Food Packaging; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 1–71. ISBN 978-1-119-16024-3. [Google Scholar]
- Arunvisut, S.; Phummanee, S.; Somwangthanaroj, A. Effect of clay on mechanical and gas barrier properties of blown film LDPE/clay nanocomposites. J. Appl. Polym. Sci. 2007, 106, 2210–2217. [Google Scholar] [CrossRef]
- Vicente, A.A. Food hydrocolloids chitosan/clay films ‘Properties as affected by biopolymer and clay micro/nanoparticles’ concentrations. Food Hydrocoll. 2009, 23, 1895–1902. [Google Scholar] [CrossRef]
- Bastarrachea, L.; Dhawan, S.; Sablani, S.S. Engineering properties of polymeric-based antimicrobial films for food packaging: A review. Food Eng. Rev. 2011, 3, 79–93. [Google Scholar] [CrossRef]
- Hyder, M.N.; Chen, P. Pervaporation dehydration of ethylene glycol with chitosan-poly(vinyl alcohol) blend membranes: Effect of CS-PVA blending ratios. J. Membr. Sci. 2009, 340, 171–180. [Google Scholar] [CrossRef]
- Lavorgna, M.; Piscitelli, F.; Mangiacapra, P.; Buonocore, G.G. Study of the combined effect of both clay and glycerol plasticizer on the properties of chitosan films. Carbohydr. Polym. 2010, 82, 291–298. [Google Scholar] [CrossRef]
- Choudalakis, G.; Gotsis, A.D. Permeability of polymer/clay nanocomposites: A review. Eur. Polym. J. 2009, 45, 967–984. [Google Scholar] [CrossRef]
- Hosseinkhanli, H.; Sharif, A.; Aalaie, J.; Khalkhali, T.; Akhlaghi, S. Oxygen permeability and the mechanical and thermal properties of (low-density polyethylene)/poly (ethylene-co-vinyl acetate)/organoclay blown film nanocomposites. J. Vinyl Addit. Technol. 2013, 19, 132–139. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.; Memic, A. Size-dependent antimicrobial properties of CuO nanoparticles against gram-positive and -negative bacterial strains. Int. J. Nanomed. 2012, 7, 3527–3535. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.-J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Cagri, A.; Ustunol, Z.; Ryser, E.T. Antimicrobial, mechanical, and moisture barrier properties of low pH whey protein-based edible films containing p-aminobenzoic or sorbic acids. J. Food Sci. 2001, 66, 865–870. [Google Scholar] [CrossRef]
- García, A.V.; Álvarez-Pérez, O.B.; Rojas, R.; Aguilar, C.N.; Garrigós, M.C. Impact of olive extract addition on corn starch-based active edible films properties for food packaging applications. Foods 2020, 9, 1339. [Google Scholar] [CrossRef]
- Amor, G.; Sabbah, M.; Caputo, L.; Idbella, M.; De Feo, V.; Porta, R.; Fechtali, T.; Mauriello, G. Basil essential oil: Composition, antimicrobial properties, and microencapsulation to produce active chitosan films for food packaging. Foods 2021, 10, 121. [Google Scholar] [CrossRef]
- Kumari, N.; Bangar, S.P.; Petrů, M.; Ilyas, R.A.; Singh, A.; Kumar, P. Development and characterization of fenugreek protein-based edible film. Foods 2021, 10, 1976. [Google Scholar] [CrossRef]


| Symbol of Samples | CS gr-wt.% | PVOH gr-wt.% | NaMt gr-wt.% | CuMt gr-wt.% | TiMt gr-wt.% |
|---|---|---|---|---|---|
| CS | 2.0-100 | - | - | - | |
| CS/PVOH | 2.0-100 | 0.25-20 | - | - | |
| CS/PVOH | 0.08-3 | ||||
| CS/PVOH/3NaMt | 0.17-6 | ||||
| CS/PVOH/6NaMt | |||||
| CS/PVOH/3CuMt | 2.0-100 | 0.5-20 | 0.08-3 | - | |
| CS/PVOH/6CuMt | 2.0-100 | 0.59 | 0.17-6 | - | |
| CS/PVOH/3TiMt | 2.0-100 | 0.5-20 | - | 0.08-3 | |
| CS/PVOH/6TiMt | 2.0-100 | 0.59-20 | - | 0.17-6 |
| Symbol of Samples | E Modulus (MPa) | σuts (MPa) | εb% | Water Sorption | WVTR (g∙m−2∙day−1) | DWV (cm2∙s−1) | OTR (cm3∙m−2∙day−1) | PeO2 (cm2∙s−1) |
|---|---|---|---|---|---|---|---|---|
| CS | 3274 ± 62 | 92.2 ± 3.5 | 10.8 ± 1.4 | 157 ± 5 | 15.5 ± 0.2 | 7.06 × 10−12 | 161.0 ± 2.4 | 6.52 × 10−10 |
| CS/PVOH | 2920 ± 105 | 86.4 ± 3.4 | 14.5 ± 0.8 | 162 ± 7 | 6.3 ± 0.2 | 2.87 × 10−12 | 70.0 ± 1.9 | 2.84 × 10−10 |
| CS/PVOH/3NaMt | 3200 ± 83 | 91.8 ± 4.2 | 7.9 ± 1.7 | 172 ± 4 | 6.0 ± 0.2 | 2.73 × 10−12 | 59.6 ± 1.4 | 2.41 × 10−10 |
| CS/PVOH/6NaMt | 3340 ± 51 | 96.4 ± 2.8 | 6.4 ± 1.2 | 176 ± 6 | 5.6 ± 0.1 | 2.55 × 10−12 | 52.3 ± 1.2 | 2.12 × 10−10 |
| CS/PVOH/3CuMt | 3270 ± 89 | 93.8 ± 4.2 | 7.4 ± 1.8 | 171 ± 5 | 5.7 ± 0.2 | 2.60 × 10−12 | 58.6 ± 1.3 | 2.37 × 10−10 |
| CS/PVOH/6CuMt | 3380 ± 51 | 97.1 ± 2.8 | 6.1 ± 1.2 | 181 ± 7 | 5.5 ± 0.1 | 2.51 × 10−12 | 50.6 ± 1.1 | 2.05 × 10−10 |
| CS/PVOH/3TiMt | 2930 ± 89 | 87.1 ± 4.2 | 7.1 ± 1.8 | 180 ± 6 | 6.1 ± 0.2 | 2.80 × 10−12 | 66.8 ± 1.4 | 2.71 × 10−10 |
| CS/PVOH/6TiMt | 3080 ± 51 | 89.1 ± 2.8 | 6.1 ± 1.2 | 186 ± 7 | 5.9 ± 0.1 | 2.69 × 10−12 | 57.2 ± 1.2 | 2.32 × 10−10 |
| Film Material | E. coli | S. aureus | S. enterica | L. monocytogenes | ||||
|---|---|---|---|---|---|---|---|---|
| Inhibition a | Contact Area b | Inhibition a | Contact Area b | Inhibition a | Contact Area b | Inhibition a | Contact Area b | |
| CS | 0.00 | - | 0.00 | - | 0.00 | - | 0.00 | + |
| CS/PVOH | 3.95 ± 0.07 | - | 4.35 ± 0.49 | - | 3.00 ± 0.00 | - | 0.00 | + |
| CS/PVOH/6NaMt | 0.00 | - | 0.00 | - | 0.00 | - | 0.00 | + |
| CS/PVOH/3CuMt | 4.75 ± 0.35 | - | 4.95 ± 0.07 | - | 3.50 ± 0.71 | - | 3.00 ± 1.41 | - |
| CS/PVOH/6CuMt | 5.50 ± 0.71 | - | 5.00 ± 0.14 | - | 3.50 ± 0.71 | - | 5.10 ± 0.14 | - |
| CS/PVOH/3TiMt | 3.50 ± 0.71 | - | 2.50 ± 0.71 | - | 3.15 ± 0.07 | - | 3.00 ± 1.41 | - |
| CS/PVOH/6TiMt | 4.05 ± 0.07 | - | 4.45 ± 0.64 | - | 3.50 ± 0.71 | - | 4.50 ± 0.42 | - |
| CS | CS/PVOH | CS/PVOH 3NaMt | CS/PVOH 6NaMt | CS/PVOH 3CuMt | CS/PVOH 6CuMt | CS/PVOH 3TiMt | CS/PVOH 6TiMt | |
|---|---|---|---|---|---|---|---|---|
| E Modulus | 3248 | 2925 | 3248 | 3360 | 3248 | 3360 | 2925 | 3080 |
| σuts | 92.6 | 86.8 | 92.6 | 96.8 | 92.6 | 96.8 | 86.8 | 89.1 |
| εb% | 10.8 | 14.5 | 7.5 | 6.2 | 7.5 | 6.2 | 7.5 | 6.2 |
| Water Sorpt. | 157 | 162 | 173 | 173 | 173 | 181 | 181 | 186 |
| WVTR | 15.5 | 6.3 | 6.0 | 5.6 | 5.6 | 5.6 | 6.0 | 6.0 |
| OTR | 161.0 | 70.0 | 58.5 | 51.5 | 58.5 | 51.5 | 66.8 | 58.5 |
| E. coli | 0.00 | 4.00 | 0.00 | 0.00 | 4.75 | 5.50 | 3.50 | 4.00 |
| S. aureus | 0.00 | 4.40 | 0.00 | 0.00 | 4.98 | 4.98 | 2.50 | 4.40 |
| S. enterica | 0.00 | 3.00 | 0.00 | 0.00 | 3.50 | 3.50 | 3.15 | 3.50 |
| L. monocytogenes | 0.00 | 0.00 | 0.00 | 0.00 | 3.00 | 5.10 | 3.00 | 4.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmas, C.E.; Giannakas, A.E.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Proestos, C. Effect of Copper and Titanium-Exchanged Montmorillonite Nanostructures on the Packaging Performance of Chitosan/Poly-Vinyl-Alcohol-Based Active Packaging Nanocomposite Films. Foods 2021, 10, 3038. https://doi.org/10.3390/foods10123038
Salmas CE, Giannakas AE, Baikousi M, Kollia E, Tsigkou V, Proestos C. Effect of Copper and Titanium-Exchanged Montmorillonite Nanostructures on the Packaging Performance of Chitosan/Poly-Vinyl-Alcohol-Based Active Packaging Nanocomposite Films. Foods. 2021; 10(12):3038. https://doi.org/10.3390/foods10123038
Chicago/Turabian StyleSalmas, Constantinos E., Aris E. Giannakas, Maria Baikousi, Eleni Kollia, Vasiliki Tsigkou, and Charalampos Proestos. 2021. "Effect of Copper and Titanium-Exchanged Montmorillonite Nanostructures on the Packaging Performance of Chitosan/Poly-Vinyl-Alcohol-Based Active Packaging Nanocomposite Films" Foods 10, no. 12: 3038. https://doi.org/10.3390/foods10123038
APA StyleSalmas, C. E., Giannakas, A. E., Baikousi, M., Kollia, E., Tsigkou, V., & Proestos, C. (2021). Effect of Copper and Titanium-Exchanged Montmorillonite Nanostructures on the Packaging Performance of Chitosan/Poly-Vinyl-Alcohol-Based Active Packaging Nanocomposite Films. Foods, 10(12), 3038. https://doi.org/10.3390/foods10123038











