Untargeted Metabolomics on Skin Mucus Extract of Channa argus against Staphylococcus aureus: Antimicrobial Activity and Mechanism
Abstract
:1. Introduction
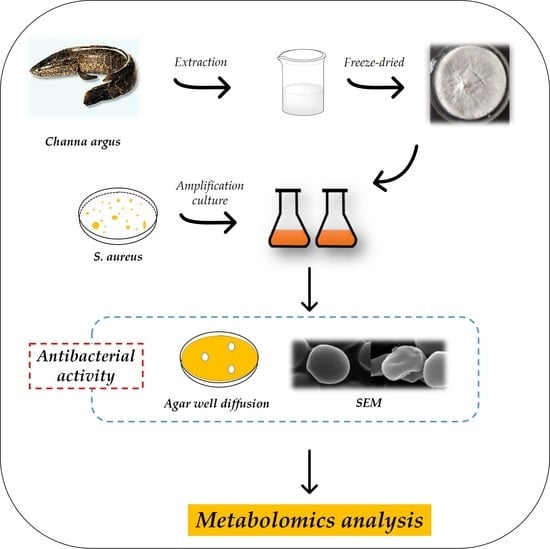
2. Materials and Methods
2.1. Reagents and Bacterial Strains
2.2. Skin Mucus Collection
2.3. Antimicrobial Activity
2.3.1. Agar Well Diffusion Method
2.3.2. Growth Curve Assay
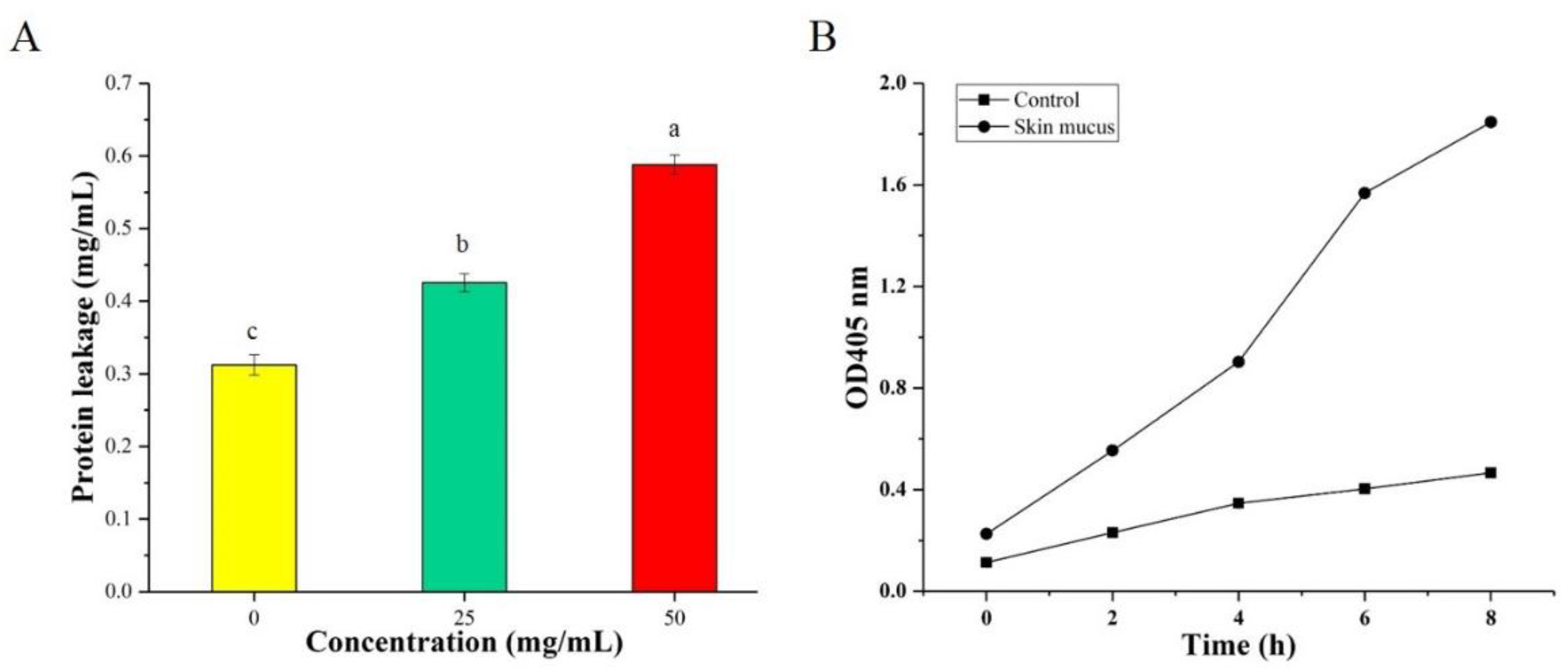
2.4. Determination of Leakage of Intracellular Proteins
2.5. Determination of Cell Membrane Damage
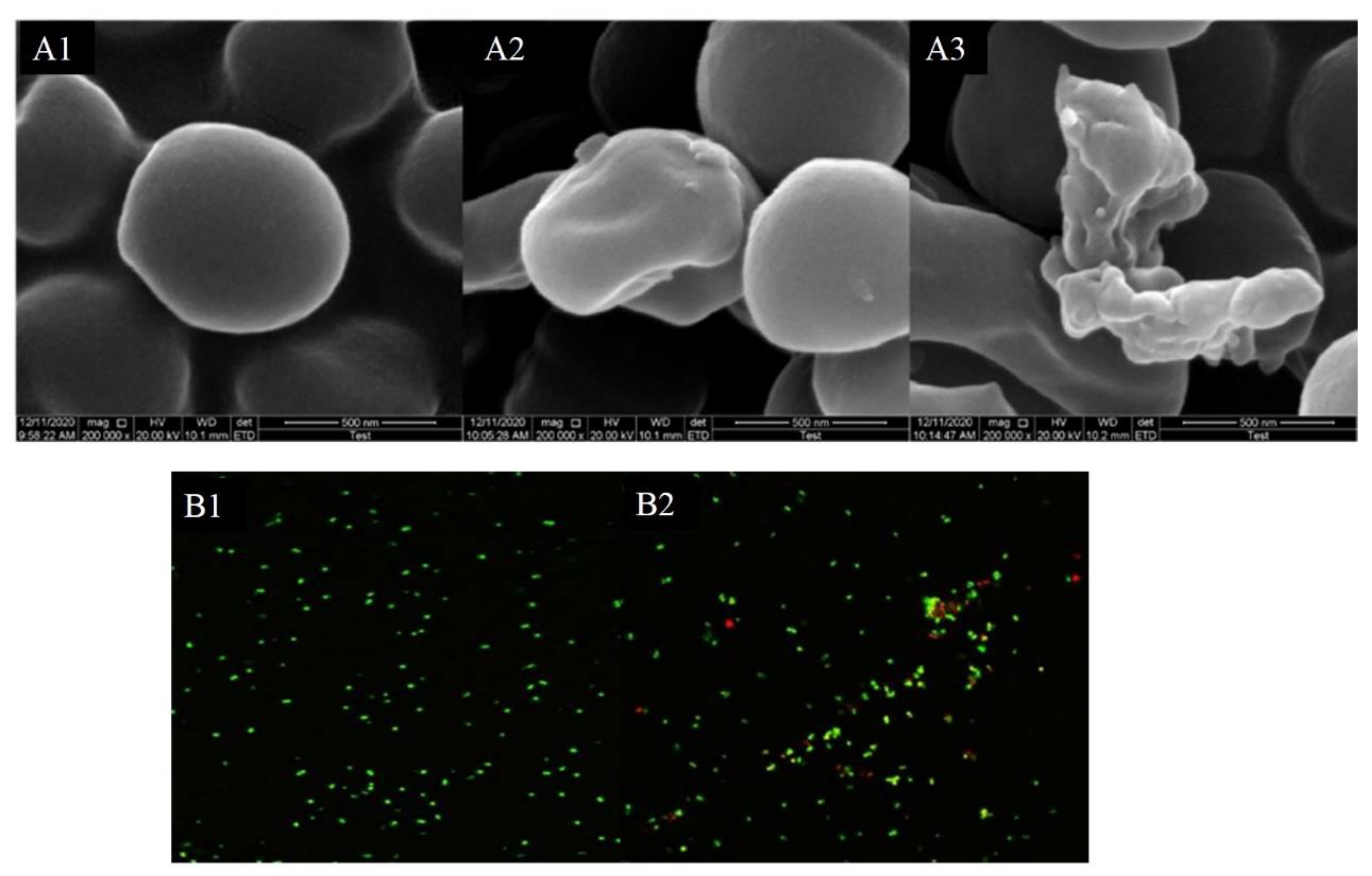
2.6. Scanning Electron Microscopy (SEM)
2.7. Laser Scanning Confocal Microscopy (CLSM)
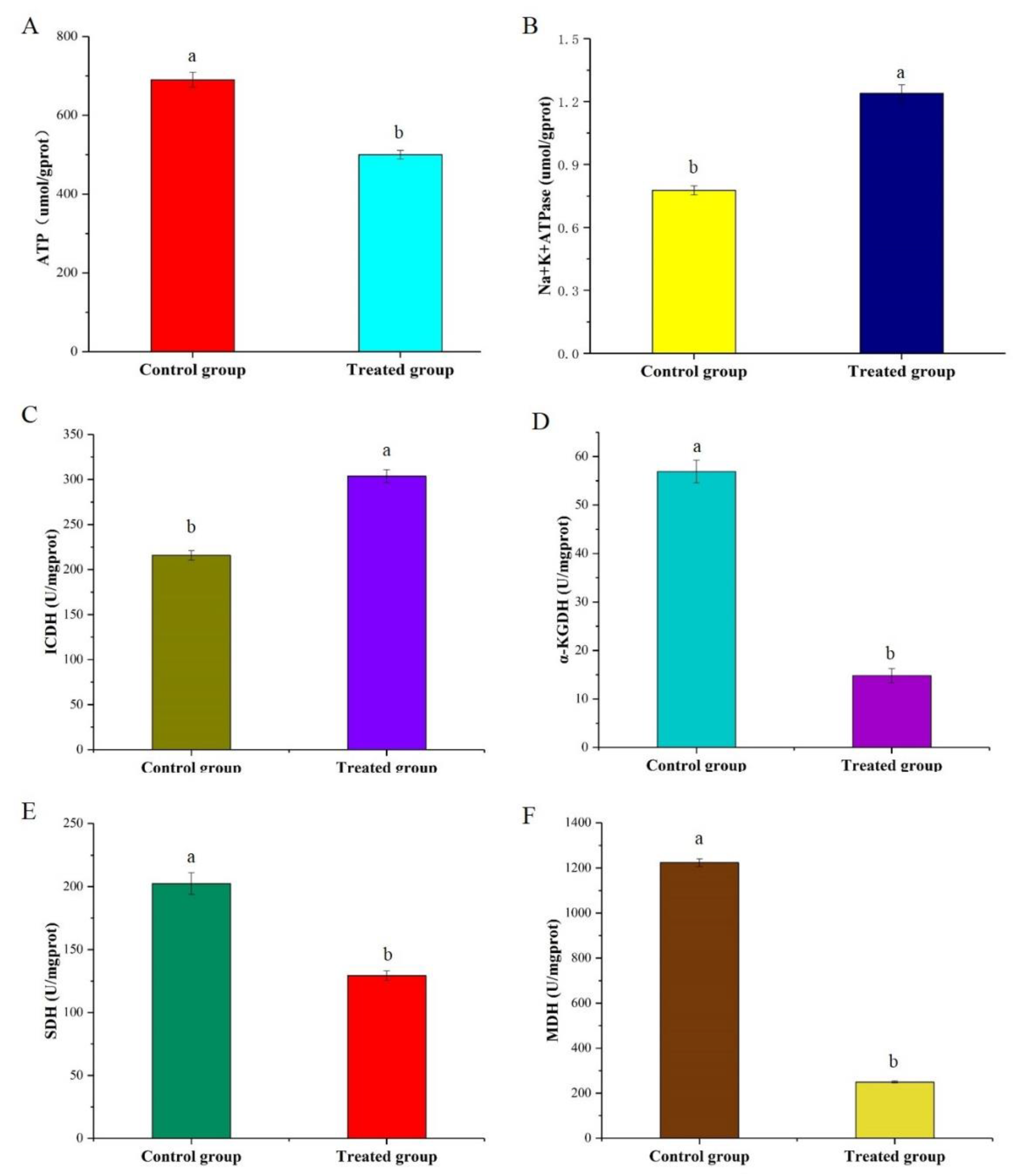
2.8. Effects of Skin Mucus on ATP Production and Na+/K+-ATPase Activity
2.9. Activities of Key Enzymes in the TCA Cycle
2.10. Sample Preparation for UHPLC–MS/MS
2.11. Untargeted LC–MS Method for Metabolomics Detection
2.12. Data Analysis
2.12.1. Statistical Analysis
2.12.2. Metabolomics Data Analysis
3. Results and Discussion
3.1. Antimicrobial Activity
3.2. Determination of Intracellular Protein Leakage
3.3. Determination of Cell Membrane Damage
3.4. Morphology of Bacteria
3.5. Laser Scanning Confocal Microscopy
3.6. Determination of ATP Production and Na+/K+-ATPase Activity
3.7. Changes in the Enzyme Activities and Metabolites Involved in the TCA Cycle
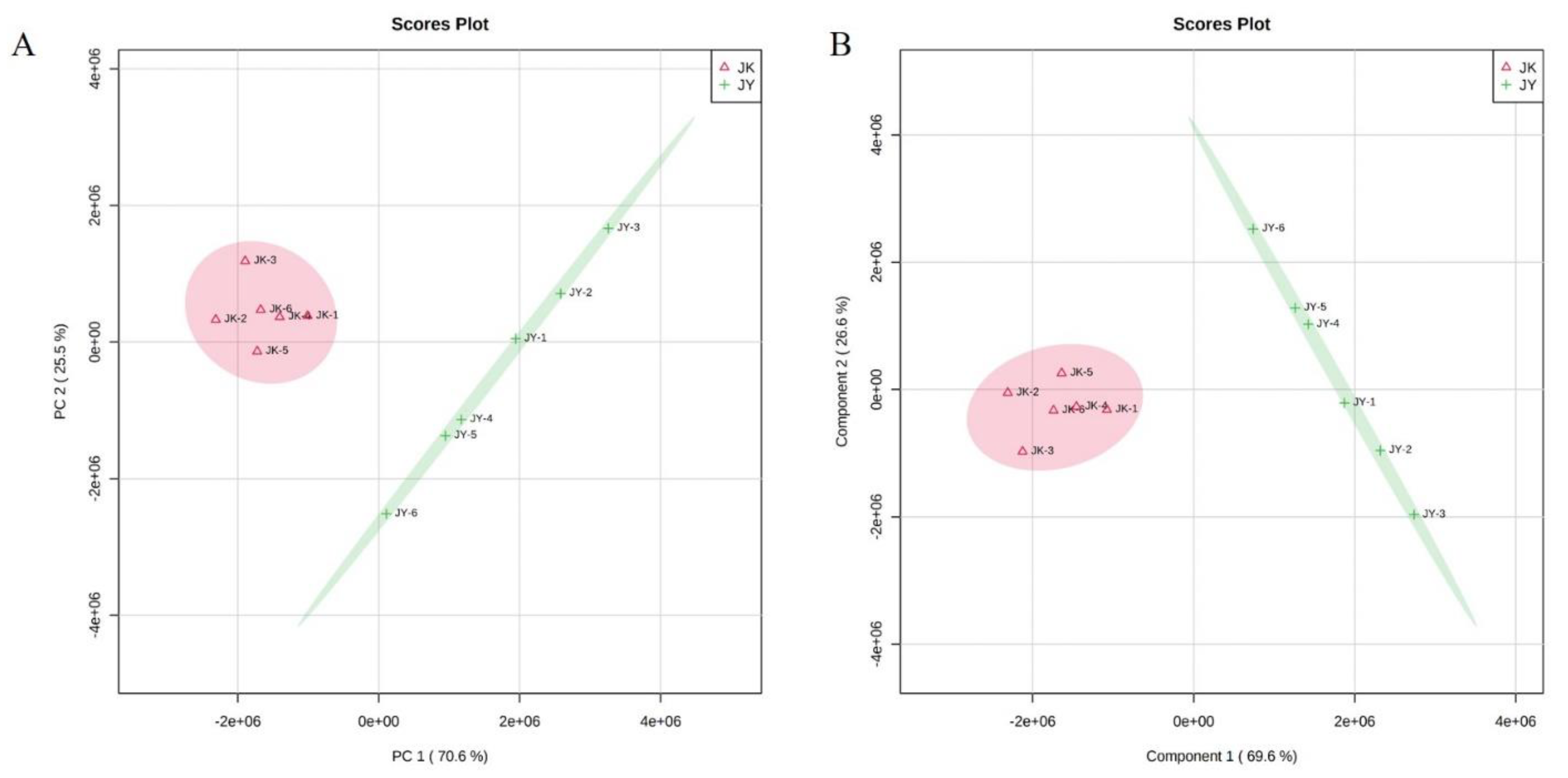
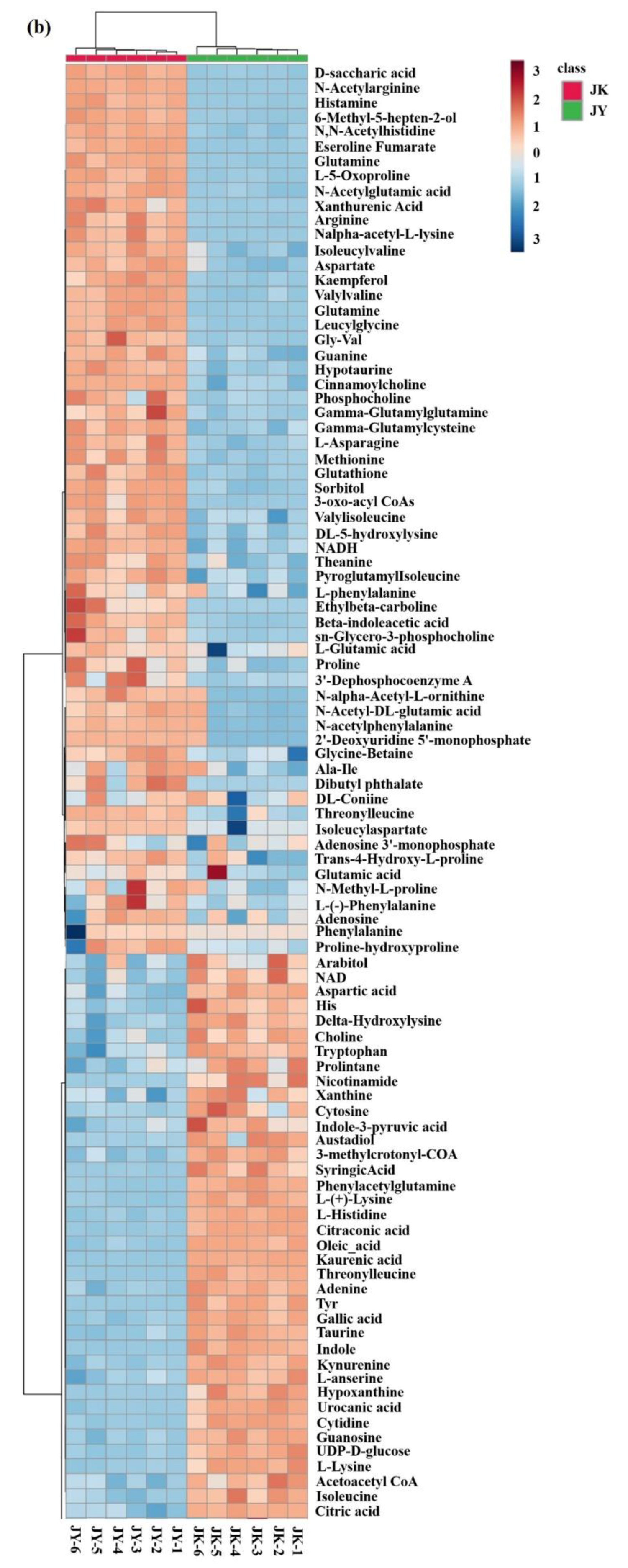
3.8. Untargeted Metabolomics Analysis
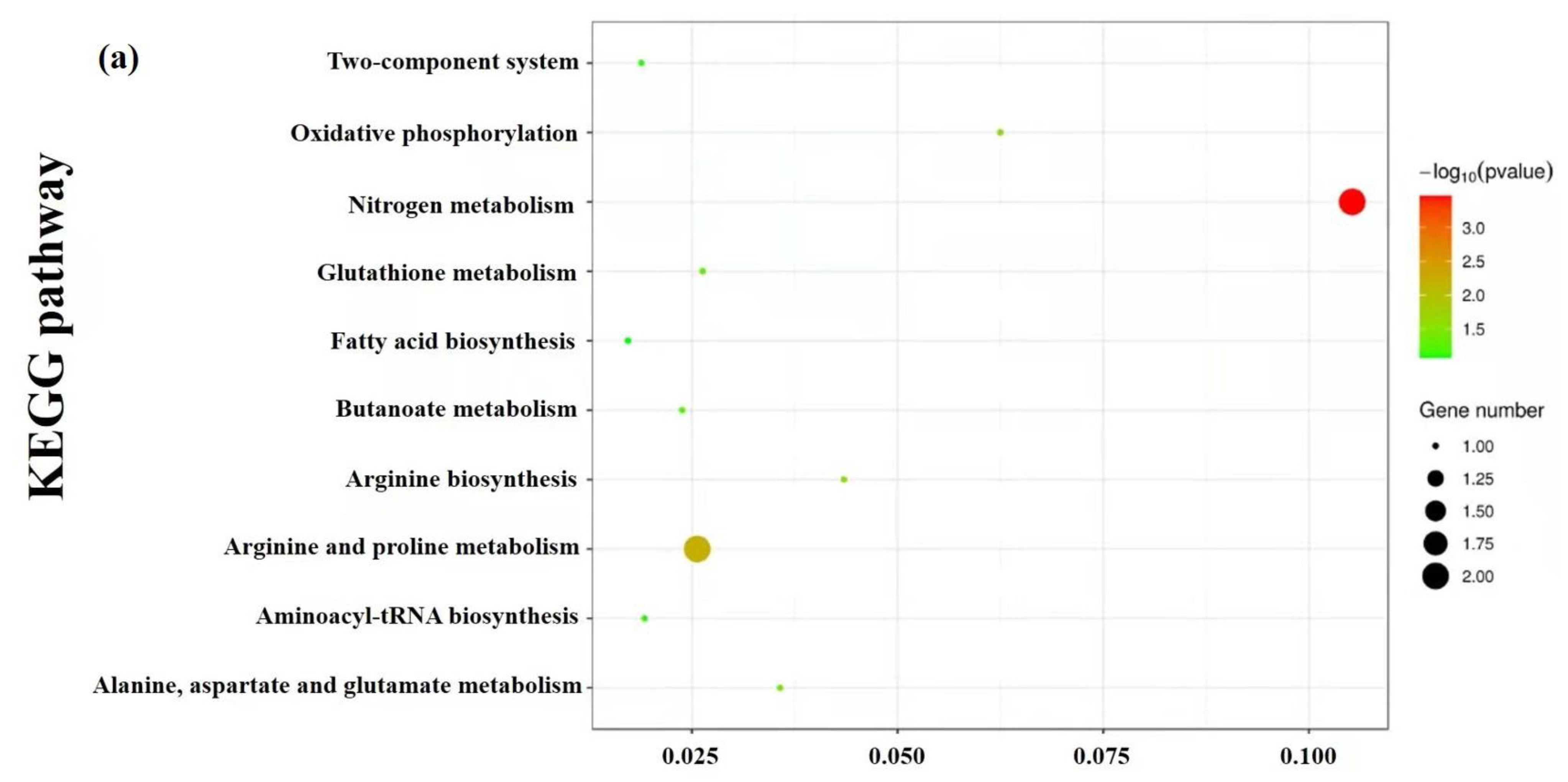
3.9. Enrichment and Metabolic Pathway Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kloos, W.E. Natural Populations of the Genus Staphylococcus. Annu. Rev. Microbiol. 1980, 34, 559–592. [Google Scholar] [CrossRef]
- Kim, M.W.; Greenfield, B.K.; Snyder, R.E.; Steinmaus, C.M.; Riley, L.W. The association between community-associated Staphylococcus aureus colonization and disease: A meta-analysis. BMC Infect. Dis. 2018, 18, 86. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, X.; Li, X.; Liu, Y.; Yu, H.; Liu, J.; Zhang, Q. Antibacterial functions of a novel fish-egg lectin from spotted knifejaw (Oplegnathus punctatus) during host defense immune responses. Dev. Comp. Immunol. 2020, 111, 103758. [Google Scholar] [CrossRef]
- Fowler, V.G.; Miro, J.M.; Hoen, B.; Cabell, C.H.; Abrutyn, E.; Rubinstein, E.; Corey, G.R.; Spelman, D.; Bradley, S.F.; Barsic, B.; et al. Investigators, Staphylococcus aureus endocarditis—A consequence of medical progress. JAMA J. Am. Med. Assoc. 2005, 293, 3012–3021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kranjec, C.; Ovchinnikov, K.V.; Gronseth, T.; Ebineshan, K.; Srikantam, A.; Diep, D.B. A bacteriocin-based antimicrobial formulation to effectively disrupt the cell viability of methicillin-resistant Staphylococcus aureus (MRSA) biofilms. NPJ Biofilms Microbiomes 2020, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holley, R.A.; Patel, D. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 2005, 22, 273–292. [Google Scholar] [CrossRef]
- Lee, K.A.; Moon, S.-H.; Lee, J.-Y.; Kim, K.-T.; Park, Y.-S.; Paik, H.-D. Antibacterial Activity of a Novel Flavonoid, 7-O-Butyl Naringenin, against Methicillin-Resistant Staphylococcus aureus (MRSA). Food Sci. Biotechnol. 2013, 22, 1725–1728. [Google Scholar] [CrossRef]
- Bo, J.; Yang, Y.; Zheng, R.; Fang, C.; Jiang, Y.; Liu, J.; Chen, M.; Hong, F.; Baile, C.; Segner, H.; et al. Antimicrobial activity and mechanisms of multiple antimicrobial peptides isolated from rockfish Sebastiscus marmoratus. Fish Shellfish Immunol. 2019, 93, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Benhamed, S.; Guardiola, F.A.; Mars, M.; Esteban, M.A. Pathogen bacteria adhesion to skin mucus of fishes. Vet. Microbiol. 2014, 171, 1–12. [Google Scholar] [CrossRef]
- Schelli, K.; Zhong, F.; Zhu, J. Comparative metabolomics revealing Staphylococcus aureus metabolic response to different antibiotics. Microb. Biotechnol. 2017, 10, 1764–1774. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shao, X.; Li, Y.; Wei, Y.; Xu, F.; Wang, H. Metabolomic Analysis and Mode of Action of Metabolites of Tea Tree Oil Involved in the Suppression of Botrytis cinerea. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Feng, M.; Yang, K.; Cao, Y.; Zhang, J.; Xu, J.; Hernandez, S.H.; Wei, X.; Fan, M. Transcriptomic and metabolomic analyses reveal antibacterial mechanism of astringent persimmon tannin against Methicillin-resistant Staphylococcus aureus isolated from pork. Food Chem. 2020, 309, 125692. [Google Scholar] [CrossRef] [PubMed]
- Go, H.-J.; Kim, C.-H.; Park, J.B.; Kim, T.Y.; Lee, T.K.; Oh, H.Y.; Park, N.G. Biochemical and molecular identification of a novel hepcidin type 2-like antimicrobial peptide in the skin mucus of the pufferfish Takifugu pardalis. Fish Shellfish Immunol. 2019, 93, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Phillips, C.A. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J. Appl. Microbiol. 2006, 101, 1232–1240. [Google Scholar] [CrossRef]
- Tang, C.; Chen, J.; Zhang, L.; Zhang, R.; Zhang, S.; Ye, S.; Zhao, Z.; Yang, D. Exploring the antibacterial mechanism of essential oils by membrane permeability, apoptosis and biofilm formation combination with proteomics analysis against methicillin-resistant Staphylococcus aureus. Int. J. Med. Microbiol. 2020, 310, 151435. [Google Scholar] [CrossRef]
- Chowdhury, T.; Mandal, S.M.; Kumari, R.; Ghosh, A.K. Purification and characterization of a novel antimicrobial peptide (QAK) from the hemolymph of Antheraea mylitta. Biochem. Biophys. Res. Commun. 2020, 527, 411–417. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Grare, M.; Fontanay, S.; Dibama, H.M.; Mourer, M.; Regnouf-de-Vains, J.B.; Finance, C.; Duval, R.E. Time-kill curves and evolution of membrane Permeability after para-guanidinoethylcalix 4 arene exposure. Pathol. Biol. 2010, 58, 46–51. [Google Scholar] [CrossRef]
- Jameel, F.; Agarwal, J.; Waseem, M.; Serajuddin, M. Antibacterial activity of epidermal mucus extracts of three freshwater air-breathing fish species against human pathogenic bacteria. Indian J. Fish. 2019, 66, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef]
- Kang, S.; Kong, F.; Shi, X.; Han, H.; Li, M.; Guan, B.; Yang, M.; Cao, X.; Tao, D.; Zheng, Y.; et al. Antibacterial activity and mechanism of lactobionic acid against Pseudomonas fluorescens and Methicillin-resistant Staphylococcus aureus and its application on whole milk. Food Control. 2020, 108, 106876. [Google Scholar] [CrossRef]
- Ma, M.; Zhao, J.; Zeng, Z.; Wan, D.; Yu, P.; Cheng, D.; Gong, D.; Deng, S. Antibacterial activity and membrane-disrupting mechanism of monocaprin against Escherichia coli and its application in apple and carrot juices. LWT Food Sci. Technol. 2020, 131. [Google Scholar] [CrossRef]
- Miao, J.; Zhou, J.; Liu, G.; Chen, F.; Chen, Y.; Gao, X.; Dixon, W.; Song, M.; Xiao, H.; Cao, Y. Membrane disruption and DNA binding of Staphylococcus aureus cell induced by a novel antimicrobial peptide produced by Lactobacillus paracasei subsp tolerans FX-6. Food Control 2016, 59, 609–613. [Google Scholar] [CrossRef]
- Panda, S.; Rout, T.K.; Prusty, A.D.; Ajayan, P.M.; Nayak, S. Electron Transfer Directed Antibacterial Properties of Graphene Oxide on Metals. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.C.P.; Sousa, R.C.S.; Otoni, C.G.; Moraes, A.R.F.; Souza, V.G.L.; Medeiros, E.A.A.; Espitia, P.J.P.; Pires, A.C.S.; Coimbra, J.S.R.; Soares, N.F.F. Nisin and other antimicrobial peptides: Production, mechanisms of action, and application in active food packaging. Innovative Food Sci. Emerg. Technol. 2018, 48, 179–194. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, L.; Wu, J.e.; He, Y.; Yang, H. Elucidating antimicrobial mechanism of nisin and grape seed extract against Listeria monocytogenes in broth and on shrimp through NMR-based metabolomics approach. Int. J. Food Microbiol. 2020, 319, 108494. [Google Scholar] [CrossRef]
- Mousavi, F.; Bojko, B.; Bessonneau, V.; Pawliszyn, J. Cinnamaldehyde Characterization as an Antibacterial Agent toward E. coli Metabolic Profile Using 96-Blade Solid-Phase Microextraction Coupled to Liquid Chromatography-Mass Spectrometry. J. Proteome Res. 2016, 15, 963–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turgis, M.; Han, J.; Caillet, S.; Lacroix, M. Antimicrobial activity of mustard essential oil against Escherichia coli O157:H7 and Salmonella typhi. Food Control 2009, 20, 1073–1079. [Google Scholar] [CrossRef]
- Shu, H.; Zhang, W.; Yun, Y.; Chen, W.; Zhong, Q.; Hu, Y.; Chen, H.; Chen, W. Metabolomics study on revealing the inhibition and metabolic dysregulation in Pseudomonas fluorescens induced by 3-carene. Food Chem. 2020, 329, 127220. [Google Scholar] [CrossRef]
- Corbet, C.; Feron, O. Cancer cell metabolism and mitochondria: Nutrient plasticity for TCA cycle fueling. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.; Rabinowitz, J.D. An unexpected trigger for calorie burning. Nature 2018, 560, 38–39. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Lin, J.; Gao, N.; Sun, X.; Meng, X.; Liu, R.; Liu, Y.; Wang, W.; Li, B.; Wang, Y. Blueberry malvidin-3-galactoside modulated gut microbial dysbiosis and microbial TCA cycle KEGG pathway disrupted in a liver cancer model induced by HepG2 cells. Food Sci. Hum. Wellness 2020, 9, 245–255. [Google Scholar] [CrossRef]
- Kubota, S.; Takeo, I.; Kume, K.; Kanai, M.; Shitamukai, A.; Mizunuma, M.; Miyakawa, T.; Shimoi, H.; Iefuji, H.; Hirata, D. Effect of ethanol on cell growth of budding yeast: Genes that are important for cell growth in the presence of ethanol. Biosci. Biotechnol. Biochem. 2004, 68, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.-Z.; Wang, X.; Yang, Y.; Yuan, Y.-J. Metabolomic Study of Interactive Effects of Phenol, Furfural, and Acetic Acid on Saccharomyces cerevisiae. OMICS J. Integr. Biol. 2011, 15, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ma, M.-L.; Luo, S.; Zhang, R.-M.; Han, P.; Hu, W. Metabolic responses to ethanol in Saccharomyces cerevisiae using a gas chromatography tandem mass spectrometry-based metabolomics approach. Int. J. Biochem. Cell Biol. 2012, 44, 1087–1096. [Google Scholar] [CrossRef]
- He, F.; Li, K.; Zhang, X.; Yang, Y.; Fang, Y.; Xiang, F. Components and antibacterial activity of a novel essential oil from the nutrient broth of Eremothecium ashbyii H4565. LWT Food Sci. Technol. 2019, 101, 389–394. [Google Scholar] [CrossRef]
- Dwyer, D.J.; Belenky, P.A.; Yang, J.H.; Macdonald, I.C.; Martell, J.D.; Takahashi, N.; Chan, C.T.Y.; Lobritz, M.A.; Braff, D.; Schwarz, E.G. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc. Natl. Acad. Sci. USA 2014, 111, 2100–2109. [Google Scholar] [CrossRef] [Green Version]
- Han, P.-P.; Yuan, Y.-J. Metabolic Profiling as a Tool for Understanding Defense Response of Taxus Cuspidata Cells to Shear Stress. Biotechnol. Progr. 2009, 25, 1244–1253. [Google Scholar] [CrossRef]
- Szeto, S.S.W.; Reinke, S.N.; Sykes, B.D.; Lemire, B.D. Mutations in the Saccharomyces cerevisiae Succinate Dehydrogenase Result in Distinct Metabolic Phenotypes Revealed through H-1 NMR-Based Metabolic Footprinting. J. Proteome Res. 2010, 9, 6729–6739. [Google Scholar] [CrossRef]
- Xu, Y.; Labedan, B.; Glansdorff, N. Surprising arginine biosynthesis: A reappraisal of the enzymology and evolution of the pathway in microorganisms. Microbiol. Mol. Biol. Rev. 2007, 71, 36–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petri, K.; Walter, F.; Persicke, M.; Rueckert, C.; Kalinowski, J. A novel type of N-acetylglutamate synthase is involved in the first step of arginine biosynthesis in Corynebacterium glutamicum. BMC Genomics 2013, 14, 713. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leng, W.; Wu, X.; Shi, T.; Xiong, Z.; Yuan, L.; Jin, W.; Gao, R. Untargeted Metabolomics on Skin Mucus Extract of Channa argus against Staphylococcus aureus: Antimicrobial Activity and Mechanism. Foods 2021, 10, 2995. https://doi.org/10.3390/foods10122995
Leng W, Wu X, Shi T, Xiong Z, Yuan L, Jin W, Gao R. Untargeted Metabolomics on Skin Mucus Extract of Channa argus against Staphylococcus aureus: Antimicrobial Activity and Mechanism. Foods. 2021; 10(12):2995. https://doi.org/10.3390/foods10122995
Chicago/Turabian StyleLeng, Weijun, Xiaoyun Wu, Tong Shi, Zhiyu Xiong, Li Yuan, Wengang Jin, and Ruichang Gao. 2021. "Untargeted Metabolomics on Skin Mucus Extract of Channa argus against Staphylococcus aureus: Antimicrobial Activity and Mechanism" Foods 10, no. 12: 2995. https://doi.org/10.3390/foods10122995
APA StyleLeng, W., Wu, X., Shi, T., Xiong, Z., Yuan, L., Jin, W., & Gao, R. (2021). Untargeted Metabolomics on Skin Mucus Extract of Channa argus against Staphylococcus aureus: Antimicrobial Activity and Mechanism. Foods, 10(12), 2995. https://doi.org/10.3390/foods10122995