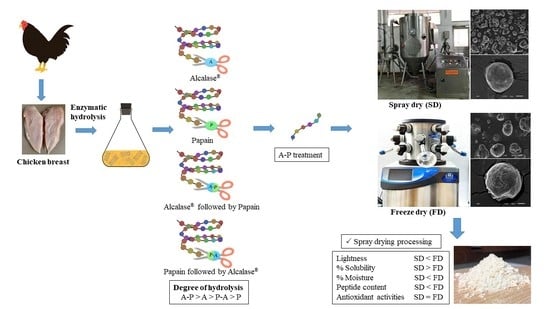
Influence of Commercial Protease and Drying Process on Antioxidant and Physicochemical Properties of Chicken Breast Protein Hydrolysates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Chicken Breast Meat
2.3. Enzymatic Hydrolysis of Chicken Breast Meat
2.4. Degree of Hydrolysis
2.5. Preparation of Microencapsulated CBH Powder
2.6. Physical and Chemical Properties of the Microencapsulated CBH Powder
2.7. Antioxidant Activities
2.7.1. Scavenging Activity on DPPH Free Radicals
2.7.2. Scavenging Activity on ABTS•+ Cation
2.7.3. Ferric Reducing Antioxidant Power
2.8. Statistical Analysis
3. Results
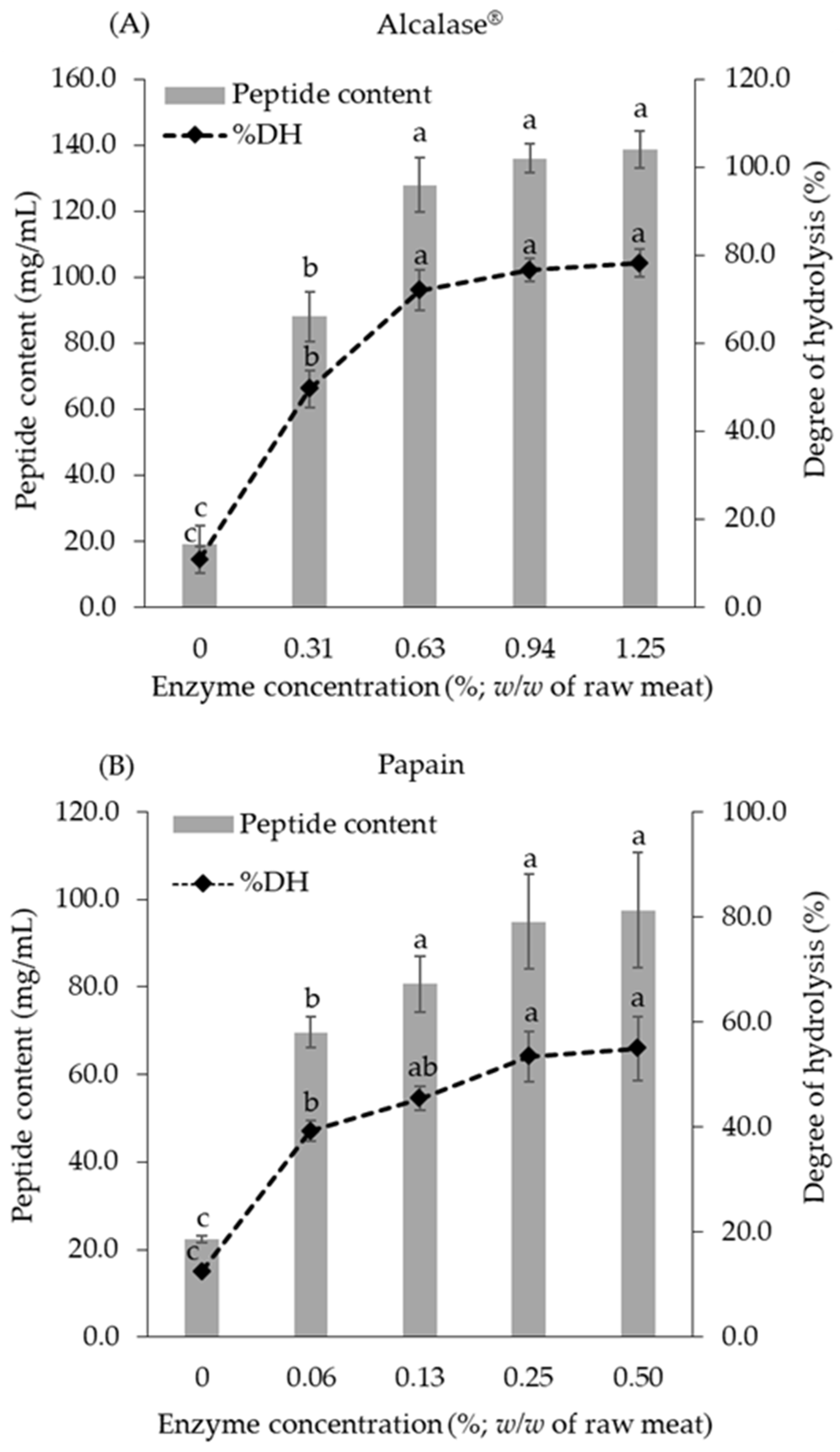
3.1. Effect of Alcalase® and Papain on Hydrolysis of Chicken Breast Meat
3.2. Effect of Sequential Dual Protease Treatment on Chemical and Antioxidant Properties of CBH
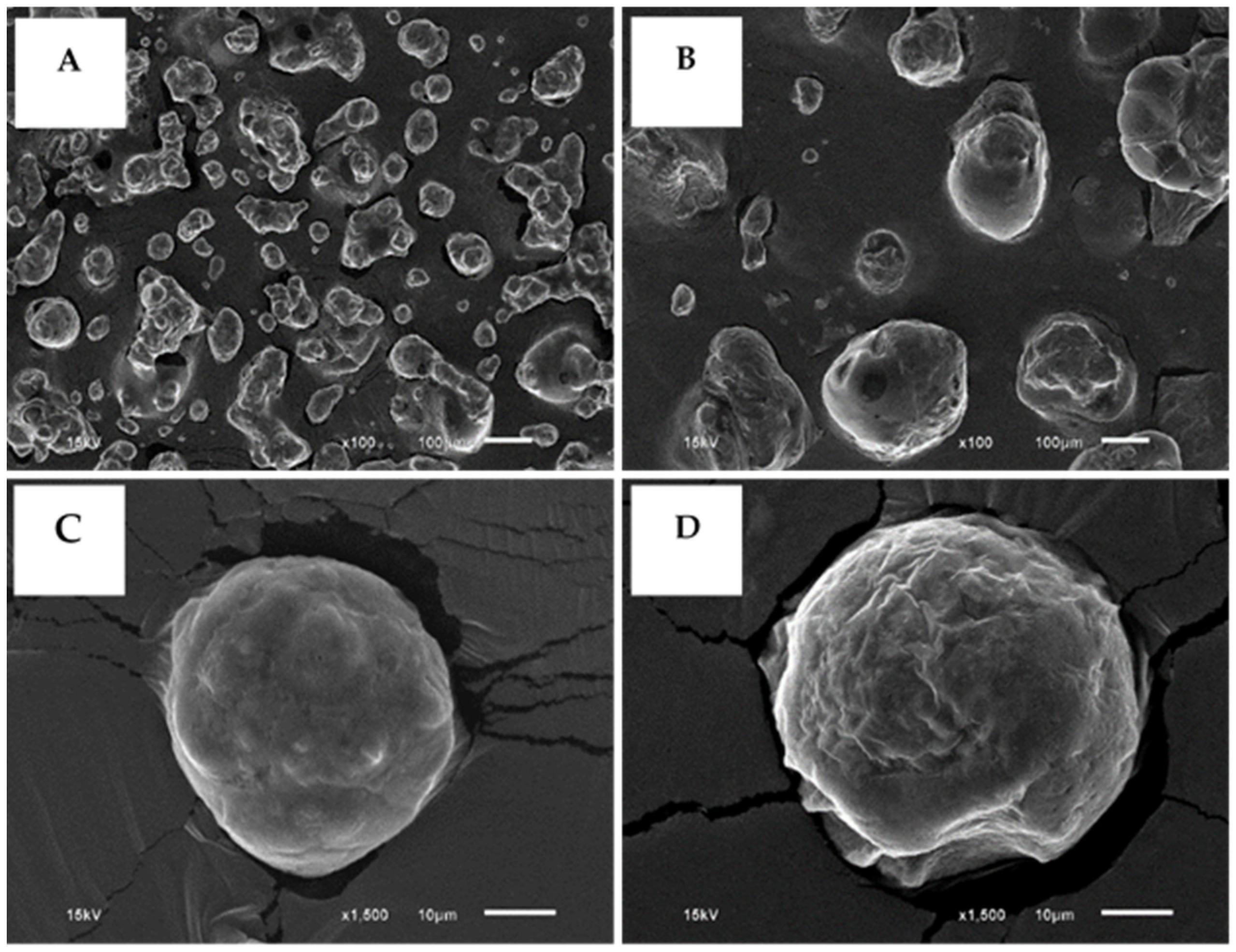
3.3. Effect of Drying Process on Physicochemical Properties of CBH Powder
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samal, J.R.K.; Samal, I.R. Protein supplements: Pros and cons. J. Diet. Suppl. 2018, 15, 365–371. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V.; Fogliano, V. Total antioxidant capacities of raw and cooked meats. Meat Sci. 2012, 90, 60–65. [Google Scholar] [CrossRef]
- Lengkidworraphiphat, P.; Wongpoomchai, R.; Taya, S.; Jaturasitha, S. Effect of genotypes on macronutrients and antioxidant capacity of chicken breast meat. Asian-Australas. J. Anim. Sci. 2020, 33, 1817–1823. [Google Scholar] [CrossRef]
- Udenigwe, C.; Aluko, R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2011, 77, 11–24. [Google Scholar] [CrossRef]
- Huang, G.-R.; Zhao, J.; Jiang, J.-X. Effect of defatting and enzyme type on antioxidative activity of shrimp processing byproducts hydrolysate. Food Sci. Biotechnol. 2011, 20, 651–657. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Idowu, A.T.; Benjakul, S.; Prodpran, T.; Yesilsu, A.F.; Kishimura, H. Effect of proteases and alcohols used for debittering on characteristics and antioxidative activity of protein hydrolysate from salmon frames. J. Food Sci. Technol. 2020, 57, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Thiansilakul, Y.; Benjakul, S.; Shahidi, F. Antioxidative activity of protein hydrolysate from round scad muscle using alcalase and flavourzyme. J. Food Biochem. 2007, 31, 266–287. [Google Scholar] [CrossRef]
- Kurozawa, L.E.; Park, K.J.; Hubinger, M.D. Spray drying of chicken meat protein hydrolysate: Influence of process conditions on powder property and dryer performance. Dry. Technol. 2011, 29, 163–173. [Google Scholar] [CrossRef]
- Borrajo, P.; Pateiro, M.; Gagaoua, M.; Franco, D.; Zhang, W.; Lorenzo, J.M. Evaluation of the antioxidant and antimicrobial activities of porcine liver protein hydrolysates obtained using alcalase, bromelain, and papain. Appl. Sci. 2020, 10, 2290. [Google Scholar] [CrossRef] [Green Version]
- López-Pedrouso, M.; Borrajo, P.; Pateiro, M.; Lorenzo, J.M.; Franco, D. Antioxidant activity and peptidomic analysis of porcine liver hydrolysates using alcalase, bromelain, flavourzyme and papain enzymes. Food Res. Int. 2020, 137, 109389. [Google Scholar] [CrossRef]
- O’Sullivan, S.M.; Lafarga, T.; Hayes, M.; O’Brien, N.M. Bioactivity of bovine lung hydrolysates prepared using papain, pepsin, and alcalase. J. Food Biochem. 2017, 41, e12406. [Google Scholar] [CrossRef]
- Ketnawa, S.; Martínez-Alvarez, O.; Gómez-Estaca, J.; Gómez-Guillén, M.D.C.; Benjakul, S.; Rawdkuen, S. Obtaining of functional components from cooked shrimp (Penaeus vannamei) by enzymatic hydrolysis. Food Biosci. 2016, 15, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Ketnawa, S.; Alvarez, M.; Benjakul, S.; Rawdkuen, S. Gelatin hydrolysates from farmed giant catfish skin using alkaline proteases and its antioxidative function of simulated gastro-intestinal digestion. Food Chem. 2016, 192, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Han, L.; Hong, H.; Pan, J.; Liu, H.; Luo, Y. Purification and identification of novel antioxidant peptides from silver carp muscle hydrolysate after simulated gastrointestinal digestion and transepithelial transport. Food Chem. 2021, 342, 128275. [Google Scholar] [CrossRef]
- Ezekiel, A.; Florence, M. Papain, a plant enzyme of biological importance: A review. Am J Biochem. Biotechnol. 2012, 8, 99–104. [Google Scholar]
- Elavarasan, K.; Shamasundar, B.A. Angiotensin-I-converting enzyme inhibitory activity and antioxidant properties of cryptides derived from natural actomyosin of Catla catla using papain. J. Food Qual. 2018, 2018, 9354829. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.-Y.; Wang, B.; Li, Z.-R.; Chi, C.-F.; Zhang, Q.-H.; He, G.-Y. Preparation and evaluation of antioxidant peptide from papain hydrolysate of Sphyrna lewini muscle protein. LWT 2013, 51, 281–288. [Google Scholar] [CrossRef]
- Liu, J.-H.; Tian, Y.-G.; Wang, Y.; Nie, S.-P.; Xie, M.-Y.; Zhu, S.; Wang, C.-Y.; Zhang, P. Characterization and in vitro antioxidation of papain hydrolysate from black-bone silky fowl (Gallus gallus domesticus Brisson) muscle and its fractions. Food Res. Int. 2011, 44, 133–138. [Google Scholar] [CrossRef]
- Sun, Y.; Pan, D.; Guo, Y.; Li, J. Purification of chicken breast protein hydrolysate and analysis of its antioxidant activity. Food Chem. Toxicol. 2012, 50, 3397–3404. [Google Scholar] [CrossRef] [PubMed]
- Saiga, A.; Tanabe, S.; Nishimura, T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J. Agric. Food Chem. 2003, 51, 3661–3667. [Google Scholar] [CrossRef]
- Kurozawa, L.E.; Park, K.J.; Hubinger, M. Effect of maltodextrin and gum arabic on water sorption and glass transition temperature of spray dried chicken meat hydrolysate protein. J. Food Eng. 2009, 91, 287–296. [Google Scholar] [CrossRef]
- Wu, P.-T.; Lau, Y.-Q.; Dai, F.-J.; Lin, J.-T.; Kao, H.-Y.; Chau, C.-F. Ability of chicken protein hydrolysate to lower serum cholesterol through its bile acid binding activity. CyTA J. Food 2020, 18, 493–499. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Kuck, L.S.; Noreña, C.P.Z. Microencapsulation of grape (Vitis labrusca var. Bordo) skin phenolic extract using gum arabic, polydextrose, and partially hydrolyzed guar gum as encapsulating agents. Food Chem. 2016, 194, 569–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Chi, Y.-J.; Xu, W. Comparisons on the functional properties and antioxidant activity of spray-dried and freeze-dried egg white protein hydrolysate. Food Bioprocess Technol. 2012, 5, 2342–2352. [Google Scholar] [CrossRef]
- Pedroche, J.; Yust, M.; Lqari, H.; Megias, C.; Giron-Calle, J.; Alaiz, M.; Vioque, J.; Millán, F. Obtaining of Brassica carinata protein hydrolysates enriched in bioactive peptides using immobilized digestive proteases. Food Res. Int. 2007, 40, 931–938. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G.W. Association of Official Analytical Chemists International. Official Methods of Analysis of AOAC International; Current Through Revision 1; AOAC International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Shittu, T.; Lawal, M. Factors affecting instant properties of powdered cocoa beverages. Food Chem. 2007, 100, 91–98. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Jo, C.; Nam, K.C.; Ahn, D.U. Enzymatic hydrolysis of ovalbumin and the functional properties of the hydrolysates. Poult. Sci. 2014, 93, 2678–2686. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J.; Goktepe, I.; Ahmedna, M. The potential of papain and alcalase enzymes and process optimizations to reduce allergenic gliadins in wheat flour. Food Chem. 2016, 196, 1338–1345. [Google Scholar] [CrossRef] [Green Version]
- Tacias-Pascacio, V.G.; Morellon-Sterling, R.; Siar, E.-H.; Tavano, O.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Use of alcalase in the production of bioactive peptides: A review. Int. J. Biol. Macromol. 2020, 165, 2143–2196. [Google Scholar] [CrossRef]
- LeDuc, A.; Fournier, V.; Henry, J. A standardized, innovative method to characterize the structure of aquatic protein hydrolysates. Heliyon 2020, 6, e04170. [Google Scholar] [CrossRef]
- Sae-Leaw, T.; Karnjanapratum, S.; O’Callaghan, Y.C.; O’Keeffe, M.B.; Fitzgerald, R.J.; O’Brien, N.M.; Benjakul, S. Purification and identification of antioxidant peptides from gelatin hydrolysate of seabass skin. J. Food Biochem. 2016, 41, e12350. [Google Scholar] [CrossRef]
- Delgado, M.C.O.; Nardo, A.; Pavlovic, M.; Rogniaux, H.; Añón, M.C.; Tironi, V.A. Identification and characterization of antioxidant peptides obtained by gastrointestinal digestion of amaranth proteins. Food Chem. 2016, 197, 1160–1167. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Soladoye, O.P.; Aluko, R.E. Maillard reaction products derived from food protein-derived peptides: Insights into flavor and bioactivity. Crit. Rev. Food Sci. Nutr. 2019, 60, 3429–3442. [Google Scholar] [CrossRef]
- Ezhilarasi, P.; Indrani, D.; Jena, B.; Anandharamakrishnan, C. Freeze drying technique for microencapsulation of Garcinia fruit extract and its effect on bread quality. J. Food Eng. 2013, 117, 513–520. [Google Scholar] [CrossRef]
- Saikia, S.; Mahnot, N.K.; Mahanta, C.L. Optimisation of phenolic extraction from Averrhoa carambola pomace by response surface methodology and its microencapsulation by spray and freeze drying. Food Chem. 2015, 171, 144–152. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of process conditions on the physicochemical properties of açai (Euterpe oleraceae Mart.) powder produced by spray drying. J. Food Eng. 2008, 88, 411–418. [Google Scholar] [CrossRef]
- Hau, E.H.; Mohd Zin, Z.; Zuraidah, N.; Shaharudin, N.A.; Zainol, M.K. Physicochemical properties of powdered protein hydrolysate from Yellowstripe scad (Selaroides leptolepis) fish. Int. Food Res. J. 2018, 25, 2553–2559. [Google Scholar]
| Samples | DH (%) | Peptide Content (mg/mL) | FRAP Value (µM Trolox Equivalent/g Sample) | Inhibition (%, 10 mg/mL) | |
|---|---|---|---|---|---|
| DPPH Assay | ABTS Assay | ||||
| CBH:A 0.63% | 62.99 ± 2.62 b | 111.82 ± 4.65 b | 0.41 ± 0.02 d | 13.83 ± 1.21 c | 34.88 ± 0.21 b |
| CBH-P 0.13% | 31.92 ± 3.27 d | 56.66 ± 5.80 d | 0.54 ± 0.03 b | 19.55 ± 0.32 b | 41.59 ± 5.95 a |
| CBH:A-P | 74.47 ± 1.78 a | 133.77 ± 3.15 a | 0.71 ± 0.02 a | 28.32 ± 0.44 a | 48.97 ± 1.93 a |
| CBH:P-A | 54.75 ± 1.76 c | 97.20 ± 3.13 c | 0.54 ± 0.03 c | 11.25 ± 2.30 d | 38.73 ± 1.48 b |
| GSH | - | - | 368 ± 14.06 | 44.27 ± 0.69 | 38.86 ± 1.56 |
| Powder Properties | Drying Conditions | |
|---|---|---|
| Spray-Drying | Freeze-Drying | |
| Color parameters | ||
| L* | 88.50 ± 0.06 b | 91.20 ± 0.02 a |
| a* | −7.45 ± 0.07 a | −8.12 ± 0.01 b |
| b* | 16.14 ± 0.03 a | 14.27 ± 0.20 b |
| Solubility (%) | 92.50 ± 1.65 a | 87.76 ± 1.09 b |
| Moisture (%) | 2.15 ± 0.24 b | 3.09 ± 0.44 a |
| Peptide content (w/w) | 0.59 ± 0.01 b | 0.63 ± 0.01 a |
| Drying Conditions | FRAP Value (µM Trolox Equivalent/g Sample) | ABTS Inhibition Activity (%) (10 mg/mL) |
|---|---|---|
| Spray-drying | 0.28 ± 0.06 a | 57.50 ± 0.87 a |
| Freeze-drying | 0.21 ± 0.08 a | 53.68 ± 3.38 a |
| GSH | 368 ± 14.06 | 38.86 ± 1.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setthaya, P.; Jaturasitha, S.; Ketnawa, S.; Chaiyaso, T.; Sato, K.; Wongpoomchai, R. Influence of Commercial Protease and Drying Process on Antioxidant and Physicochemical Properties of Chicken Breast Protein Hydrolysates. Foods 2021, 10, 2994. https://doi.org/10.3390/foods10122994
Setthaya P, Jaturasitha S, Ketnawa S, Chaiyaso T, Sato K, Wongpoomchai R. Influence of Commercial Protease and Drying Process on Antioxidant and Physicochemical Properties of Chicken Breast Protein Hydrolysates. Foods. 2021; 10(12):2994. https://doi.org/10.3390/foods10122994
Chicago/Turabian StyleSetthaya, Phatthawin, Sanchai Jaturasitha, Sunantha Ketnawa, Thanongsak Chaiyaso, Kenji Sato, and Rawiwan Wongpoomchai. 2021. "Influence of Commercial Protease and Drying Process on Antioxidant and Physicochemical Properties of Chicken Breast Protein Hydrolysates" Foods 10, no. 12: 2994. https://doi.org/10.3390/foods10122994
APA StyleSetthaya, P., Jaturasitha, S., Ketnawa, S., Chaiyaso, T., Sato, K., & Wongpoomchai, R. (2021). Influence of Commercial Protease and Drying Process on Antioxidant and Physicochemical Properties of Chicken Breast Protein Hydrolysates. Foods, 10(12), 2994. https://doi.org/10.3390/foods10122994