Edible Mushrooms: A Comprehensive Review on Bioactive Compounds with Health Benefits and Processing Aspects
Abstract
1. Introduction
2. Bioactive Components in Edible Mushrooms
3. Techniques Involved in Extraction of Bioactive Components from Edible Mushrooms
3.1. Conventional Techniques Used in Extraction of Bioactive Components
3.2. Use of Novel Extraction Techniques
3.2.1. Enzyme-Assisted Extraction
3.2.2. Supercritical and Subcritical Fluid Extraction
3.2.3. Ultrasound-Assisted Extraction (UAE)
3.2.4. Extraction Using Pulsed Electric Fields
3.2.5. Extraction Using Microwaves
3.2.6. Subcritical Water Extraction
4. Health Benefits of Bioactive Components Present in the Mushroom
4.1. Anti-Carcinogenic Properties
4.2. Anti-Oxidative Properties
4.3. Hypo-Cholesterolemic Agents
4.4. Hepatoprotective Effects
4.5. Anti-Diabetic Effects
4.6. Anti-Microbial Effects
4.7. Mushrooms as Natural Resources of Immunotherapy
5. Processing Aspects of Edible Mushrooms
6. Conclusions and Future Aspects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kakon, A.J.; Choudhury, M.B.K.; Saha, S. Mushroom is an ideal food supplement. J. Dhaka Natl. Med. Coll. Hosp. 2012, 18, 58–62. [Google Scholar] [CrossRef]
- Erbiai, E.H.; da Silva, L.P.; Saidi, R.; Lamrani, Z.; da Silva, J.C.E.; Maouni, A. Chemical composition, bioactive compounds, and antioxidant activity of two wild edible mushrooms Armillaria Mellea and macrolepiota procera from two countries (Morocco and Portugal). Biomolecules 2021, 11, 575. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Vaida, N.; Ahmad Dar, M. Medicinal importance of mushrooms: A review. Int. J. Adv. Res. 2014, 2, 1–4. [Google Scholar]
- Roncero-Ramos, I.; Mendiola-Lanao, M.; Pérez-Clavijo, M.; Delgado-Andrade, C. Effect of different cooking methods on nutritional value and antioxidant activity of cultivated mushrooms. Int. J. Food Sci. Nutr. 2017, 68, 287–297. [Google Scholar] [CrossRef]
- Khatun, S.; Islam, A.; Cakilcioglu, U.; Chatterjee, N.C. Research on mushroom as a potential source of nutraceuticals: A review on Indian perspective. J. Exp. Agric. Int. 2012, 2, 47–73. [Google Scholar] [CrossRef]
- Kumar, K. Role of edible mushrooms as functional foods—A review. S. Asian J. Food Technol. Environ. 2015, 1, 211–218. [Google Scholar] [CrossRef]
- Perera, P.K.; Li, Y. Mushrooms as a functional food mediator in preventing and ameliorating diabetes. Funct. Foods Health Dis. 2011, 1, 161–171. [Google Scholar] [CrossRef]
- Leong, Y.K.; Yang, F.-C.; Chang, J.-S. Extraction of polysaccharides from edible mushrooms: Emerging technologies and recent advances. Carbohydr. Polym. 2021, 251, 117006. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.C. Nutritional value and health benefits of mushrooms. Mushrooms Funct. Foods 2008, 2, 71–109. [Google Scholar]
- Elisashvili, V.I.; Wasser, S.P.; Tan, K.-K. Hypoglycemic, interferonogenous, and immunomodulatory activity of tremellastin from the submerged culture of Tremella Mesenterica Retz.: Fr.(Heterobasidiomycetes). Int. J. Med. Mushrooms 2002, 4, 13. [Google Scholar]
- Ooi, V.E. Antitumor and immunomodulatory activities of mushroom polysaccharides. In Mushrooms as Functional Foods; Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 147–198. [Google Scholar]
- Reshetnikov, S.V.; Tan, K.-K. Higher basidiomycota as a source of antitumor and immunostimulating polysaccharides. Int. J. Med. Mushrooms 2001, 3, 361–394. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, S.W.; Cheung, P.C.K.; Wang, Q. Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol. 2007, 18, 4–19. [Google Scholar] [CrossRef]
- Moro, C.; Palacios, I.; Lozano, M.; D’Arrigo, M.; Guillamón, E.; Villares, A.; Martínez, J.A.; García-Lafuente, A. Anti-inflammatory activity of methanolic extracts from edible mushrooms in LPS activated RAW 264.7 macrophages. Food Chem. 2012, 130, 350–355. [Google Scholar] [CrossRef]
- Ndungutse, V.; Mereddy, R.; Sultanbawa, Y. Bioactive properties of mushroom (A Garicus bisporus) stipe extracts. J. Food Process. Preserv. 2015, 39, 2225–2233. [Google Scholar] [CrossRef]
- Lakhanpal, T.N.; Rana, M. Medicinal and nutraceutical genetic resources of mushrooms. Plant Genet. Resour. 2005, 3, 288–303. [Google Scholar] [CrossRef]
- Zhang, D.W.; Zhao, L.; Wu, T.X. Optimization of Auricularia auricula exopolysaccharide fermentation medium by orthogonal experiment design. J. Guizhou Univ. Technol. Nat. Sci. Ed. 2007, 36, 40–43. [Google Scholar]
- Chang, H.-H.; Hsieh, K.-Y.; Yeh, C.-H.; Tu, Y.-P.; Sheu, F. Oral administration of an Enoki mushroom protein FVE activates innate and adaptive immunity and induces anti-tumor activity against murine hepatocellular carcinoma. Int. Immunopharmacol. 2010, 10, 239–246. [Google Scholar] [CrossRef]
- Chang, C.; Jiu-Gang, X.U.E.; Kai-Song, Z.; Yan, L.I.; Han-Xing, Z.; Chang-Kai, Z. Purification and characterization of flammulin, a basic protein with anti-tumor activities from Flammulina velutipes. J. Chin. Pharm. Sci. 2003, 12, 60. [Google Scholar]
- Wu, D.; Duan, W.; Liu, Y.; Cen, Y. Anti-Inflammatory effect of the polysaccharides of golden needle mushroom in burned rats. Int. J. Biol. Macromol. 2010, 46, 100–103. [Google Scholar] [CrossRef]
- Yin, H.; Wang, Y.; Wang, Y.; Chen, T.; Tang, H.; Wang, M. Purification, characterization and immuno-modulating properties of polysaccharides isolated from Flammulina velutipes mycelium. Am. J. Chin. Med. 2010, 38, 191–204. [Google Scholar] [CrossRef]
- Xu, J.-W.; Zhao, W.; Zhong, J.-J. Biotechnological production and application of ganoderic acids. Appl. Microbiol. Biotechnol. 2010, 87, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Walton, E. Buried treasure: Unlocking the secrets of medicinal mushrooms. Biomed. J. 2014, 37, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Attarat, J.; Phermthai, T. Bioactive compounds in three edible Lentinus mushrooms. Walailak J. Sci. Technol. WJST 2015, 12, 491–504. [Google Scholar]
- Chowdhury, M.M.H.; Kubra, K.; Ahmed, S.R. Screening of antimicrobial, antioxidant properties and bioactive compounds of some edible mushrooms cultivated in Bangladesh. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 1–6. [Google Scholar] [CrossRef]
- Israilides, C.; Kletsas, D.; Arapoglou, D.; Philippoussis, A.; Pratsinis, H.; Ebringerová, A.; Hříbalová, V.; Harding, S.E. In vitro cytostatic and immunomodulatory properties of the medicinal mushroom Lentinula edodes. Phytomedicine 2008, 15, 512–519. [Google Scholar] [CrossRef]
- Ngai, P.H.; Ng, T.B. Lentin, a novel and potent antifungal protein from Shitake Mushroom with inhibitory effects on activity of human immunodeficiency virus-1 reverse transcriptase and proliferation of leukemia cells. Life Sci. 2003, 73, 3363–3374. [Google Scholar] [CrossRef]
- Sasaki, T.; Takasuka, N. Further study of the structure of lentinan, an anti-tumor polysaccharide from Lentinus edodes. Carbohydr. Res. 1976, 47, 99–104. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Pardhi, P.; Bhadoriya, S.S.; Jain, N.; Rai, G.; Jain, A.P. Antioxidant potential of White Oyster culinary-medicinal mushroom, Pleurotus Florida (higher basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 491–498. [Google Scholar] [CrossRef]
- Menaga, D.; Mahalingam, P.U.; Rajakumar, S.; Ayyasamy, P.M. Evaluation of phytochemical characteristics and antimicrobial activity of Pleurotus Florida mushroom. Asian J. Pharm. Clin. Res. 2012, 5, 102–106. [Google Scholar]
- El Enshasy, H.; Maftoun, P.; Abd Malek, R. Pleuran: Immunomodulator polysaccharide from Pleurotus ostreatus, structure, production and application. In Mushrooms Types, Properties and Nutrition; Nova Publisher: Hauppauge, NY, USA, 2012; pp. 153–172. [Google Scholar]
- Tong, H.; Xia, F.; Feng, K.; Sun, G.; Gao, X.; Sun, L.; Jiang, R.; Tian, D.; Sun, X. Structural characterization and in vitro antitumor activity of a novel polysaccharide isolated from the fruiting bodies of Pleurotus ostreatus. Bioresour. Technol. 2009, 100, 1682–1686. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.T.; Xia, L.; Ng, T.B. Pleurostrin, an antifungal peptide from the Oyster mushroom. Peptides 2005, 26, 2098–2103. [Google Scholar] [CrossRef]
- El-Fakharany, M.E.; Haroun, M.B.; Ng, T.; Redwan, M.E.-R. Oyster mushroom laccase inhibits Hepatitis C virus entry into peripheral blood cells and hepatoma cells. Protein Pept. Lett. 2010, 17, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Oloke, J.K.; Adebayo, E.A. Effectiveness of Immunotherapies from Oyster mushroom (Pleurotus species) in the management of immunocompromised patients. Int. J. Immunol. 2015, 3, 8. [Google Scholar]
- Lavi, I.; Nimri, L.; Levinson, D.; Peri, I.; Hadar, Y.; Schwartz, B. Glucans from the edible mushroom Pleurotus Pulmonarius inhibit colitis-associated colon carcinogenesis in mice. J. Gastroenterol. 2012, 47, 504–518. [Google Scholar] [CrossRef] [PubMed]
- Smiderle, F.R.; Olsen, L.M.; Carbonero, E.R.; Baggio, C.H.; Freitas, C.S.; Marcon, R.; Santos, A.R.; Gorin, P.A.; Iacomini, M. Anti-inflammatory and analgesic properties in a rodent model of a (1 → 3),(1 → 6)-linked β-glucan isolated from Pleurotus pulmonarius. Eur. J. Pharmacol. 2008, 597, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-C.; Hsu, C.-I.; Lin, R.-H.; Kao, C.-L.; Lin, J.-Y. Fip-Vvo, a new fungal immunomodulatory protein isolated from Volvariella volvacea. Biochem. J. 1997, 323, 557–565. [Google Scholar] [CrossRef]
- Badalyan, S.M. Potential of Mushroom Bioactive Molecules to Develop Healthcare Biotech Products. In Proceedings of the 8th International Conference on Mushroom Biology and Mushroom Products (ICMBMP8), New Delhi, India, 19–22 November 2014; Yugantar Prakashan Pvt. Ltd.: New Delhi, India, 2014; pp. 373–378. [Google Scholar]
- Wasser, S.P. Medicinal mushroom science: History, current status, future trends, and unsolved problems. Int. J. Med. Mushrooms 2010, 12, 1–16. [Google Scholar] [CrossRef]
- Erjavec, J.; Kos, J.; Ravnikar, M.; Dreo, T.; Sabotič, J. Proteins of higher fungi–from forest to application. Trends Biotechnol. 2012, 30, 259–273. [Google Scholar] [CrossRef]
- Jiang, J.; Sliva, D. Novel medicinal mushroom blend suppresses growth and invasiveness of human breast cancer cells. Int. J. Oncol. 2010, 37, 1529–1536. [Google Scholar]
- Kumar, K. Nutraceutical potential and processing aspects of Oyster mushrooms (Pleurotus species). Curr. Nutr. Food Sci. 2020, 16, 3–14. [Google Scholar] [CrossRef]
- Barros, L.; Cruz, T.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C. Wild and commercial mushrooms as source of nutrients and nutraceuticals. Food Chem. Toxicol. 2008, 46, 2742–2747. [Google Scholar] [CrossRef] [PubMed]
- Roselló-Soto, E.; Parniakov, O.; Deng, Q.; Patras, A.; Koubaa, M.; Grimi, N.; Boussetta, N.; Tiwari, B.K.; Vorobiev, E.; Lebovka, N. Application of non-conventional extraction methods: Toward a sustainable and green production of valuable compounds from mushrooms. Food Eng. Rev. 2016, 8, 214–234. [Google Scholar] [CrossRef]
- Mondal, S.; Chakraborty, I.; Pramanik, M.; Rout, D.; Islam, S.S. Structural studies of water-soluble polysaccharides of an edible mushroom, Termitomyces Eurhizus. A reinvestigation. Carbohydr. Res. 2004, 339, 1135–1140. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Z.; Lei, Z.; Yang, Y.; Sugiura, N. Comparative study of antioxidant activity and antiproliferative effect of hot water and ethanol extracts from the mushroom Inonotus obliquus. J. Biosci. Bioeng. 2009, 107, 42–48. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, M.; Zhao, Y.; Miao, K.; Pan, S.; Cao, F.; Dai, Y. Analysis of antioxidant metabolites by solvent extraction from sclerotia of Inonotus obliquus (Chaga). Phytochem. Anal. 2011, 22, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Shnyreva, A.V.; Song, W.; Van Griensven, L.J.L.D. Extracts of medicinal mushrooms Agaricus bisporus and Phellinus Linteus induce proapoptotic effects in the human leukemia cell line K562. Int. J. Med. Mushrooms 2010, 12, 70. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Adams, D.J. Fungal cell wall chitinases and glucanases. Microbiology 2004, 150, 2029–2035. [Google Scholar] [CrossRef]
- Enman, J.; Rova, U.; Berglund, K.A. Quantification of the bioactive compound eritadenine in selected strains of Shiitake mushroom (Lentinus edodes). J. Agric. Food Chem. 2007, 55, 1177–1180. [Google Scholar] [CrossRef]
- De Castro, M.L.; Jiménez-Carmona, M.M.; Fernandez-Perez, V. Towards more rational techniques for the isolation of valuable essential oils from plants. TrAC Trends Anal. Chem. 1999, 18, 708–716. [Google Scholar] [CrossRef]
- Goto, M.; Sato, M.; Hirose, T. Extraction of peppermint oil by supercritical carbon dioxide. J. Chem. Eng. Jpn. 1993, 26, 401–407. [Google Scholar] [CrossRef]
- Mhemdi, H.; Rodier, E.; Kechaou, N.; Fages, J. A supercritical tuneable process for the selective extraction of fats and essential oil from coriander seeds. J. Food Eng. 2011, 105, 609–616. [Google Scholar] [CrossRef]
- Vidović, S.; Mujić, I.; Zeković, Z.; Lepojević, Ž.; Milošević, S.; Jokić, S. Extraction of fatty acids from Boletus edulis by subcritical and supercritical carbon dioxide. J. Am. Oil Chem. Soc. 2011, 88, 1189–1196. [Google Scholar] [CrossRef]
- Seo, H.-K.; Lee, S.-C. Antioxidant activity of subcritical water extracts from Chaga mushroom (Inonotus obliquus). Sep. Sci. Technol. 2010, 45, 198–203. [Google Scholar] [CrossRef]
- Yang, L.; Qu, H.; Mao, G.; Zhao, T.; Li, F.; Zhu, B.; Zhang, B.; Wu, X. Optimization of subcritical water extraction of polysaccharides from Grifola Frondosa using response surface methodology. Pharmacogn. Mag. 2013, 9, 120. [Google Scholar] [PubMed]
- Roselló-Soto, E.; Galanakis, C.M.; Brnčić, M.; Orlien, V.; Trujillo, F.J.; Mawson, R.; Knoerzer, K.; Tiwari, B.K.; Barba, F.J. Clean recovery of antioxidant compounds from plant foods, by-products and algae assisted by ultrasounds processing. Modeling approaches to optimize processing conditions. Trends Food Sci. Technol. 2015, 42, 134–149. [Google Scholar] [CrossRef]
- Soria, A.C.; Villamiel, M. Effect of ultrasound on the technological properties and bioactivity of food: A review. Trends Food Sci. Technol. 2010, 21, 323–331. [Google Scholar] [CrossRef]
- Barba, F.J.; Grimi, N.; Vorobiev, E. New approaches for the use of non-conventional cell disruption technologies to extract potential food additives and nutraceuticals from microalgae. Food Eng. Rev. 2015, 7, 45–62. [Google Scholar] [CrossRef]
- Cheung, Y.-C.; Siu, K.-C.; Wu, J.-Y. Kinetic models for ultrasound-assisted extraction of water-soluble components and polysaccharides from medicinal fungi. Food Bioprocess Technol. 2013, 6, 2659–2665. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Wang, W. Optimization of ultrasonic-assisted extraction and in vitro antioxidant activities of polysaccharides from Trametes orientalis. Carbohydr. Polym. 2014, 111, 315–323. [Google Scholar] [CrossRef]
- You, Q.; Yin, X.; Ji, C. Pulsed counter-current ultrasound-assisted extraction and characterization of polysaccharides from Boletus edulis. Carbohydr. Polym. 2014, 101, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Lebovka, N.I.; Shynkaryk, M.V.; El-Belghiti, K.; Benjelloun, H.; Vorobiev, E. Plasmolysis of sugarbeet: Pulsed electric fields and thermal treatment. J. Food Eng. 2007, 80, 639–644. [Google Scholar] [CrossRef]
- Vorobiev, E.; Lebovka, N. Enhanced extraction from solid foods and biosuspensions by pulsed electrical energy. Food Eng. Rev. 2010, 2, 95–108. [Google Scholar] [CrossRef]
- Boussetta, N.; Vorobiev, E. Extraction of valuable biocompounds assisted by high voltage electrical discharges: A review. Comptes Rendus Chim. 2014, 17, 197–203. [Google Scholar] [CrossRef]
- Grimi, N.; Dubois, A.; Marchal, L.; Jubeau, S.; Lebovka, N.I.; Vorobiev, E. Selective extraction from microalgae Nannochloropsis Sp. using different methods of cell disruption. Bioresour. Technol. 2014, 153, 254–259. [Google Scholar] [CrossRef]
- Liu, D.; Lebovka, N.I.; Vorobiev, E. Impact of electric pulse treatment on selective extraction of intracellular compounds from Saccharomyces Cerevisiae yeasts. Food Bioprocess Technol. 2013, 6, 576–584. [Google Scholar] [CrossRef]
- Xue, D.; Farid, M.M. Pulsed electric field extraction of valuable compounds from white button mushroom (Agaricus bisporus). Innov. Food Sci. Emerg. Technol. 2015, 29, 178–186. [Google Scholar] [CrossRef]
- Parniakov, O.; Lebovka, N.I.; Van Hecke, E.; Vorobiev, E. Pulsed electric field assisted pressure extraction and solvent extraction from mushroom (Agaricus bisporus). Food Bioprocess Technol. 2014, 7, 174–183. [Google Scholar] [CrossRef]
- Deng, Q.; Zinoviadou, K.G.; Galanakis, C.M.; Orlien, V.; Grimi, N.; Vorobiev, E.; Lebovka, N.; Barba, F.J. The effects of conventional and non-conventional processing on glucosinolates and its derived forms, isothiocyanates: Extraction, degradation, and applications. Food Eng. Rev. 2015, 7, 357–381. [Google Scholar] [CrossRef]
- Lebovka, N.; Vorobiev, E.; Chemat, F. Enhancing Extraction Processes in the Food Industry; CRC Press: Boca Raton, FL, USA, 2012; ISBN 1-4398-4593-X. [Google Scholar]
- Haswell, S.J.; Kingston, H.M. Microwave-Enhanced Chemistry: Fundamentals, Sample Preparation, and Applications; American Chemical Society: Washington, DC, USA, 1997; ISBN 0-8412-3375-6. [Google Scholar]
- Özyürek, M.; Bener, M.; Güçlü, K.; Apak, R. Antioxidant/antiradical properties of microwave-assisted extracts of three wild edible mushrooms. Food Chem. 2014, 157, 323–331. [Google Scholar] [CrossRef]
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub-and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Smith, R.M. Superheated Water: The Ultimate Green Solvent for Separation Science; Springer: Cham, Switzerland, 2006; ISBN 1618-2650. [Google Scholar]
- Kumar, H.; Choudhary, N.; Varsha, K.N.; Suman, S.R. Phenolic compounds and their health benefits: A review. J. Food Res. Technol. 2014, 2, 46–59. [Google Scholar]
- Daba, A.S.; Ezeronye, O.U. Anti-cancer effect of polysaccharides isolated from higher Basidiomycetes mushrooms. Afr. J. Biotechnol. 2003, 2, 672–678. [Google Scholar]
- Patel, S.; Goyal, A. Recent developments in mushrooms as anti-cancer therapeutics: A review. 3 Biotech 2012, 2, 1–15. [Google Scholar] [CrossRef]
- Baker, J.R.; Kim, J.-S.; Park, S.-Y. Composition and proposed structure of a water-soluble glycan from the Keumsa Sangwhang mushroom (Phellinus Linteus). Fitoterapia 2008, 79, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Lavi, I.; Friesem, D.; Geresh, S.; Hadar, Y.; Schwartz, B. An aqueous polysaccharide extract from the edible mushroom Pleurotus ostreatus induces anti-proliferative and pro-apoptotic effects on HT-29 colon cancer cells. Cancer Lett. 2006, 244, 61–70. [Google Scholar] [CrossRef]
- Niu, Y.-C.; Liu, J.-C.; Zhao, X.-M.; Cao, J. A low molecular weight polysaccharide isolated from Agaricus Blazei Murill (LMPAB) exhibits its anti-metastatic effect by down-regulating metalloproteinase-9 and up-regulating Nm23-H1. Am. J. Chin. Med. 2009, 37, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Ajith, T.A.; Janardhanan, K.K. Indian medicinal mushrooms as a source of antioxidant and antitumor agents. J. Clin. Biochem. Nutr. 2007, 40, 157–162. [Google Scholar] [CrossRef]
- Chen, S.; Oh, S.-R.; Phung, S.; Hur, G.; Ye, J.J.; Kwok, S.L.; Shrode, G.E.; Belury, M.; Adams, L.S.; Williams, D. Anti-aromatase activity of phytochemicals in White Button Mushrooms (Agaricus bisporus). Cancer Res. 2006, 66, 12026–12034. [Google Scholar] [CrossRef]
- Shin, A.; Kim, J.; Lim, S.-Y.; Kim, G.; Sung, M.-K.; Lee, E.-S.; Ro, J. Dietary mushroom intake and the risk of breast cancer based on hormone receptor status. Nutr. Cancer 2010, 62, 476–483. [Google Scholar] [CrossRef]
- Mehra, R.; Kumar, H.; Kumar, N.; Kaushik, R. Red rice conjugated with Barley and Rhododendron extracts for new variant of beer. J. Food Sci. Technol. 2020, 57, 4152–4159. [Google Scholar] [CrossRef]
- Dubost, N.J.; Ou, B.; Beelman, R.B. Quantification of polyphenols and ergothioneine in cultivated mushrooms and correlation to total antioxidant capacity. Food Chem. 2007, 105, 727–735. [Google Scholar] [CrossRef]
- Mau, J.-L.; Tsai, S.-Y.; Tseng, Y.-H.; Huang, S.-J. Antioxidant properties of hot water extracts from Ganoderma Tsugae Murrill. LWT-Food Sci. Technol. 2005, 38, 589–597. [Google Scholar] [CrossRef]
- Wei, S. Pro-and antioxidative properties of medicinal mushroom extracts. Int. J. Med. Mushrooms 2008, 10, 315–324. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrović, P.; Niksic, M.; Vrvic, M.M.; Van Griensven, L. Antioxidants of edible mushrooms. Molecules 2015, 20, 19489–19525. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ooi, V.E.C.; Chang, S.T. Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci. 1997, 60, 763–771. [Google Scholar] [CrossRef]
- Kosanic, M.; Rankovic, B.; Dasic, M. Antioxidant and antimicrobial properties of mushrooms. Bulg. J. Agric. Sci. 2013, 19, 1040–1046. [Google Scholar]
- Ishikawa, Y.; Morimoto, K.; Hamasaki, T. Flavoglaucin, a metabolite of Eurotium Chevalieri, its antioxidation and synergism with tocopherol. J. Am. Oil Chem. Soc. 1984, 61, 1864–1868. [Google Scholar] [CrossRef]
- Nabubuya, A.; Muyonga, J.H.; Kabasa, J.D. Nutritional and hypocholesterolemic properties of termitomyces Microcarpus mushrooms. Afr. J. Food Agric. Nutr. Dev. 2010, 10, 54081. [Google Scholar] [CrossRef]
- Yang, B.-K.; Park, J.-B.; Song, C.-H. Hypolipidemic effect of exo-polymer produced in submerged mycelial culture of five different mushrooms. J. Microbiol. Biotechnol. 2002, 12, 957–961. [Google Scholar]
- Rathee, S.; Rathee, D.; Rathee, D.; Kumar, V.; Rathee, P. Mushrooms as therapeutic agents. Rev. Bras. Farmacogn. 2012, 22, 459–474. [Google Scholar] [CrossRef]
- Kodavanti, P.R.S.; Joshi, U.M.; Young, R.A.; Meydrech, E.F.; Mehendale, H.M. Protection of hepatotoxic and lethal effects of CCl by partial hepatectomy. Toxicol. Pathol. 1989, 17, 494–505. [Google Scholar] [CrossRef]
- Navarro, V.J.; Senior, J.R. Drug-related hepatotoxicity. N. Engl. J. Med. 2006, 354, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Dey, A.; Dutta, R.; Dey, S.; Acharya, K. hepatoprotective effect of the ethanolic extract of Calocybe Indica on mice with CCl4 hepatic intoxication. Int. J. Pharm. Technol. Res. 2011, 3, 2162–2168. [Google Scholar]
- Hirotani, M.; Ino, C.; Furuya, T.; Shiro, M. Ganoderic acids T, S and R, new triterpenoids from the cultured mycelia of Ganoderma lucidum. Chem. Pharm. Bull. 1986, 34, 2282–2285. [Google Scholar] [CrossRef]
- Ooi, V.E.C. Hepatoprotective effect of some edible mushrooms. Phytother. Res. 1996, 10, 536–538. [Google Scholar] [CrossRef]
- Sumy, A.K.; Jahan, N.; Sultana, N.; Sikder, A.M. Effect of Oyster mushroom in Paracetamol induced toxicity of liver in Wistar albino rats. J. Enam Med. Coll. 2014, 4, 161–167. [Google Scholar] [CrossRef]
- Zhou, C.Y.; Jia, W.; Yang, Y.; Bai, Y.Q. Experimental studies on prevention of several kinds of fungi polysaccharides against alcohol-induced hepatic injury. Edible Fungi 2002, 24, 36–37. [Google Scholar]
- Kim, D.-H.; Shim, S.-B.; Kim, N.-J.; Jang, I.-S. β-Glucuronidase-Inhibitory activity and hepatoprotective effect of Ganoderma lucidum. Biol. Pharm. Bull. 1999, 22, 162–164. [Google Scholar] [CrossRef]
- Refaie, F.M.; Esmat, A.Y.; Daba, A.S.; Osman, W.M.; Taha, S.M. Hepatoprotective activity of polysaccharopeptides from Pleurotus ostreatus mycelium on thioacetamide-intoxicated mice. Micol. Appl. Int. 2010, 22, 1–13. [Google Scholar]
- Zhang, Z.; Lv, G.; Pan, H.; Pandey, A.; He, W.; Fan, L. Antioxidant and hepatoprotective potential of endo-polysaccharides from Hericium Erinaceus grown on Tofu whey. Int. J. Biol. Macromol. 2012, 51, 1140–1146. [Google Scholar] [CrossRef]
- Chen, J.; Mao, D.; Yong, Y.; Li, J.; Wei, H.; Lu, L. Hepatoprotective and hypolipidemic effects of water-soluble polysaccharidic extract of Pleurotus eryngii. Food Chem. 2012, 130, 687–694. [Google Scholar] [CrossRef]
- Soares, A.A.; de Oliveira, A.L.; Sá-Nakanishi, A.B.; Comar, J.F.; Rampazzo, A.P.; Vicentini, F.A.; Natali, M.R.; Gomes da Costa, S.M.; Bracht, A.; Peralta, R.M. Effects of an Agaricus Blazei aqueous extract pretreatment on Paracetamol-induced brain and liver injury in rats. BioMed Res. Int. 2013, 2013, 469180. [Google Scholar] [CrossRef]
- De Silva, D.D.; Rapior, S.; Hyde, K.D.; Bahkali, A.H. Medicinal mushrooms in prevention and control of diabetes mellitus. Fungal Divers. 2012, 56, 1–29. [Google Scholar] [CrossRef]
- Cui, B.; Han, L.; Qu, J.; Lv, Y. Hypoglycemic activity of Grifola Frondosa rich in vanadium. Biol. Trace Elem. Res. 2009, 131, 186–191. [Google Scholar] [CrossRef]
- Kaur, A.; Dhingra, G.S.; Shri, R. Antidiabetic potential of mushrooms. Asian J. Pharm. Res. 2015, 5, 111–125. [Google Scholar]
- Cho, E.J.; Hwang, H.J.; Kim, S.W.; Oh, J.Y.; Baek, Y.M.; Choi, J.W.; Bae, S.H.; Yun, J.W. Hypoglycemic effects of exopolysaccharides produced by mycelial cultures of two different mushrooms Tremella Fuciformis and Phellinus Baumii in Ob/Ob mice. Appl. Microbiol. Biotechnol. 2007, 75, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Rushita, S.; Vijayakumar, M.; Noorlidah, A.; Abdulla, M.A.; Vikineswary, S. Effect of Pleurotus Citrinopileatus on blood glucose, insulin and catalase of streptozotocin-induced type 2 diabetes mellitus rats. J. Anim. Plant Sci. 2013, 23, 1566–1571. [Google Scholar]
- Ahmad, N.; Bansal, R.; Rastogi, A.K.; Kidwai, J.R. Effect of PHA-B fraction of Agaricus bisporus lectin on insulin release and 45Ca2+ uptake by islets of langerhans in vitro. Acta Diabetol. Lat. 1984, 21, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Ravi, B.; Renitta, R.E.; Prabha, M.L.; Issac, R.; Naidu, S. Evaluation of antidiabetic potential of Oyster mushroom (Pleurotus ostreatus) in alloxan-induced diabetic mice. Immunopharmacol. Immunotoxicol. 2013, 35, 101–109. [Google Scholar] [CrossRef]
- Sharma, A.K.; Jana, A.M.; Srivastav, A.; Gupta, M.; Sharma, S.; Gill, S.S. Antimicrobial properties of some edible mushrooms: A review. World J. Pharm. Pharm. Sci. 2014, 3, 1009–1023. [Google Scholar]
- Ali, N.A.; Mothana, R.A.A.; Lesnau, A.; Pilgrim, H.; Lindequist, U. Antiviral activity of Inonotus hispidus. Fitoterapia 2003, 74, 483–485. [Google Scholar]
- Chen, J.-T.; Huang, J.-W. Antimicrobial activity of edible mushroom culture filtrates on plant pathogens. Plant Pathol. Bull. 2010, 19, 261–270. [Google Scholar]
- Alves, M.J.; Ferreira, I.C.; Martins, A.; Pintado, M. Antimicrobial activity of wild mushroom extracts against clinical isolates resistant to different antibiotics. J. Appl. Microbiol. 2012, 113, 466–475. [Google Scholar] [CrossRef]
- Shen, H.-S.; Shao, S.; Chen, J.-C.; Zhou, T. Antimicrobials from mushrooms for assuring food safety. Compr. Rev. Food Sci. Food Saf. 2017, 16, 316–329. [Google Scholar] [CrossRef]
- Wang, H.X.; Liu, W.K.; Ng, T.B.; Ooi, V.E.C.; Chang, S.T. The immunomodulatory and antitumor activities of lectins from the mushroom Tricholoma mongolicum. Immunopharmacology 1996, 31, 205–211. [Google Scholar] [CrossRef]
- Guggenheim, A.G.; Wright, K.M.; Zwickey, H.L. Immune modulation from five major mushrooms: Application to integrative oncology. Integr. Med. Clin. J. 2014, 13, 32. [Google Scholar]
- Wu, D.; Pae, M.; Ren, Z.; Guo, Z.; Smith, D.; Meydani, S.N. Dietary supplementation with white button mushroom enhances natural killer cell activity in C57BL/6 mice. J. Nutr. 2007, 137, 1472–1477. [Google Scholar] [CrossRef]
- Arola, H.; Koivula, T.; Karvonen, A.-L.; Jokela, H.; Ahola, T.; Isokoski, M. Low trehalase activity is associated with abdominal symptoms caused by edible mushrooms. Scand. J. Gastroenterol. 1999, 34, 898–903. [Google Scholar] [PubMed]
- Petermann, I.; Triggs, C.M.; Huebner, C.; Han, D.Y.; Gearry, R.B.; Barclay, M.L.; Demmers, P.S.; McCulloch, A.; Ferguson, L.R. Mushroom intolerance: A novel diet–gene interaction in Crohn’s disease. Br. J. Nutr. 2009, 102, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Che, T.; Yin, Y.; Yu, G.; Yang, Q.; Liu, W.; Ye, X.; Yu, W.; Alok, S.; Chen, Y. lethal protein in mass consumption edible mushroom Agrocybe Aegerita linked to strong hepatic toxicity. Toxicon 2014, 90, 273–285. [Google Scholar] [CrossRef]
- Argyropoulos, D.; Heindl, A.; Müller, J. Assessment of convection, hot-air combined with microwave-vacuum and freeze-drying methods for mushrooms with regard to product quality. Int. J. Food Sci. Technol. 2011, 46, 333–342. [Google Scholar] [CrossRef]
- Kulshreshtha, M.; Singh, A.; Vipul, D. Effect of drying conditions on mushroom quality. J. Eng. Sci. Technol. 2009, 4, 90–98. [Google Scholar]
- Kumar, K.; Barmanray, A. Nutritional evaluation and storage studies of button mushroom powder fortified biscuits. Proteins 2007, 96, 325. [Google Scholar]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Kumar, K.; Barmanray, A.; Kumar, S. Shelf-life studies on osmo-air dried white button mushroom (Agaricus bisporus L.). Curr. Res. Nutr. Food Sci. J. 2017, 5, 144–153. [Google Scholar] [CrossRef]
- Lin, Q.; Lu, Y.; Zhang, J.; Liu, W.; Guan, W.; Wang, Z. Effects of high CO2 in-package treatment on flavor, quality and antioxidant activity of button mushroom (Agaricus bisporus) during postharvest storage. Postharvest Biol. Technol. 2017, 123, 112–118. [Google Scholar] [CrossRef]
- Farokhian, F.; Jafarpour, M.; Goli, M.; Askari-Khorasgani, O. Quality preservation of air-dried sliced button mushroom (Agaricus bisporus) by lavender (Lavendula Angustifolia Mill.) essential oil. J. Food Process Eng. 2017, 40, e12432. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, F.; Yang, H.; Ibrahim, S.A.; Wang, Y.; Huang, W. Effects of preservation methods on amino acids and 5′-nucleotides of Agaricus bisporus mushrooms. Food Chem. 2014, 149, 221–225. [Google Scholar] [CrossRef]
- Coşkuner, Y.; Özdemir, Y. Acid and EDTA blanching effects on the essential element content of mushrooms (Agaricus bisporus). J. Sci. Food Agric. 2000, 80, 2074–2076. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Mau, J.-L. Contents of sugars, free amino acids and free 5′-nucleotides in mushrooms, Agaricus bisporus, during post-harvest storage. J. Sci. Food Agric. 1999, 79, 1519–1523. [Google Scholar] [CrossRef]
- Fernandes, Â.; Barros, L.; Barreira, J.C.; Antonio, A.L.; Oliveira, M.B.P.; Martins, A.; Ferreira, I.C. Effects of different processing technologies on chemical and antioxidant parameters of Macrolepiota Procera wild mushroom. LWT-Food Sci. Technol. 2013, 54, 493–499. [Google Scholar] [CrossRef]
- Muyanja, C.; Kyambadde, D.; Namugumya, B. Effect of pretreatments and drying methods on chemical composition and sensory evaluation of Oyster mushroom (Pluerotus oestreatus) powder and soup. J. Food Process. Preserv. 2014, 38, 457–465. [Google Scholar] [CrossRef]
- Jaworska, G.; Bernaś, E.; Mickowska, B. Effect of production process on the amino acid content of frozen and canned Pleurotus ostreatus mushrooms. Food Chem. 2011, 125, 936–943. [Google Scholar] [CrossRef]
- Ziarati, P.; Rabizadeh, H. Safety and nutritional comparison of fresh, cooked and frozen mushroom (Agaricus bisporus). Intl. J. Farm. Allied Sci. 2013, 2, 1141–1147. [Google Scholar]
- Barros, L.; Baptista, P.; Correia, D.M.; Sá Morais, J.; Ferreira, I.C. Effects of conservation treatment and cooking on the chemical composition and antioxidant activity of Portuguese wild edible mushrooms. J. Agric. Food Chem. 2007, 55, 4781–4788. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, S.M.; Chun, J.; Lee, H.B.; Lee, J. Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shiitake (Lentinus edodes) mushroom. Food Chem. 2006, 99, 381–387. [Google Scholar] [CrossRef]
- Reid, T.; Munyanyi, M.; Mduluza, T. Effect of cooking and preservation on nutritional and phytochemical composition of the mushroom Amanita zambiana. Food Sci. Nutr. 2017, 5, 538–544. [Google Scholar] [CrossRef]
- Robertson, R.E.; Hoy, J. Food Irradiation: Available Research Indicates That Benefits Outweigh Risks; Report to Congressional Requesters; US General Accounting Office: Washington, DC, USA, 2000.
- Minnaar, A.; Taylor, J.R.N.; Dersley, N.N.; McGill, A.E.J. Technological feasibility of heat-irradiation combination treatments for low-acid food products. Radiat. Phys. Chem. 1996, 48, 371–372. [Google Scholar] [CrossRef]
- Kwon, J.-H.; Byun, M.-W.; Cho, H.-O. Browning end color characteristics in mushrooms (Agaricus bisporus) as influenced by ionizing energy. Korean J. Food Sci. Technol. 1990, 22, 509–513. [Google Scholar]
- Xiong, Q.; Xing, Z.; Feng, Z.; Tan, Q.; Bian, Y. Effect of 60Co γ-irradiation on postharvest quality and selected enzyme activities of Pleurotus nebrodensis. LWT-Food Sci. Technol. 2009, 42, 157–161. [Google Scholar] [CrossRef]
- Jasinghe, V.J.; Perera, C.O. Ultraviolet irradiation: The generator of vitamin D2 in edible mushrooms. Food Chem. 2006, 95, 638–643. [Google Scholar] [CrossRef]
- Huang, S.-J.; Lin, C.-P.; Tsai, S.-Y. Vitamin D2 content and antioxidant properties of fruit body and Mycelia of edible mushrooms by UV-B irradiation. J. Food Compos. Anal. 2015, 42, 38–45. [Google Scholar] [CrossRef]
- Fernandes, Â.; Barreira, J.C.; Antonio, A.L.; Oliveira, M.B.P.; Martins, A.; Ferreira, I.C. Effects of Gamma irradiation on chemical composition and antioxidant potential of processed samples of the wild mushroom Macrolepiota procera. Food Chem. 2014, 149, 91–98. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Yu, H.-T.; Kao, J.-P.; Yang, C.-C.; Chiang, S.-S.; Mishchuk, D.O.; Mau, J.-L.; Slupsky, C.M. Consumption of vitamin D2 enhanced mushrooms is associated with improved bone health. J. Nutr. Biochem. 2015, 26, 696–703. [Google Scholar] [CrossRef]
- Jiang, T.; Luo, S.; Chen, Q.; Shen, L.; Ying, T. Effect of integrated application of Gamma irradiation and modified atmosphere packaging on physicochemical and microbiological properties of Shiitake mushroom (Lentinus edodes). Food Chem. 2010, 122, 761–767. [Google Scholar] [CrossRef]
- Benoit, M.A.; D’Aprano, G.; Lacroix, M. Effect of γ-irradiation on phenylalanine ammonia-lyase activity, total phenolic content, and respiration of mushrooms (Agaricus bisporus). J. Agric. Food Chem. 2000, 48, 6312–6316. [Google Scholar] [CrossRef]
- Sommer, I. Effect of Gamma Irradiation on Selected Compounds of Fresh Mushrooms. Ph.D. Thesis, University of Wien, Wien, Austria, 2008. [Google Scholar]
- Kortei, N.K.; Odamtten, G.T.; Appiah, V.; Obodai, M.; Narh, D.L.; Akonor, P.T.; Wiafe-Kwagyan, M.; Akonor, M.A.; Adaboro, R.M. Preliminary shelf life studies of in-vitro antioxidant potential of Gamma irradiated dried mushrooms (Pleurotus ostreatus Ex. Fries) Kummer in Ghana. J. Pharm. Res. Int. 2016, 9, 1–13. [Google Scholar] [CrossRef]
- Krings, U.; Berger, R.G. Dynamics of sterols and fatty acids during UV-B treatment of Oyster mushroom. Food Chem. 2014, 149, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Dawoud, M.E.; Taleb, A.A. Evaluation of nutritional substrate and physical stress (Gamma irradiation) in β-glucan productivity by mushroom (Pleurotus ostreatus). Afr. J. Biotechnol. 2011, 10, 15578–15586. [Google Scholar] [CrossRef]
- Bhat, R.; Deshpande, R.; Ganachari, S.V.; Huh, D.S.; Venkataraman, A. Photo-irradiated biosynthesis of silver nanoparticles using edible mushroom Pleurotus Florida and their antibacterial activity studies. Bioinorg. Chem. Appl. 2011, 2011, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Sharanabasava, V.G.; Deshpande, R.; Shetti, U.; Sanjeev, G.; Venkataraman, A. Photo-bio-synthesis of irregular shaped functionalized gold nanoparticles using edible mushroom Pleurotus Florida and its anticancer evaluation. J. Photochem. Photobiol. B 2013, 125, 63–69. [Google Scholar] [CrossRef]
- Kumar, K.; Barmanray, A. Shelf-life studies on pickled button mushroom (Agaricus bisporus). Mushroom Res. 2008, 17, 25–30. [Google Scholar]
- Kumar, K. Studies on development and shelf life evaluation of soup powder prepared by incorporation of White Button mushroom (Agaricus bisporus L.). S. Asian J. Food Technol. Environ. 2015, 1, 219–224. [Google Scholar] [CrossRef]
- Singh, S.; Ghosh, S.; Patil, G.R. Development of a mushroom-whey soup powder. Int. J. Food Sci. Technol. 2003, 38, 217–224. [Google Scholar] [CrossRef]
- Krishan, K.; Ray, A.B. Nutritional evaluation and storage studies of Chutney prepared from White Button mushroom (Agaricus bisporus L.). Haryana J. Hortic. Sci. 2008, 37, 236–239. [Google Scholar]
- Okafor, J.N.C.; Okafor, G.I.; Ozumba, A.U.; Elemo, G.N. Quality characteristics of bread made from wheat and Nigerian Oyster mushroom (Pleurotus plumonarius) powder. Pak. J. Nutr. 2012, 11, 5–10. [Google Scholar] [CrossRef]
- Nurhanan, A.; Aishah, M. Effect of partial replacement of wheat flour with Oyster mushroom (Pleurotus Sajor-Caju) powder on nutritional composition and sensory properties of butter biscuit. Sains Malays. 2012, 41, 1565–1570. [Google Scholar]
- Kumar, K.; Ray, A.B. Development and shelf-life evaluation of tomato-mushroom mixed ketchup. J. Food Sci. Technol. 2016, 53, 2236–2243. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Ray, A. Effect of incorporation of mushroom pulp with tomato pulp on physico-chemical characteristics of mixed soup. Beverage Food World 2010, 37, 71–72. [Google Scholar]
- Wakchaure, G.C.; Shirur, M.; Manikandan, K.; Rana, L. Development and evaluation of Oyster Mushroom value added products. Mushroom Res. 2010, 19, 40–44. [Google Scholar]
- Farzana, T.; Mohajan, S. Effect of incorporation of soy flour to wheat flour on nutritional and sensory quality of biscuits fortified with mushroom. Food Sci. Nutr. 2015, 3, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Prodhan, U.K.; Linkon, K.M.M.R.; Al-Amin, M.F.; Alam, M.J. Development and quality evaluation of mushroom (Pleurotus Sajor-Caju) enriched biscuits. Emir. J. Food Agric. 2015, 542–547. [Google Scholar] [CrossRef]
- Singh, K.; Thakur, M. Formulation, organoleptic and nutritional evaluation of value added baked product incorporating Oyster mushrooms (Pleurotus ostearus) powder. Int. J. Food Sci. Nutr. 2016, 1, 16–20. [Google Scholar]
- Hong, G.-H.; Kim, Y.-S.; Song, G.-S. Effect of Oyster mushroom (Pleurotus ostreatus) powder on bread quality. Prev. Nutr. Food Sci. 2005, 10, 214–218. [Google Scholar] [CrossRef]
- Mahamud, M.M.; Shirshir, M.R.I.; Hasan, M.R. Fortification of wheat bread using mushroom powder. Bangladesh Res. Publ. J. 2012, 7, 60–68. [Google Scholar]
- Verma, A.; Singh, V. Formulation and quality evaluation of mushroom (Oyster mushroom) powder fortified potato pudding. Asian J. Dairy Food Res. 2017, 36, 72–75. [Google Scholar] [CrossRef][Green Version]
- Park, M.J.; Lee, J.S.; Lee, B.; Lee, J.S. Development of natural seasoning based on mushroom. J. East Asian Soc. Diet. Life 2001, 11, 196–203. [Google Scholar]
- Kumar, K.; Barmanray, A. Studies on drying characteristics of White Button mushroom dried by different drying techniques. Mushroom Res. 2007, 16, 37–40. [Google Scholar]
- Kulkarni, S.K.; Sakhale, B.K.; Pawar, V.D.; Miniyar, U.G.; Patil, B.M. Studies on sensory quality of cookies enriched with mushroom powder. Food Sci. Res. J. 2010, 1, 90–93. [Google Scholar]
- Kim, K.; Choi, B.; Lee, I.; Lee, H.; Kwon, S.; Oh, K.; Kim, A.Y. Bioproduction of mushroom mycelium of Agaricus bisporus by commercial submerged fermentation for the production of meat analogue. J. Sci. Food Agric. 2011, 91, 1561–1568. [Google Scholar] [CrossRef]
- Singh, J.; Sindhu, S.C.; Sindhu, A.; Yadav, A. Development and evaluation of value added biscuits from dehydrated Shiitake (Lentinus edodes) mushroom. Int. J. Curr. Res. 2016, 8, 27155–27159. [Google Scholar]
- Lin, L.-Y.; Tseng, Y.-H.; Li, R.-C.; Mau, J.-L. Quality of Shiitake stipe bread. J. Food Process. Preserv. 2008, 32, 1002–1015. [Google Scholar] [CrossRef]
- Kim, B.-R.; Joo, N.-M. Optimization of sweet rice muffin processing prepared with Oak mushroom (Lentinus edodes) powder. J. Korean Soc. Food Cult. 2012, 27, 202–210. [Google Scholar] [CrossRef]
- Yoo, S.-J.; Kim, S.-H.; Choi, H.-T.; Oh, H.-T.; Choi, H.-J.; Ham, S.-S. Antioxidative, antimutagenic and cytotoxic effects of natural seasoning using Lentinus edodes powder. J. Korean Soc. Food Sci. Nutr. 2007, 36, 515–520. [Google Scholar] [CrossRef]
- Han, C.-W.; Lee, M.-Y.; Seong, S.-K. Quality characteristics of the brown sauce prepared with Lentinus edodes and Agaricus bisporus. J. East Asian Soc. Diet. Life 2006, 16, 364–370. [Google Scholar]
- Chung, H.C.; Lee, J.T.; Kwon, O.J. Bread properties utilizing extracts of Ganoderma lucidum (GL). J. Korean Soc. Food Sci. Nutr. 2004, 33, 1201–1205. [Google Scholar]
- Leskosek-Cukalovic, I.; Despotovic, S.; Lakic, N.; Niksic, M.; Nedovic, V.; Tesevic, V. Ganoderma lucidum—Medical mushroom as a raw material for beer with enhanced functional properties. Food Res. Int. 2010, 43, 2262–2269. [Google Scholar] [CrossRef]
- Aishah, M.S.; Rosli, W.W. The effect of addition of Oyster mushroom (Pleurotus Sajor-Caju) on nutrient composition and sensory acceptation of selected wheat-and rice-based products. Int. Food Res. J. 2013, 20, 183. [Google Scholar]
- Jeong, C.H.; Shim, K.H. Quality characteristics of sponge cakes with addition of Pleurotus eryngii mushroom powders. J. Korean Soc. Food Sci. Nutr. 2004, 19, 254–260. [Google Scholar]

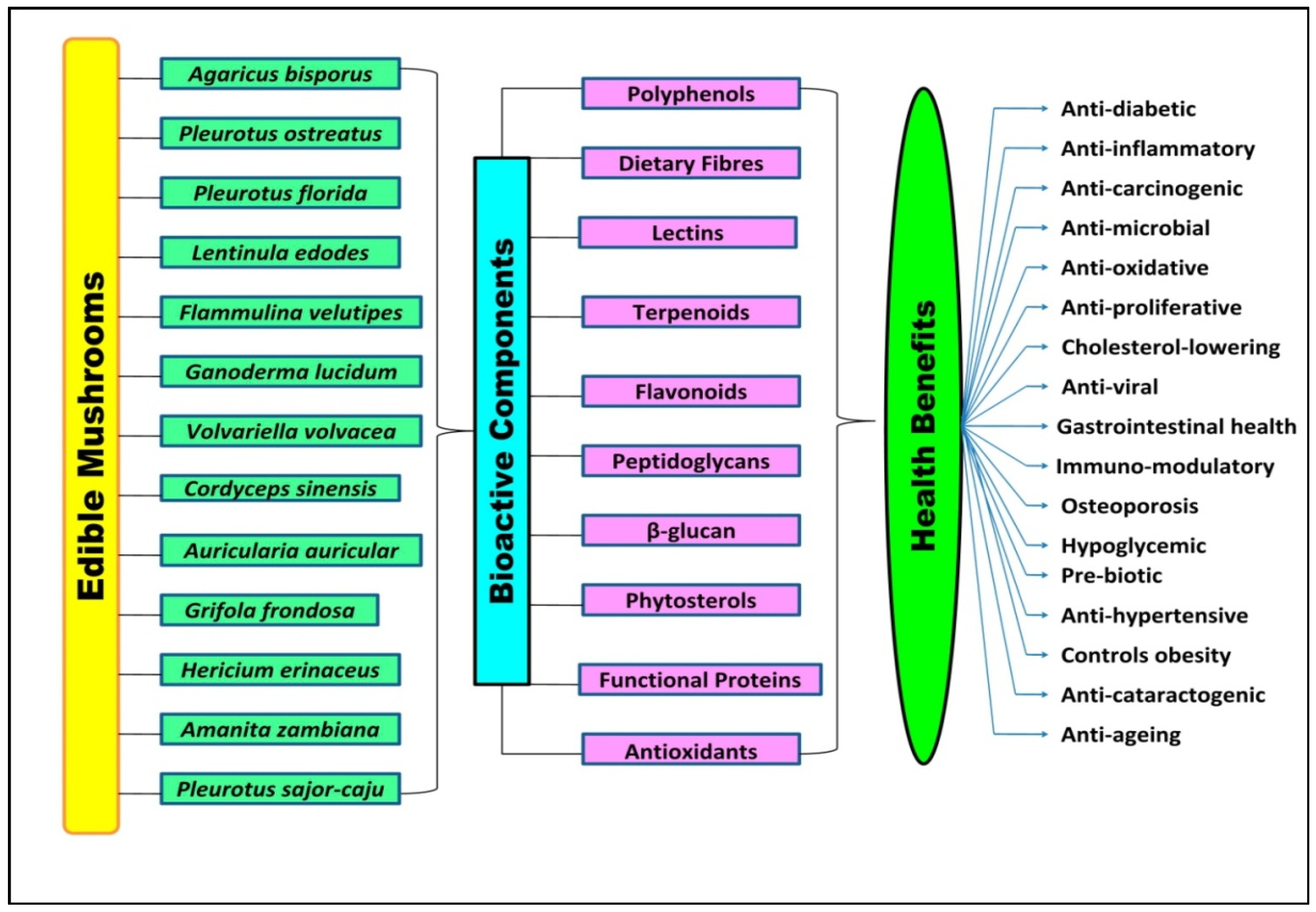
| Mushroom (Common Name) | Bioactive Compounds | Health Benefits | References |
|---|---|---|---|
| Agaricus bisporus (White mushroom) | Pyrogallol, hydroxybenzoic acid derivatives, flavonoids, lectins | Anti-inflammatory, enhanced insulin secretion, anti-ageing property | [14,15,16] |
| Auricularia auricular (Jew’s ear mushroom) | Glucan, acidic polysaccharides | Immunomodulatory, anti-tumour, anti-inflammatory, lowers cholesterol and triglycerides, hypoglycaemic activity, immune tonic, and beneficial in coronary heart disease | [16,17] |
| Flammulina velutipes (Golden needle mushroom) | Peptidoglycan, polysaccharides, flammulin, FVP (flammulina polysaccharide-protein), proflamin (glycoprotein), a prolamin (active sugar protein) | Anti-inflammatory, antiviral, anti-tumour, antioxidant, activity, immuno-modulatory, anti-ageing property, anti-viral action | [16,18,19,20,21] |
| Ganoderma lucidum (Reishi, lingzhi) | Ganoderic acids, ganodermanontriol, ganoderiol, polysaccharides, germanium, triterpenoids, nucleotides and nucleosides, β-glucan | Anti-metastatic, anti-tumour, anti-viral, anti-HIV, immunomodulatory, antibiotic properties, liver protection, prevents cholesterol synthesis | [22,23] |
| Lentinula edodes (Shiitake) | Lentinan, glucan, mannoglucan, fucomannogalactan, lentin (protein), catechinflavonoids, eritadenine | Immunomodulatory, anti-tumour, anti-inflammatory, anti-fungal, antioxidant, anti-bacterial, antifungal, antioxidant, hypolipidemic activity | [24,25,26,27,28] |
| Cordyceps sinensis (Caterpillar fungus) | Cordycepin | treat lung infection, hypo-glycemic activity, cellular health properties, antidepressant activity | [16] |
| Pleurotus florida (White oyster) | β-glucans | Antioxidant, anti-microbial | [29,30] |
| Pleurotus ostreatus (Oyster mushroom) | Functional proteins (ubiquinone-9, ubiquitin-like peptide, nebrodeolysin, and glycoprotein), proteoglycans pleuran (β-1, 3-glucan with galactose, and mannose), glucans, proteoglycan, laccase, pleurostrin (peptide) | Immunomodulatory, hyperglycemia, anti-tumour, antioxidant, anti-viral, anti-fungal | [31,32,33,34,35] |
| Grifola frondosa (Ram’s head) | Lectins, polysaccharides | Decrease blood glucose improves insulin secretion and ovulation | [16] |
| Pleurotus pulmonarius (Lung oyster mushroom) | Polysaccharides such as β (1,3)-glucopyranosyl, and Polysaccharides (1,3), (1,6)-linked β-glucan | Anti-inflammatory | [36,37] |
| Volvariella volvacea (Paddy straw mushroom) | Fip-vvo | Immunomodulatory | [38] |
| Hericiumerinaceus (Monkey head mushroom) | Hericenones and erinacines | Neuritogenic effects | [16] |
| Mushrooms | Methods of Processing/Storage | Effect on Nutritional Composition | References |
|---|---|---|---|
| Agaricus bisporus | Freezing at −25 °C, canning and salting for 6 months | The protein content was reduced to 24.3 percent, 22.2 percent, 16.54 percent, in canning, freezing, and salting respectively; decrease in free amino acids (cysteine, tyrosine, glutamine, alanine) in all treatments. | [135] |
| Blanching at 95–100 °C for 15 min | Decreased levels of minerals | [136] | |
| Stored at 12 °C for 12 days | The decrease in sugar content, fructose, and mannitol; increase in free amino acids from 77.92 to 140.57 g/kg | [137] | |
| Macrolepiota procera | Freezing, drying, and gamma irradiation | Higher DPPH scavenging activity was reported in dried samples while freeze and irradiated samples showed higher reducing power | [138] |
| P. ostreatus | Oven-dried at 60 °C till a constant weight obtained, Blanching at 88 °C for 1 min, Brining (25% salt solution) for 30–60 min | The protein content decreased and carbohydrates get enhanced during oven drying. It was also observed that protein, fat, and carbohydrate contents get reduced during blanching and brining | [139] |
| Freezer storage for 12 months | The decrease in some amino acids such as alanine, glycine, histidine, threonine serine, and methionine) | [140] | |
| Microwave processing and frying | Reduction in the amount of Fe, Zn, Mn, Ca, and Cu during microwave processing and increase in Iron content during frying | [141] | |
| Macrolepiota mastoidea, Lactarius deliciosus, Sarcodon imbricatus, and Macrolepiota procera | Drying, freezing, and cooking | Antioxidant activities and nutrient concentrations of cooked samples was lower than either of dried or frozen mushroom samples | [142] |
| Lentinus edodes | Heat treatment | There was a significant increase in DPPH and ABTS radical scavenging activities by 2.2-fold and 2.0-fold, respectively as compared to the raw sample | [143] |
| Amanita zambiana | Frying, microwave heating, boiling, drying | Frying increased proteins, lipids, and carbohydrates, microwave heating increased the proteins and carbohydrates content while boiling only increased the carbohydrate content and decreased the phenolic contents, drying increased the proteins, carbohydrates, and total phenolic components | [144] |
| Lentinula edodes, Agaricus bisporus, Pleurotus eryngii, and Pleurotus ostreatus | Boiling, microwaving, grilling, and deep-frying | Significant loss of ash, carbohydrates and protein contents, during frying but increase in energy as well as fat contents. Further, boiling enhanced the total glucan contents decreased the antioxidant activity significant especially after frying and, boiling as compared to microwaved and grilled mushrooms | [4] |
| Edible Fungi | Treatment Conditions | Major Findings | References |
|---|---|---|---|
| Fresh shiitake mushrooms, oyster mushroom, button mushroom, and abalone mushroom | Ultra Violet-A (wavelength 315 to 400 nm) Ultra Violet-B (wavelength 290 to 315 nm) Ultra Violet-C (wavelength 190 to 290 nm) for 1 h | Increased amounts of vitamin D2 content | [149] |
| Six species from genus Agaricus, Auricularia, Agrocybe, Lentinula, Hypsizigus, and Pholiota, and five species from Pleurotus genus | Ultra Violet-B for 2 h | Increase in Vitamin D2 content and antioxidant activity | [150] |
| Macrolepiota prolera | γ-Irradiation (0.5 and 1 kGy) | The freezing and over-drying were attenuated by irradiation treatment | [151] |
| Pleurotus ferulae | Pulsed irradiation (19 to 700 nm; 60 pulses) | Increase in vitamin D2 and bone density of PM mice with increased osteoblast and lower osteoclast cells | [152] |
| Lentinula edodes | γ-Irradiation (1 kGy) | Increase in phenolic compounds and antioxidant activity of mushroom | [153] |
| Agaricus bisporus | γ-irradiation | Increased total phenolic components and phenylalanine ammonia-lyase activity | [154] |
| γ-Irradiation (1, 3, and 5 kGy) | Irradiation significantly reduced the concentration of guanosine 5′-diphosphate (22%) and adenosine 5′-monophosphate (AMP) (46%). | [155] | |
| Pleurotus ostreatus | 60Co γ-Irradiation | Irradiation treatment increased phenolic content, flavonoids, and antioxidant activity of dried mushroom | [156] |
| Mushrooms were illuminated with UV-B with a light intensity of 310–320 nm and 11.5 W/m2 for 60 min at 20 °C | The accumulation of vitamin D2 > 100 μg. The concentration of Photo-products such as lumisterol, tachysterol, and pre-vitamin D2 increased concurrently. | [157] | |
| γ-Irradiation | The increased antioxidant potential, hygienic quality and extended shelf-life | [156] | |
| γ-Irradiation | Irradiation with 1 to 6 kGy as physical stress factors increased protein, carbohydrates, and glucans | [158] | |
| UV-B radiation | Increase in vitamin D2 content in irradiated mycelia of golden and pink oyster mushrooms as 0.28–5.93 and 66.03–81.71 μg/g, respectively. | [150] | |
| Pleurotus florida | Photo-irradiation | Extracellular synthesis of silver nanoparticles from aqueous extract of the mushroom | [159] |
| Synthesis of biofunctionalized gold nanoparticles | [160] |
| Mushroom | Products | References |
|---|---|---|
| Pleurotus ostreatus | Value-added products (biscuits, soups, pickles, jam, snacks) | [169] |
| Butter biscuits, biscuits | [166,170,171] | |
| Cake | [172] | |
| Bread | [165,173,174] | |
| Potato Puddings | [175] | |
| Seasoning | [176] | |
| Agaricus bisporus | Soup powder | [162,163] |
| Drying | [128,177] | |
| Pickle | [161] | |
| Chutney | [164] | |
| Biscuits | [130,178] | |
| Ketchup | [167] | |
| Meat analogue | [179] | |
| Llentinus edodes | Biscuits | [180] |
| Bread | [181] | |
| Muffin | [182] | |
| Seasoning | [183] | |
| Brown sauce | [184] | |
| Ganoderma lucidum | Functional bread | [185] |
| Drink (Beer, Yakju) | [186] | |
| Pleurotus plumonarius | Bread | [165] |
| Pleurotus sajor-caju | Flat bread, rice-porridge and conventional cake | [187] |
| Biscuits | [171] | |
| Pleurotus eryngii | Sponge cake | [188] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, K.; Mehra, R.; Guiné, R.P.F.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible Mushrooms: A Comprehensive Review on Bioactive Compounds with Health Benefits and Processing Aspects. Foods 2021, 10, 2996. https://doi.org/10.3390/foods10122996
Kumar K, Mehra R, Guiné RPF, Lima MJ, Kumar N, Kaushik R, Ahmed N, Yadav AN, Kumar H. Edible Mushrooms: A Comprehensive Review on Bioactive Compounds with Health Benefits and Processing Aspects. Foods. 2021; 10(12):2996. https://doi.org/10.3390/foods10122996
Chicago/Turabian StyleKumar, Krishan, Rahul Mehra, Raquel P. F. Guiné, Maria João Lima, Naveen Kumar, Ravinder Kaushik, Naseer Ahmed, Ajar Nath Yadav, and Harish Kumar. 2021. "Edible Mushrooms: A Comprehensive Review on Bioactive Compounds with Health Benefits and Processing Aspects" Foods 10, no. 12: 2996. https://doi.org/10.3390/foods10122996
APA StyleKumar, K., Mehra, R., Guiné, R. P. F., Lima, M. J., Kumar, N., Kaushik, R., Ahmed, N., Yadav, A. N., & Kumar, H. (2021). Edible Mushrooms: A Comprehensive Review on Bioactive Compounds with Health Benefits and Processing Aspects. Foods, 10(12), 2996. https://doi.org/10.3390/foods10122996










