Microalgae as Sources of High-Quality Protein for Human Food and Protein Supplements
Abstract
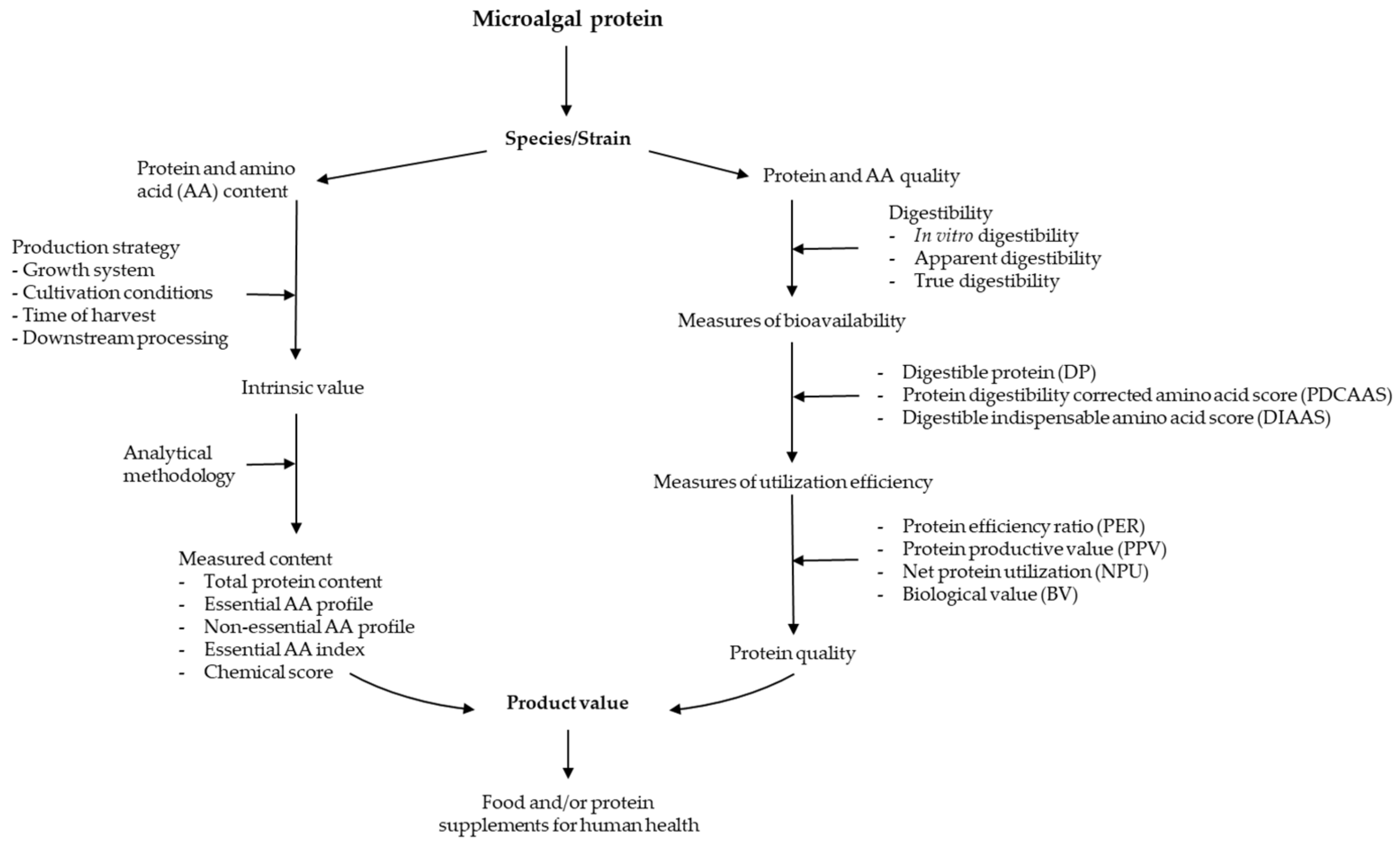
:1. Introduction
2. Historical Use of Microalgae as Human Food
3. Protein Quantity and Difference among Microalgae Species
4. Influence of Analytical Methods on the Protein Content of Microalgal Biomass
5. Influence of Growing Conditions on Microalgae Protein Content
6. Protein Content of Microalgae Collected at Different Growth Phases
7. Protein Quality of Microalgae Biomass
8. Current Challenges and Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tukker, A.; Jansen, B. Environmental Impacts of Products: A Detailed Review of Studies. J. Ind. Ecol. 2006, 10, 159–182. [Google Scholar] [CrossRef]
- Vermeulen, S.J.; Campbell, B.M.; Ingram, J.S.I. Climate Change and Food Systems. Annu. Rev. Environ. Resour. 2012, 37, 195–222. [Google Scholar] [CrossRef] [Green Version]
- Crippa, M.; Solazzo, E.; Guizzardi, D.; Monforti-Ferrario, F.; Tubiello, F.N.; Leip, A. Food systems are responsible for a third of global anthropogenic GHG emissions. Nat. Food 2021, 2, 198–209. [Google Scholar] [CrossRef]
- Tester, M.; Langridge, P. Breeding Technologies to Increase Crop Production in a Changing World. Science 2010, 327, 818–822. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Delgado, C.L. Rising Consumption of Meat and Milk in Developing Countries Has Created a New Food Revolution. J. Nutr. 2003, 133, 3907S–3910S. [Google Scholar] [CrossRef] [Green Version]
- Caporgno, M.P.; Mathys, A. Trends in Microalgae Incorporation into Innovative Food Products with Potential Health Benefits. Front. Nutr. 2018, 5, 58. [Google Scholar] [CrossRef]
- Smetana, S.; Mathys, A.; Knoch, A.; Heinz, V. Meat alternatives: Life cycle assessment of most known meat substitutes. Int. J. Life Cycle Assess. 2015, 20, 1254–1267. [Google Scholar] [CrossRef]
- Pimentel, D.; Pimentel, M. Sustainability of meat-based and plant-based diets and the environment. Am. J. Clin. Nutr. 2003, 78, 660S–663S. [Google Scholar] [CrossRef]
- Storlien, L.H.; Higgins, J.A.; Thomas, T.C.; Brown, M.A.; Wang, H.Q.; Huang, X.F.; Else, P.L. Diet composition and insulin action in animal models. Br. J. Nutr. 2000, 83, S85–S90. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Gagnon, J.; Nair, S.; Sha, S. Herring Milt Protein Hydrolysate Improves Insulin Resistance in High-Fat-Diet-Induced Obese Male C57BL/6J Mice. Mar. Drugs 2019, 17, 456. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Nair, S.; Gagnon, J. Herring Milt and Herring Milt Protein Hydrolysate Are Equally Effective in Improving Insulin Sensitivity and Pancreatic Beta-Cell Function in Diet-Induced Obese- and Insulin-Resistant Mice. Mar. Drugs 2020, 18, 635. [Google Scholar] [CrossRef]
- MacLeod, M.J.; Vellinga, T.; Opio, C.; Falcucci, A.; Tempio, G.; Henderson, B.; Makkar, H.; Mottet, A.; Robinson, T.; Steinfeld, H.; et al. Invited review: A position on the Global Livestock Environmental Assessment Model (GLEAM). Animal 2018, 12, 383–397. [Google Scholar] [CrossRef] [Green Version]
- Murphy, S.P.; Allen, L.H. Nutritional Importance of Animal Source Foods. J. Nutr. 2003, 133, 3932S–3935S. [Google Scholar] [CrossRef] [Green Version]
- Randolph, T.F.; Schelling, E.; Grace, D.; Nicholson, C.F.; Leroy, J.L.; Cole, D.; Demment, M.W.; Omore, A.; Zinsstag, J.; Ruel, M. Invited Review: Role of livestock in human nutrition and health for poverty reduction in developing countries. J. Anim. Sci. 2007, 85, 2788–2800. [Google Scholar] [CrossRef] [Green Version]
- Gerber, P.J.; Mottet, A.; Opio, C.I.; Falcucci, A.; Teillard, F. Environmental impacts of beef production: Review of challenges and perspectives for durability. Meat Sci. 2015, 109, 2–12. [Google Scholar] [CrossRef]
- Herrero, M.; Havlik, P.; Valin, H.; Notenbaert, A.M.O.; Rufino, M.; Thornton, P.K.; Blümmel, M.; Weiss, F.; Grace, D.; Obersteiner, M. Biomass use, production, feed efficiencies, and greenhouse gas emissions from global livestock systems. Proc. Natl. Acad. Sci. USA 2013, 110, 20888–20893. [Google Scholar] [CrossRef] [Green Version]
- Tredici, M.R.; Rodolfi, L.; Biondi, N.; Bassi, N.; Sampietro, G. Techno-economic analysis of microalgal biomass production in a 1-ha Green Wall Panel (GWP®) plant. Algal Res. 2016, 19, 253–263. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.F.; Ferreira, A.; Dias, A.P.S.; Gouveia, L. Pyrolysis of Scenedesmus obliquus Biomass Following the Treatment of Different Wastewaters. BioEnergy Res. 2020, 13, 896–906. [Google Scholar] [CrossRef]
- Navarro-López, E.; Ruíz-Nieto, A.; Ferreira, A.; Acién, F.G.; Gouveia, L. Biostimulant Potential of Scenedesmus obliquus Grown in Brewery Wastewater. Molecules 2020, 25, 664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borowitzka, M.A.; Vonshak, A. Scaling up microalgal cultures to commercial scale. Eur. J. Phycol. 2017, 52, 407–418. [Google Scholar] [CrossRef]
- Vonshak, A. Chapter 15—Micro-algae: Laboratory growth techniques and outdoor biomass production. In Techniques in Bioproductivity and Photosynthesis, 2nd ed.; Coombs, J., Hall, D.O., Long, S.P., Scurlock, J.M.O., Eds.; Pergamon Press: Oxford, UK, 1985; pp. 188–200. [Google Scholar]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Optimization of Nannochloropsis oculata growth using the response surface method. J. Chem. Technol. Biotechnol. 2006, 81, 1049–1056. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richmond, A. CRC Handbook of Microalgal Mass Culture, 1st ed.; CRC Press: Boca Raton, FL, USA, 1986. [Google Scholar]
- Burlew, J.S. Algal Culture from Laboratory to Pilot Plant; Carnegie Institution of Washington Publication: Washington, DC, USA, 1953. [Google Scholar]
- Ismail, B.P.; Senaratne-Lenagala, L.; Stube, A.; Brackenridge, A. Protein demand: Review of plant and animal proteins used in alternative protein product development and production. Anim. Front. 2020, 10, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Milledge, J.J. Commercial application of microalgae other than as biofuels: A brief review. Rev. Environ. Sci. Bio/Technol. 2011, 10, 31–41. [Google Scholar] [CrossRef]
- Aaronson, S.; Berner, T.; Dubinsky, Z. Microalgae as a source of chemicals and natural products. In Algae Biomass: Production and Use/[Sponsored by the National Council for Research and Development, Israel and the Gesellschaft fur Strahlen-und Umweltforschung (GSF), Munich, Germany]; Shelef, G., Soeder, C.J., Eds.; Elsevier/North-Holland Biomedical Press: Amsterdam, The Netherlands, 1980; pp. 595–601. [Google Scholar]
- Habib, M.A.B. Review on Culture, Production and Use of Spirulina as Food for Humans and Feeds for Domestic Animals and Fish; Food and Agriculture Organization of the United Nations: Rome, Italy, 2008. [Google Scholar]
- Becker, E. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Burlew, J.S. Algal Culture from Laboratory to Pilot Plant. AIBS Bull. 1953, 3, 11. [Google Scholar]
- Goldman, J.C. Outdoor algal mass cultures—I. Applications. Water Res. 1979, 13, 1–19. [Google Scholar] [CrossRef]
- Goldman, J.C. Outdoor algal mass cultures—II. Photosynthetic yield limitations. Water Res. 1979, 13, 119–136. [Google Scholar] [CrossRef]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef]
- Chacón-Lee, T.; González-Mariño, G. Microalgae for “Healthy” Foods-Possibilities and Challenges. Compr. Rev. Food Sci. Food Saf. 2010, 9, 655–675. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Bursic, I.; Sousa, I.; Raymundo, A.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae as Functional Ingredients in Savory Food Products: Application to Wheat Crackers. Foods 2019, 8, 611. [Google Scholar] [CrossRef] [Green Version]
- Niccolai, A.; Bažec, K.; Rodolfi, L.; Biondi, N.; Zlatić, E.; Jamnik, P.; Tredici, M.R. Lactic Acid Fermentation of Arthrospira platensis (Spirulina) in a Vegetal Soybean Drink for Developing New Functional Lactose-Free Beverages. Front. Microbiol. 2020, 11, 560684. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Functional and Bioactive Properties of Protein Extracts Generated from Spirulina platensis and Isochrysis galbana T-Iso. Appl. Sci. 2021, 11, 3964. [Google Scholar] [CrossRef]
- Sousa, I.; Gouveia, L.; Batista, A.P.; Raymundo, A.; Bandarra, N.M. Microalgae in novel food products. Food Chem. Res. Dev. 2008, 75–112. [Google Scholar]
- Norton, T.A.; Melkonian, M.; Andersen, R.A. Algal biodiversity. Phycologia 1996, 35, 308–326. [Google Scholar] [CrossRef]
- Acquah, C.; Tibbetts, S.M.; Pan, S.; Udenigwe, C. Chapter 19—Nutritional quality and bioactive properties of proteins and peptides from microalgae. In Handbook of Microalgae-Based Processes and Products; Jacob-Lopes, E., Maroneze, M.M., Queiroz, M.I., Zepka, L.Q., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 493–531. [Google Scholar]
- Brown, M.R. The amino-acid and sugar composition of 16 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 1991, 145, 79–99. [Google Scholar] [CrossRef]
- Tipnee, S.; Ramaraj, R.; Unpaprom, Y. Nutritional evaluation of edible freshwater green macroalga Spirogyra varians. Emergent Life Sci. Res. 2015, 1, 1–7. [Google Scholar]
- Saragih, H.T.; Muhamad, A.A.K.; Alfianto, A.; Viniwidihastuti, F.; Untari, L.F.; Lesmana, I.; Widyatmoko, H.; Rohmah, Z. Effects of Spirogyra jaoensis as a dietary supplement on growth, pectoralis muscle performance, and small intestine morphology of broiler chickens. Vet. World 2019, 12, 1233–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, E.W. Microalgae for Human and Animal Nutrition. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Richmong, A., Hu, Q., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 461–503. [Google Scholar]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.-H.; Joo, S.-T. Meat analog as future food: A review. J. Anim. Sci. Technol. 2020, 62, 111–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. Environ. Boil. Fishes 2015, 27, 1109–1119. [Google Scholar] [CrossRef] [Green Version]
- Islam, R.; Hassan, A.; Sulebele, G.; Orosco, C.; Roustaian, P. Influence of temperature on growth and biochemical composition of Spirulina platensis and S. fusiformis. Ital. Int. J. Sci. 2003, 4, 97–106. [Google Scholar]
- Tokuşoglu, O.; Uunal, M. Biomass Nutrient Profiles of Three Microalgae: Spirulina platensis, Chlorella vulgaris, and Isochrisis galbana. J. Food Sci. 2003, 68, 1144–1148. [Google Scholar] [CrossRef]
- Ferreira, L.; Rodrigues, M.; Converti, A.; Sato, S.; Carvalho, J. Arthrospira (Spirulina) platensis cultivation in tubular photobioreactor: Use of no-cost CO2 from ethanol fermentation. Appl. Energy 2012, 92, 379–385. [Google Scholar] [CrossRef]
- Coca, M.; Barrocal, V.M.; Lucas, S.; Benito, G.G.; García-Cubero, M.T. Protein production in Spirulina platensis biomass using beet vinasse-supplemented culture media. Food Bioprod. Process. 2015, 94, 306–312. [Google Scholar] [CrossRef]
- Salla, A.C.V.; Margarites, A.C.F.; Seibel, F.I.; Holz, L.C.; Brião, V.B.; Bertolin, T.E.; Colla, L.; Costa, J.A.V. Increase in the carbohydrate content of the microalgae Spirulina in culture by nutrient starvation and the addition of residues of whey protein concentrate. Bioresour. Technol. 2016, 209, 133–141. [Google Scholar] [CrossRef]
- Colla, L.; Reinehr, C.; Reichert, C.; Costa, J.A.V. Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Bioresour. Technol. 2007, 98, 1489–1493. [Google Scholar] [CrossRef]
- Matsudo, M.C.; Bezerra, R.P.; Sato, S.; Perego, P.; Converti, A.; Carvalho, J.C.M. Repeated fed-batch cultivation of Arthrospira (Spirulina) platensis using urea as nitrogen source. Biochem. Eng. J. 2009, 43, 52–57. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Tornabene, T.G.; Thomas, W.H. Chemical profile of selected species of microalgae with emphasis on lipids1. J. Phycol. 1985, 21, 72–81. [Google Scholar] [CrossRef]
- Lubitz, J.A. The Protein Quality, Digestibility, and Composition of Algae, Chlorella 71105. J. Food Sci. 1963, 28, 229–232. [Google Scholar] [CrossRef]
- Leveille, G.A.; Sauberlich, H.E.; Shockley, J.W. Protein Value and the Amino Acid Deficiencies of Various Algae for Growth of Rats and Chicks. J. Nutr. 1962, 76, 423–428. [Google Scholar] [CrossRef]
- Sousa, I.; Gouveia, L.; Batista, A.; Raymundo, A.; Bandarra, N. Microalgae in novel food products. In Food Chemistry Research Developments; Papadopoulos, K.N., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2008. [Google Scholar]
- Rebolloso-Fuentes, M.M.; Navarro-Pérez, A.; García-Camacho, F.; Ramos-Miras, J.J.; Guil-Guerrero, J.L. Biomass Nutrient Profiles of the Microalga Nannochloropsis. J. Agric. Food Chem. 2001, 49, 2966–2972. [Google Scholar] [CrossRef]
- Fuentes, M.R.; Fernández, G.A.; Pérez, J.S.; Guerrero, J.G. Biomass nutrient profiles of the microalga Porphyridium cruentum. Food Chem. 2000, 70, 345–353. [Google Scholar] [CrossRef]
- Tomás-Almenar, C.; Larrán, A.; de Mercado, E.; Sanz-Calvo, M.; Hernández, D.; Riaño, B.; González, M.C.G. Scenedesmus almeriensis from an integrated system waste-nutrient, as sustainable protein source for feed to rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 497, 422–430. [Google Scholar] [CrossRef]
- Alkhamis, Y.; Qin, J.G. Comparison of pigment and proximate compositions of Tisochrysis lutea in phototrophic and mixotrophic cultures. Environ. Boil. Fishes 2016, 28, 35–42. [Google Scholar] [CrossRef]
- Clayton, J.R.; Dortch, Q.; Thoresen, S.S.; Ahmed, S. Evaluation of methods for the separation and analysis of proteins and free amino acids in phytoplankton samples. J. Plankton Res. 1988, 10, 341–358. [Google Scholar] [CrossRef]
- Lohrenz, S.; Taylor, C.D. Inorganic 14C as a probe of growth rate-dependent variations in intracellular free amino acid and protein composition of NH+4 -limited continuous cultures of Nannochloris atomis Butcher. J. Exp. Mar. Biol. Ecol. 1987, 106, 31–55. [Google Scholar] [CrossRef]
- López, C.V.G.; del Carmen Cerón García, M.; Fernandez, F.G.A.; Bustos, C.S.; Chisti, Y.; Sevilla, J.M.F. Protein measurements of microalgal and cyanobacterial biomass. Bioresour. Technol. 2010, 101, 7587–7591. [Google Scholar] [CrossRef]
- Lourenço, S.O.; Barbarino, E.; Lavin, P.; Marquez, U.M.L.; Aidar, E. Distribution of intracellular nitrogen in marine microalgae: Calculation of new nitrogen-to-protein conversion factors. Eur. J. Phycol. 2004, 39, 17–32. [Google Scholar] [CrossRef]
- Laurens, L.M.; Van Wychen, S.; McAllister, J.P.; Arrowsmith, S.; Dempster, T.A.; McGowen, J.; Pienkos, P.T. Strain, biochemistry, and cultivation-dependent measurement variability of algal biomass composition. Anal. Biochem. 2014, 452, 86–95. [Google Scholar] [CrossRef]
- Templeton, D.; Laurens, L.M. Nitrogen-to-protein conversion factors revisited for applications of microalgal biomass conversion to food, feed and fuel. Algal Res. 2015, 11, 359–367. [Google Scholar] [CrossRef] [Green Version]
- Finkel, Z.V.; Follows, M.J.; Liefer, J.; Brown, C.M.; Benner, I.; Irwin, A.J. Phylogenetic Diversity in the Macromolecular Composition of Microalgae. PLoS ONE 2016, 11, e0155977. [Google Scholar] [CrossRef] [Green Version]
- Slocombe, S.P.; Ross, M.; Thomas, N.; McNeill, S.; Stanley, M.S. A rapid and general method for measurement of protein in micro-algal biomass. Bioresour. Technol. 2013, 129, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Waterborg, J. The Lowry Method for Protein Quantitation; Humana Press: Totowa, NJ, USA, 2009; pp. 7–10. [Google Scholar]
- Shen, C.-H. Chapter 8—Quantification and Analysis of Proteins. In Diagnostic Molecular Biology; Shen, C.-H., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 187–214. [Google Scholar]
- Peterson, G.L. Review of the folin phenol protein quantitation method of lowry, rosebrough, farr and randall. Anal. Biochem. 1979, 100, 201–220. [Google Scholar] [CrossRef]
- Wang, Y.; Tibbetts, S.M.; Berrue, F.; McGinn, P.J.; MacQuarrie, S.P.; Puttaswamy, A.; Patelakis, S.; Schmidt, D.; Melanson, R.; MacKenzie, S.E. A Rat Study to Evaluate the Protein Quality of Three Green Microalgal Species and the Impact of Mechanical Cell Wall Disruption. Foods 2020, 9, 1531. [Google Scholar] [CrossRef]
- Laurens, L.M.L.; Olstad, J.L.; Templeton, D.W. Total Protein Content Determination of Microalgal Biomass by Elemental Nitrogen Analysis and a Dedicated Nitrogen-to-Protein Conversion Factor. In Biofuels from Algae: Methods and Protocols; Spilling, K., Ed.; Springer New York: New York, NY, USA, 2020; pp. 233–242. [Google Scholar]
- Metsoviti, M.N.; Katsoulas, N.; Karapanagiotidis, I.T.; Papapolymerou, G. Effect of nitrogen concentration, two-stage and prolonged cultivation on growth rate, lipid and protein content of Chlorella vulgaris. J. Chem. Technol. Biotechnol. 2019, 94, 1466–1473. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Y.; Shang, C.; Wang, Z.; Xu, J.; Yuan, Z. Characterization of lipid and fatty acids composition of Chlorella zofingiensis in response to nitrogen starvation. J. Biosci. Bioeng. 2015, 120, 205–209. [Google Scholar] [CrossRef]
- Ruangsomboon, S. Effect of light, nutrient, cultivation time and salinity on lipid production of newly isolated strain of the green microalga, Botryococcus braunii KMITL 2. Bioresour. Technol. 2012, 109, 261–265. [Google Scholar] [CrossRef]
- Sukenik, A.; Carmeli, Y.; Berner, T. Regulation of fatty acid composition by irradiance level in the eustigmatophyte Nannochloropsis sp. J. Phycol. 1989, 25, 686–692. [Google Scholar] [CrossRef]
- George, B.; Pancha, I.; Desai, C.; Chokshi, K.; Paliwal, C.; Ghosh, T.; Mishra, S. Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus—A potential strain for bio-fuel production. Bioresour. Technol. 2014, 171, 367–374. [Google Scholar] [CrossRef]
- Nzayisenga, J.C.; Farge, X.; Groll, S.L.; Sellstedt, A. Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnol. Biofuels 2020, 13, 1–8. [Google Scholar] [CrossRef]
- Renaud, S.M.; Thinh, L.-V.; Lambrinidis, G.; Parry, D.L. Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 2002, 211, 195–214. [Google Scholar] [CrossRef]
- De Oliveira, M.; Monteiro, M.; Robbs, P.; Leite, S. Growth and Chemical Composition of Spirulina maxima and Spirulina platensis Biomass at Different Temperatures. Aquac. Int. 1999, 7, 261–275. [Google Scholar] [CrossRef]
- Thompson, P.; Guo, M.-X.; Harrison, P.J. Effects of variation in temperature. I. On the biochemical composition of eight species of marine phytoplankton. J. Phycol. 1992, 28, 481–488. [Google Scholar] [CrossRef]
- Anderson, S.L.; Vacek, J.R.; Macharg, M.A.; Holtkamp, D.J. Occurrence of Incisional Complications and Associated Risk Factors Using a Right Ventral Paramedian Celiotomy Incision in 159 Horses. Vet. Surg. 2011, 40, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Sydney, E.B.; Sturm, W.; de Carvalho, J.C.; Thomaz-Soccol, V.; Larroche, C.; Pandey, A.; Soccol, C.R. Potential carbon dioxide fixation by industrially important microalgae. Bioresour. Technol. 2010, 101, 5892–5896. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Han, W.; Li, P.; Miao, X.; Zhong, J.-J. CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour. Technol. 2011, 102, 3071–3076. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.J.; Pirt, S.J. Carbon Dioxide Inhibition of Photosynthetic Growth of Chlorella. Microbiology 1984, 130, 2833–2838. [Google Scholar] [CrossRef] [Green Version]
- Patil, L.; Kaliwal, B. Effect of CO2 Concentration on Growth and Biochemical Composition of Newly Isolated Indigenous Microalga Scenedesmus bajacalifornicus BBKLP-07. Appl. Biochem. Biotechnol. 2016, 182, 335–348. [Google Scholar] [CrossRef]
- Kandasamy, L.; Neves, M.; Demura, M.; Nakajima, M. The Effects of Total Dissolved Carbon Dioxide on the Growth Rate, Biochemical Composition, and Biomass Productivity of Nonaxenic Microalgal Polyculture. Sustainability 2021, 13, 2267. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of CO2 concentration on algal growth: A review. Renew. Sustain. Energy Rev. 2014, 38, 172–179. [Google Scholar] [CrossRef]
- Wang, J.; Sommerfeld, M.R.; Lu, C.; Hu, Q. Combined effect of initial biomass density and nitrogen concentration on growth and astaxanthin production of Haematococcus pluvialis (Chlorophyta) in outdoor cultivation. Algae 2013, 28, 193–202. [Google Scholar] [CrossRef]
- AnanadhiPadmanabhan, M.R.; Renita, A.; Stanley, S.A. Studies on the effect of nitrogen source and the growth of Marine microalgae algae. In Proceedings of the Recent Advances in Space Technology Services and Climate Change 2010 (RSTS & CC-2010), Chennai, India, 13–15 November 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 350–352. [Google Scholar]
- Zarrinmehr, M.J.; Farhadian, O.; Heyrati, F.P.; Keramat, J.; Koutra, E.; Kornaros, M.; Daneshvar, E. Effect of nitrogen concentration on the growth rate and biochemical composition of the microalga, Isochrysis galbana. Egypt. J. Aquat. Res. 2020, 46, 153–158. [Google Scholar] [CrossRef]
- Solovchenko, A.; Solovchenko, O.; Khozin-Goldberg, I.; Didi-Cohen, S.; Pal, D.; Cohen, Z.; Boussiba, S. Probing the effects of high-light stress on pigment and lipid metabolism in nitrogen-starving microalgae by measuring chlorophyll fluorescence transients: Studies with a Δ5 desaturase mutant of Parietochloris incisa (Chlorophyta, Trebouxiophyceae). Algal Res. 2013, 2, 175–182. [Google Scholar] [CrossRef]
- Ho, S.-H.; Ye, X.; Hasunuma, T.; Chang, J.-S.; Kondo, A. Perspectives on engineering strategies for improving biofuel production from microalgae—A critical review. Biotechnol. Adv. 2014, 32, 1448–1459. [Google Scholar] [CrossRef]
- Kim, G.; Mujtaba, G.; Lee, K. Effects of nitrogen sources on cell growth and biochemical composition of marine chlorophyte Tetraselmis sp. for lipid production. Algae 2016, 31, 257–266. [Google Scholar] [CrossRef]
- Fidalgo, J.; Cid, A.; Torres, E.; Sukenik, A.; Herrero, C. Effects of nitrogen source and growth phase on proximate biochemical composition, lipid classes and fatty acid profile of the marine microalga Isochrysis galbana. Aquaculture 1998, 166, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Qiu, R.; Gao, S.; Lopez, P.A.; Ogden, K.L. Effects of pH on cell growth, lipid production and CO2 addition of microalgae Chlorella sorokiniana. Algal Res. 2017, 28, 192–199. [Google Scholar] [CrossRef]
- Danilov, R.A.; Ekelund, N.G.A. Effects of pH on the growth rate, motility and photosynthesis in Euglena gracilis. Folia Microbiol. 2001, 46, 549–554. [Google Scholar] [CrossRef]
- Moheimani, N.R. Inorganic carbon and pH effect on growth and lipid productivity of Tetraselmis suecica and Chlorella sp. (Chlorophyta) grown outdoors in bag photobioreactors. Environ. Boil. Fishes 2012, 25, 387–398. [Google Scholar] [CrossRef]
- Moheimani, N.R.; Borowitzka, M.A. Increased CO2 and the effect of pH on growth and calcification of Pleurochrysis carterae and Emiliania huxleyi (Haptophyta) in semicontinuous cultures. Appl. Microbiol. Biotechnol. 2011, 90, 1399–1407. [Google Scholar] [CrossRef]
- Khalil, Z.I.; Asker, M.M.S.; El-Sayed, S.; Kobbia, I.A. Effect of pH on growth and biochemical responses of Dunaliella bardawil and Chlorella ellipsoidea. World J. Microbiol. Biotechnol. 2009, 26, 1225–1231. [Google Scholar] [CrossRef]
- Goldman, J.C.; Azov, Y.; Riley, C.B.; Dennett, M.R. The effect of pH in intensive microalgal cultures. I. Biomass regulation. J. Exp. Mar. Biol. Ecol. 1982, 57, 1–13. [Google Scholar] [CrossRef]
- Bartley, M.; Boeing, W.J.; Dungan, B.N.; Holguin, F.O.; Schaub, T. pH effects on growth and lipid accumulation of the biofuel microalgae Nannochloropsis salina and invading organisms. Environ. Boil. Fishes 2013, 26, 1431–1437. [Google Scholar] [CrossRef]
- Valdés, F.; Hernández, M.; Catalá, L.; Marcilla, A. Estimation of CO2 stripping/CO2 microalgae consumption ratios in a bubble column photobioreactor using the analysis of the pH profiles. Application to Nannochloropsis oculata microalgae culture. Bioresour. Technol. 2012, 119, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Danquah, M.K.; Gladman, B.; Moheimani, N.; Forde, G.M. Microalgal growth characteristics and subsequent influence on dewatering efficiency. Chem. Eng. J. 2009, 151, 73–78. [Google Scholar] [CrossRef]
- Salim, S.; Shi, Z.; Vermuë, M.; Wijffels, R. Effect of growth phase on harvesting characteristics, autoflocculation and lipid content of Ettlia texensis for microalgal biodiesel production. Bioresour. Technol. 2013, 138, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, F.; Le Grand, F.; Quéré, C.; Bougaran, G.; Cadoret, J.-P.; Robert, R.; Soudant, P. Effects of growth phase and nitrogen limitation on biochemical composition of two strains of Tisochrysis lutea. Algal Res. 2017, 27, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.R.; Garland, C.D.; Jeffrey, S.W.; Jameson, I.D.; Leroi, J.M. The gross and amino acid compositions of batch and semi-continuous cultures OfIsochrysis sp. (clone T.ISO), Pavlova lutheri and Nannochloropsis oculata. Environ. Boil. Fishes 1993, 5, 285–296. [Google Scholar] [CrossRef]
- Sánchez-Bayo, A.; Morales, V.; Rodríguez, R.; Vicente, G.; Bautista, L.F. Cultivation of Microalgae and Cyanobacteria: Effect of Operating Conditions on Growth and Biomass Composition. Molecules 2020, 25, 2834. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Falvo, M.J. Protein—Which is Best? J. Sports Sci. Med. 2004, 3, 118–130. [Google Scholar]
- Rutherfurd, S.M.; Fanning, A.; Miller, B.J.; Moughan, P.J. Protein Digestibility-Corrected Amino Acid Scores and Digestible Indispensable Amino Acid Scores Differentially Describe Protein Quality in Growing Male Rats. J. Nutr. 2015, 145, 372–379. [Google Scholar] [CrossRef] [Green Version]
- FAO; WHO. Protein Quality Evaluation: Report of Joint FAO/WHO Expert Consultation; Food and Agriculture Organization: Rome, Italy, 1991; p. 51. [Google Scholar]
- FAO; WHO. Energy and Protein Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation; WHO Technical Report Series 724; World Health Organization: Geneva, Switzerland, 1985. [Google Scholar]
- FAO. Dietary Protein Quality Evaluation in Human Nutrition: Report of an FAO Expert Consultation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; p. 92. [Google Scholar]
- FAO. Protein Quality Assessment in Follow-Up Formula for Young Children and Ready to Use Therapeutic Foods; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017. [Google Scholar]
- Nosworthy, M.G.; Franczyk, A.J.; Medina, G.; Neufeld, J.; Appah, P.; Utioh, A.; Frohlich, P.; House, J.D. Effect of Processing on the in Vitro and in Vivo Protein Quality of Yellow and Green Split Peas (Pisum sativum). J. Agric. Food Chem. 2017, 65, 7790–7796. [Google Scholar] [CrossRef]
- Sarwar, G. The Protein Digestibility—Corrected Amino Acid Score Method Overestimates Quality of Proteins Containing Antinutritional Factors and of Poorly Digestible Proteins Supplemented with Limiting Amino Acids in Rats. J. Nutr. 1997, 127, 758–764. [Google Scholar] [CrossRef] [Green Version]
- Kent, M.; Welladsen, H.M.; Mangott, A.; Li, Y. Nutritional Evaluation of Australian Microalgae as Potential Human Health Supplements. PLoS ONE 2015, 10, e0118985. [Google Scholar] [CrossRef]
- Waghmare, A.G.; Salve, M.K.; Leblanc, J.G.; Arya, S. Concentration and characterization of microalgae proteins from Chlorella pyrenoidosa. Bioresour. Bioprocess. 2016, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Machado, M.; Machado, S.; Pimentel, F.B.; Freitas, V.; Alves, R.C.; Oliveira, M.B.P.P. Amino Acid Profile and Protein Quality Assessment of Macroalgae Produced in an Integrated Multi-Trophic Aquaculture System. Foods 2020, 9, 1382. [Google Scholar] [CrossRef]
- Andreeva, A.; Budenkova, E.; Babich, O.; Sukhikh, S.; Ulrikh, E.; Ivanova, S.; Prosekov, A.; Dolganyuk, V. Production, Purification, and Study of the Amino Acid Composition of Microalgae Proteins. Molecules 2021, 26, 2767. [Google Scholar] [CrossRef]
- Hendriks, W.H.; Van Baal, J.; Bosch, G. Ileal and faecal protein digestibility measurement in humans and other non-ruminants—A comparative species view. Br. J. Nutr. 2012, 108, S247–S257. [Google Scholar] [CrossRef] [Green Version]
- Health Canada. 1981. Available online: https://www.hc-sc.gc.ca/fn-an/alt_formats/hpfb-dgpsa/pdf/res-rech/fo-1-eng.pdf (accessed on 15 July 2021).
- Tessier, R.; Calvez, J.; Khodorova, N.; Gaudichon, C. Protein and amino acid digestibility of 15N Spirulina in rats. Eur. J. Nutr. 2021, 60, 2263–2269. [Google Scholar] [CrossRef]
- Amorim, M.L.; Soares, J.; Coimbra, J.S.D.R.; Leite, M.D.O.; Albino, L.F.T.; Martins, M.A. Microalgae proteins: Production, separation, isolation, quantification, and application in food and feed. Crit. Rev. Food Sci. Nutr. 2021, 61, 1976–2002. [Google Scholar] [CrossRef]
- Nosworthy, M.; Neufeld, J.; Frohlich, P.; Young, G.; Malcolmson, L.; House, J.D. Determination of the protein quality of cooked Canadian pulses. Food Sci. Nutr. 2017, 5, 896–903. [Google Scholar] [CrossRef]
- Jiménez-Munoz, L.M.; Tavares, G.M.; Corredig, M. Design future foods using plant protein blends for best nutritional and technological functionality. Trends Food Sci. Technol. 2021, 113, 139–150. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Patelakis, S.J. Apparent digestibility coefficients (ADCs) of intact-cell marine microalgae meal (Pavlova sp. 459) for juvenile Atlantic salmon (Salmo salar L.). Aquaculture 2022, 546, 737236. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Chen, Y.; Kaur, A.; Yu, L. Pulse proteins: Secondary structure, functionality and applications. J. Food Sci. Technol. 2019, 56, 2787–2798. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Banaszek, A.; Townsend, J.R.; Bender, D.; Vantrease, W.C.; Marshall, A.C.; Johnson, K.D. The Effects of Whey vs. Pea Protein on Physical Adaptations Following 8-Weeks of High-Intensity Functional Training (HIFT): A Pilot Study. Sports 2019, 7, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norsker, N.-H.; Barbosa, M.; Vermuë, M.H.; Wijffels, R.H. Microalgal production—A close look at the economics. Biotechnol. Adv. 2011, 29, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Van der Spiegel, M.; Noordam, M.; van der Fels-Klerx, H. Safety of Novel Protein Sources (Insects, Microalgae, Seaweed, Duckweed, and Rapeseed) and Legislative Aspects for Their Application in Food and Feed Production. Compr. Rev. Food Sci. Food Saf. 2013, 12, 662–678. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [Green Version]
| Species | Protein Content (% Dry Matter) | Reference |
|---|---|---|
| Acutodesmus dimorphus | 28 | [54] |
| Anabaena cylindrica | 43–56 | [34] |
| Aphanizomenon flos-aquae | 62 | [34] |
| Arthrospira fusiformis | 62 | [55] |
| Arthrospira maxima | 60–71 | [31,34] |
| 65 | [32] | |
| Arthrospira platensis (Bangladesh) | 60 | [33] |
| Arthrospira platensis (France) | 65 | [33] |
| Arthrospira platensis (Malaysia) | 61 | [33] |
| Arthrospira platensis (Thailand) | 55–70 | [33] |
| Arthrospira platensis | 63 | [34] |
| 53–70 | [7] | |
| 45–62 | [8] | |
| 22–38 | [9] | |
| 61 | [56] | |
| 64 | [55] | |
| 17–32 | [57] | |
| 26–72 | [58] | |
| 22–51 | [59] | |
| 57–70 | [60] | |
| 45–62 | [61] | |
| 56 | [54] | |
| Botryococcus braunii | 22 | [62] |
| 39–40 | [54] | |
| Chaetoceros calcitrans | 34 | [48] |
| Chaetoceros gracilis | 12 | [48] |
| Chlamydomonas rheinhardii | 48 | [34] |
| Chlorella 71105 | 56 | [63] |
| Chlorella pyrenoidosa | 57 | [34] |
| 60 | [64] | |
| Chlorella pyrenoidosa and Chlorella vulgaris | 53 | [54] |
| Chlorella vulgaris | 51–58 | [34] |
| 48 | [56] | |
| Chroomonas salina | 29 | [48] |
| Diacronema vlkianum | 57 | [1] |
| Dunaliella hardawil | 10 | [62] |
| Dunaliella salina | 57 | [34] |
| 29 | [62] | |
| Dunaliella tertiolecta | 20 | [48] |
| Euglena gracilis | 39–61 | [34] |
| Haematococcus pluvialis | 48 | [65] |
| Isochrisis aff.galbana (T-iso) | 23 | [48] |
| Isochrysis galbana | 29 | [48] |
| 27 | [56] | |
| Nannochloris atomus | 30 | [48] |
| Nannochloropsis granulata | 18–34 | [54] |
| Nannochloropsis oculata | 35 | [48] |
| Nannochloropsis spp. | 29 | [66] |
| Neochloris oleoabundans | 30 | [54] |
| Nitzschia closterium | 26 | [48] |
| Nitzschia sp. | 17 | [62] |
| Nochloris oleoabundans | 30 | [1] |
| Pavlova lutheri | 29 | [48] |
| Pavlova salina | 26 | [48] |
| Phaeodactylum tricornutum | 30 | [48] |
| 40 | [54] | |
| Porphyridium aerugineum | 32 | [54] |
| Porphyridium cruentum | 28–39 | [34] |
| 34 | [67] | |
| Scenedesmus almeriensis | 47 | [68] |
| Scenedesmus obliquus | 50–56 | [34] |
| Skeletonema costatum | 25 | [48] |
| Spirogyra sp. | 6–20 | [34] |
| Spirogyra varians | 17 | [49] |
| Spongiococcum excentricum | 32 | [64] |
| Synechococcus sp. | 46–63 | [34] |
| Tetraselmis chuii | 31 | [48] |
| 47 | [54] | |
| Tetraselmis suecica | 31 | [48] |
| Thalassiosira pseudonana | 34 | [48] |
| Tisochrysis lutea | 37–42 | [69] |
| Microalgal Species | His | ISO | Leu | Lys | SAA | AAA | Thr | Trp | Val | AAS # | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference protein | 16 | 30 | 61 | 48 | 23 | 41 | 25 | 6.6 | 40 | [123] | |
| Acutodesmus obliquus | 14& | 36 | 85 | 41 | 33 | 92 | 59 | 20 | 60 | 0.86 | [81] |
| Acutodesmus obliquus * | 12 | 38 | 89 | 36 | 34 | 90 | 61 | 22 | 62 | 0.76 | [81] |
| Arthrospira maxima | 18 | 60 | 80 | 46 | 18 | 88 | 46 | 14 | 65 | 0.78 | [34] |
| Arthrospira platensis | 22 | 45 | 98 | 71 | 39 | 157 | 46 | 12 | 78 | 1.37 | [130] |
| Botryococcus braunii (A) | 15 | 34 | 71 | 47 | 39 | 72 | 37 | 22 | 44 | 0.94 | [54] |
| Chaetoceros calcitrans | 19 | 55 | 82 | 63 | 30 | 112 | 45 | 14 | 59 | 1.19 | [48] |
| Chaetoceros gracilis | 24 | 58 | 72 | 51 | 29 | 125 | 59 | 16 | 62 | 1.06 | [48] |
| Chlorella pyrenoidosa | 16 | 62 | 34 | 81 | 61 | 51 | 35 | 52 | 0.56 | [128] | |
| Chlorella sorokiniana | 20 | 35 | 84 | 57 | 27 | 87 | 53 | 20 | 59 | 1.16 | [81] |
| Chlorella sorokiniana * | 19 | 35 | 83 | 58 | 28 | 86 | 50 | 22 | 59 | 1.16 | [81] |
| Chlorella vulgaris | 18 | 36 | 92 | 52 | 29 | 98 | 43 | 23 | 58 | 1.10 | [81] |
| Chlorella vulgaris | 20 | 38 | 88 | 84 | 36 | 84 | 48 | 21 | 55 | 1.25 | [34] |
| Chlorella vulgaris * | 18 | 37 | 93 | 48 | 25 | 95 | 45 | 23 | 60 | 1.10 | [81] |
| Chroomonas salina | 18 | 41 | 78 | 61 | 30 | 111 | 54 | 13 | 61 | 1.13 | [48] |
| Dunaliella bardawil | 18 | 42 | 110 | 70 | 35 | 95 | 54 | 7 | 58 | 1.06 | [34] |
| Dunaliella tertiolecta | 21 | 48 | 84 | 60 | 18 | 117 | 47 | 15 | 62 | 0.80 | [48] |
| Isochrisis aff.galbana (T-iso) | 20 | 46 | 87 | 60 | 31 | 105 | 45 | 16 | 61 | 1.25 | [48] |
| Isochrysis galbana | 21 | 48 | 87 | 62 | 27 | 108 | 52 | 13 | 62 | 1.15 | [48] |
| Nannochloris atomus | 18 | 34 | 75 | 52 | 25 | 94 | 40 | 11 | 59 | 1.07 | [48] |
| Nannochloropsis granulata (A) | 23 | 56 | 110 | 85 | 51 | 104 | 54 | 28 | 71 | 1.44 | [54] |
| Nannochloropsis oculata | 21 | 48 | 78 | 61 | 20 | 104 | 55 | 16 | 65 | 0.87 | [48] |
| Nitzschia closterium | 14 | 50 | 81 | 57 | 22 | 108 | 55 | 14 | 62 | 0.88 | [48] |
| Nostoc sp. | 20 | 37 | 95 | 65 | 38 | 140 | 53 | 10 | 72 | 1.23 | [130] |
| Pavlova lutheri | 50 | 49 | 81 | 56 | 37 | 111 | 43 | 15 | 67 | 1.17 | [48] |
| Pavlova salina | 15 | 44 | 90 | 62 | 20 | 92 | 52 | 9 | 61 | 0.94 | [48] |
| Phaeodactylum tricornutum | 15 | 46 | 70 | 64 | 42 | 82 | 48 | 26 | 51 | 0.94 | [54] |
| Phaeodactylum tricornutum | 17 | 49 | 77 | 56 | 23 | 107 | 54 | 16 | 59 | 1.06 | [48] |
| Pleurochrysis carterae | 19 | 42 | 99 | 72 | 44 | 154 | 57 | 11 | 76 | 1.18 | [130] |
| Porphyridium aerugineum | 19 | 71 | 119 | 80 | 59 | 121 | 58 | 33 | 73 | 1.19 | [54] |
| Scenedesmus obliquus | 21 | 36 | 73 | 56 | 21 | 80 | 51 | 3 | 60 | 0.45 | [34] |
| Skeletonema costatum | 16 | 52 | 83 | 57 | 26 | 109 | 51 | 13 | 63 | 1.00 | [48] |
| Spirulina platensis | 22 | 67 | 98 | 48 | 34 | 106 | 62 | 3 | 71 | 0.45 | [34] |
| Tetraselmis chuii | 18 | 35 | 75 | 57 | 25 | 91 | 42 | 10 | 58 | 1.07 | [48] |
| Tetraselmis chuii | 16 | 34 | 73 | 56 | 52 | 77 | 40 | 23 | 48 | 1.00 | [54] |
| Tetraselmis suecica | 18 | 35 | 80 | 60 | 30 | 97 | 41 | 12 | 57 | 1.13 | [48] |
| Thalassiosira pseudonana | 16 | 55 | 84 | 59 | 27 | 110 | 52 | 8.7 | 61 | 1.00 | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Tibbetts, S.M.; McGinn, P.J. Microalgae as Sources of High-Quality Protein for Human Food and Protein Supplements. Foods 2021, 10, 3002. https://doi.org/10.3390/foods10123002
Wang Y, Tibbetts SM, McGinn PJ. Microalgae as Sources of High-Quality Protein for Human Food and Protein Supplements. Foods. 2021; 10(12):3002. https://doi.org/10.3390/foods10123002
Chicago/Turabian StyleWang, Yanwen, Sean M. Tibbetts, and Patrick J. McGinn. 2021. "Microalgae as Sources of High-Quality Protein for Human Food and Protein Supplements" Foods 10, no. 12: 3002. https://doi.org/10.3390/foods10123002
APA StyleWang, Y., Tibbetts, S. M., & McGinn, P. J. (2021). Microalgae as Sources of High-Quality Protein for Human Food and Protein Supplements. Foods, 10(12), 3002. https://doi.org/10.3390/foods10123002







