Modeling and Optimization of Process Parameters for Nutritional Enhancement in Enzymatic Milled Rice by Multiple Linear Regression (MLR) and Artificial Neural Network (ANN)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rice Samples
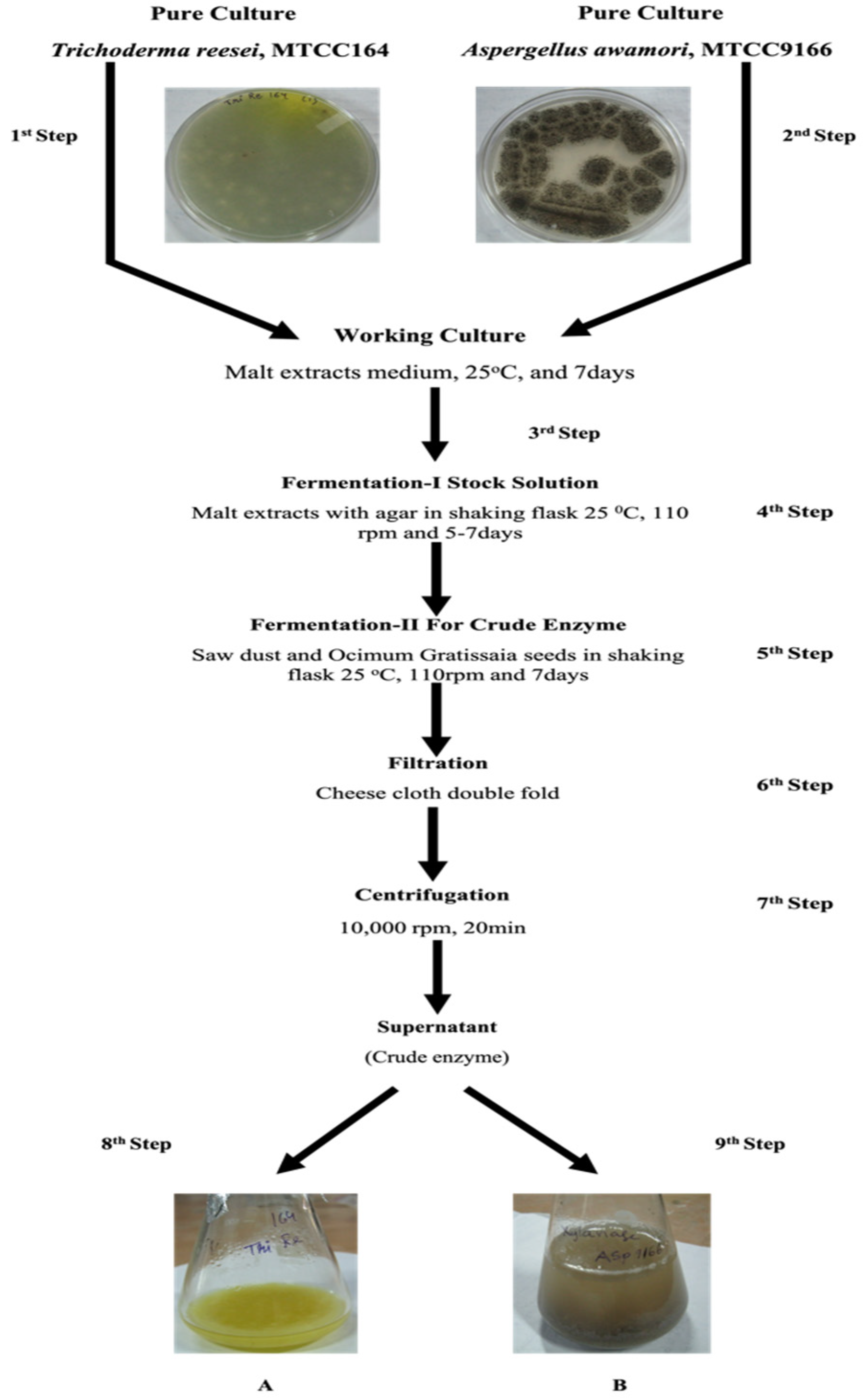
2.2. Enzyme Preparation
2.3. Experimental Process
2.3.1. Estimation of Mineral Content
2.3.2. Total Phenolic Substance Estimation
2.3.3. Total Free Amino Acid Assessment
2.3.4. Grain Hardness Assay
2.3.5. Total Protein Content Determination
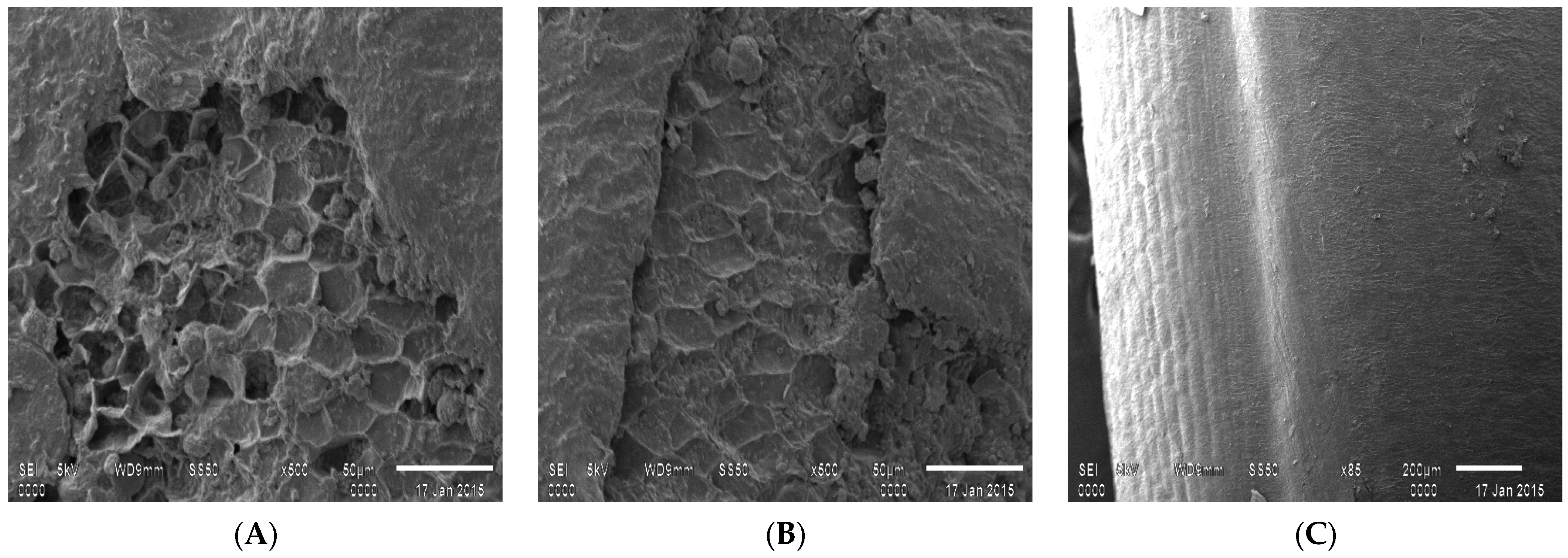
2.3.6. Enzyme Interaction through Scanning Electron Microscopy (SEM)
2.4. Statistical Analysis
2.4.1. Multiple Linear Regression (MLR)
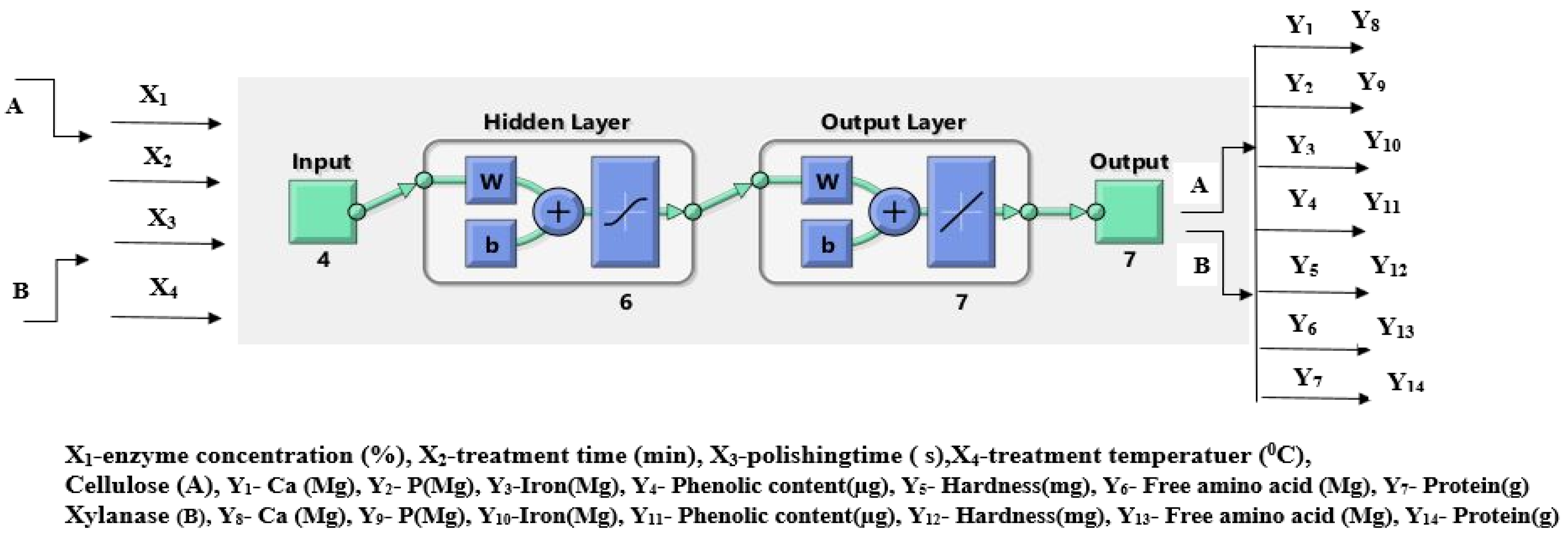
2.4.2. Artificial Neural Network
3. Results and Discussion
3.1. Multiple Linear Regression (MLR)
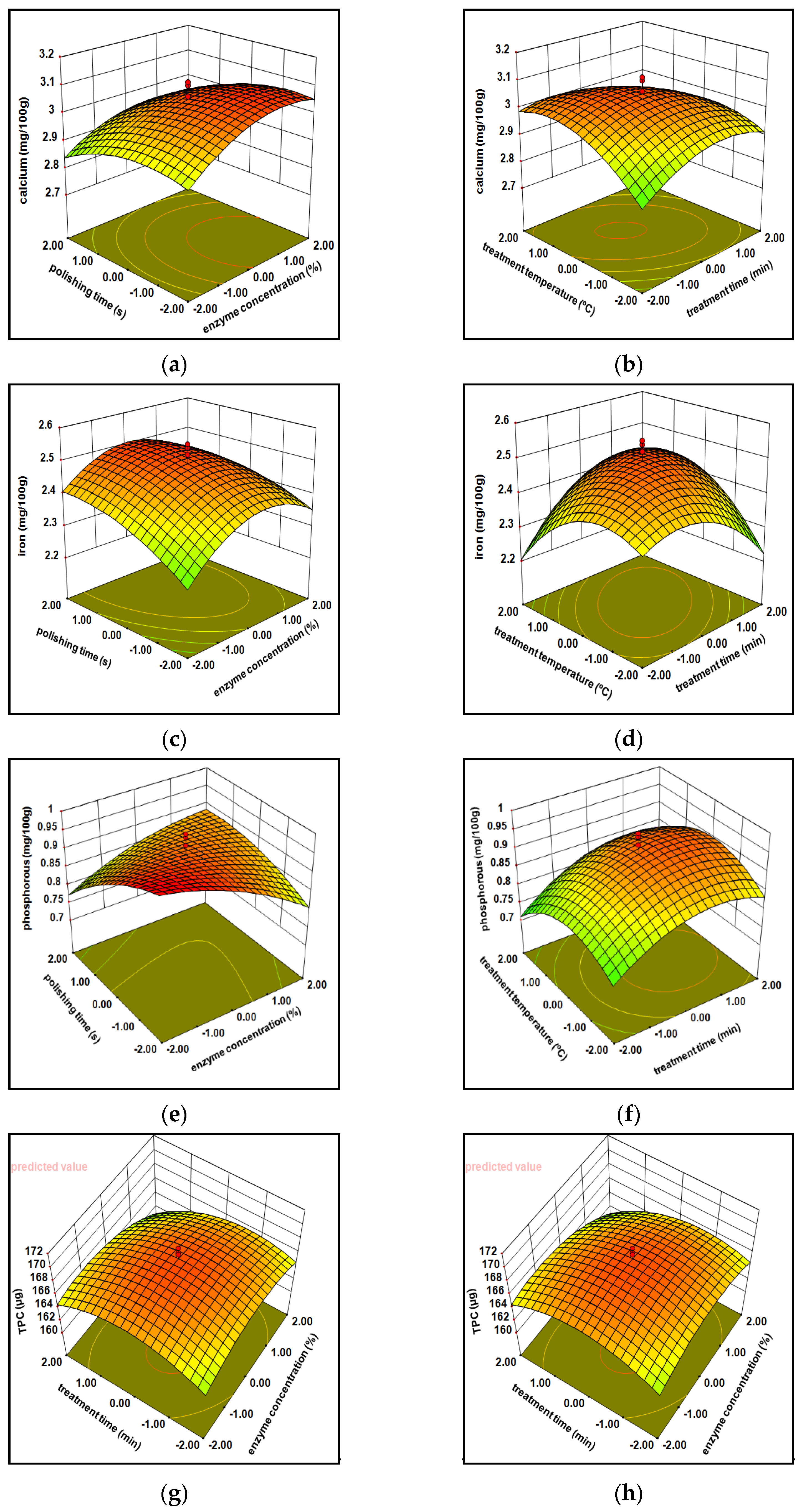
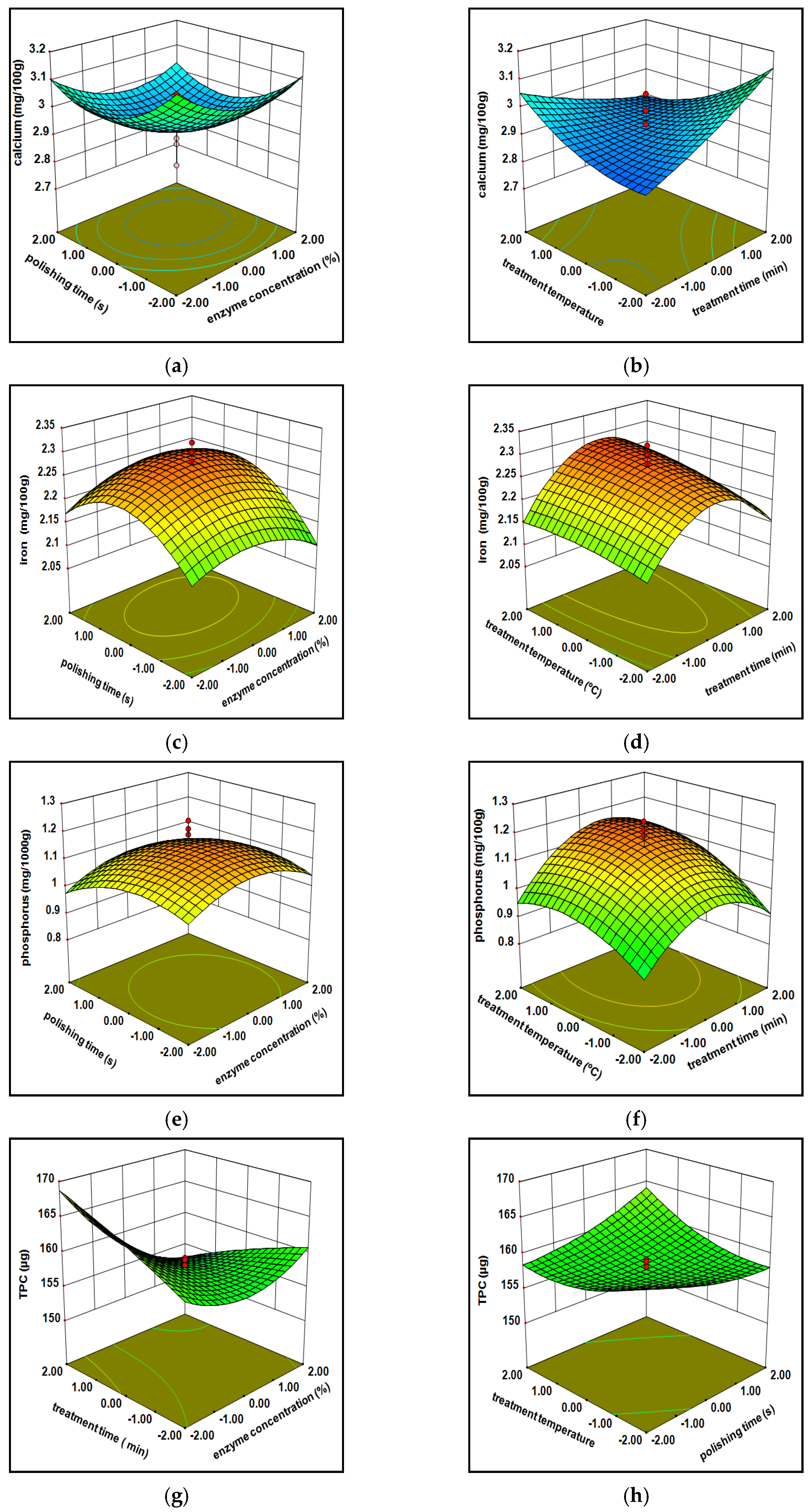
3.1.1. Minerals
3.1.2. Total Phenolic Content (TPC)
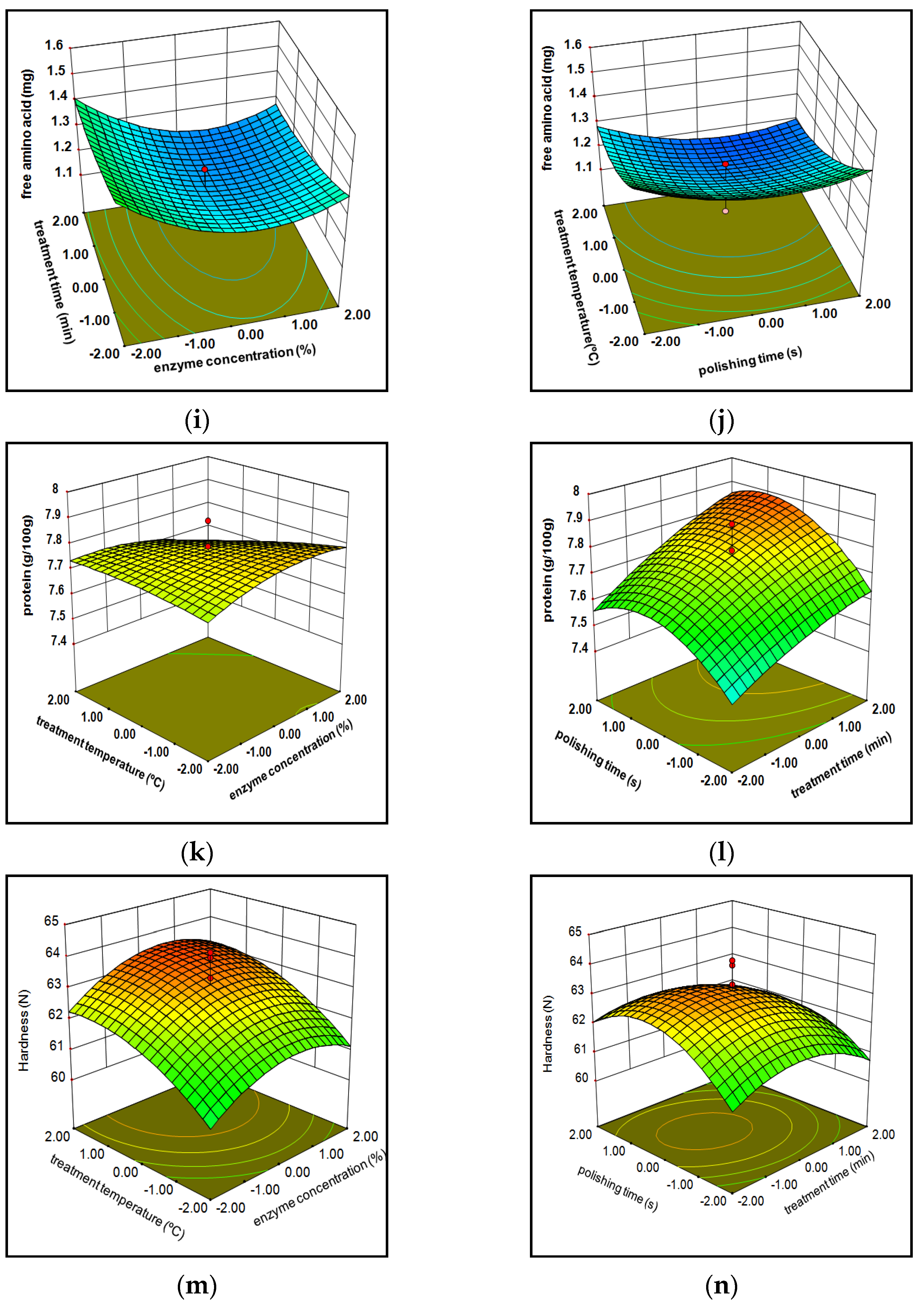
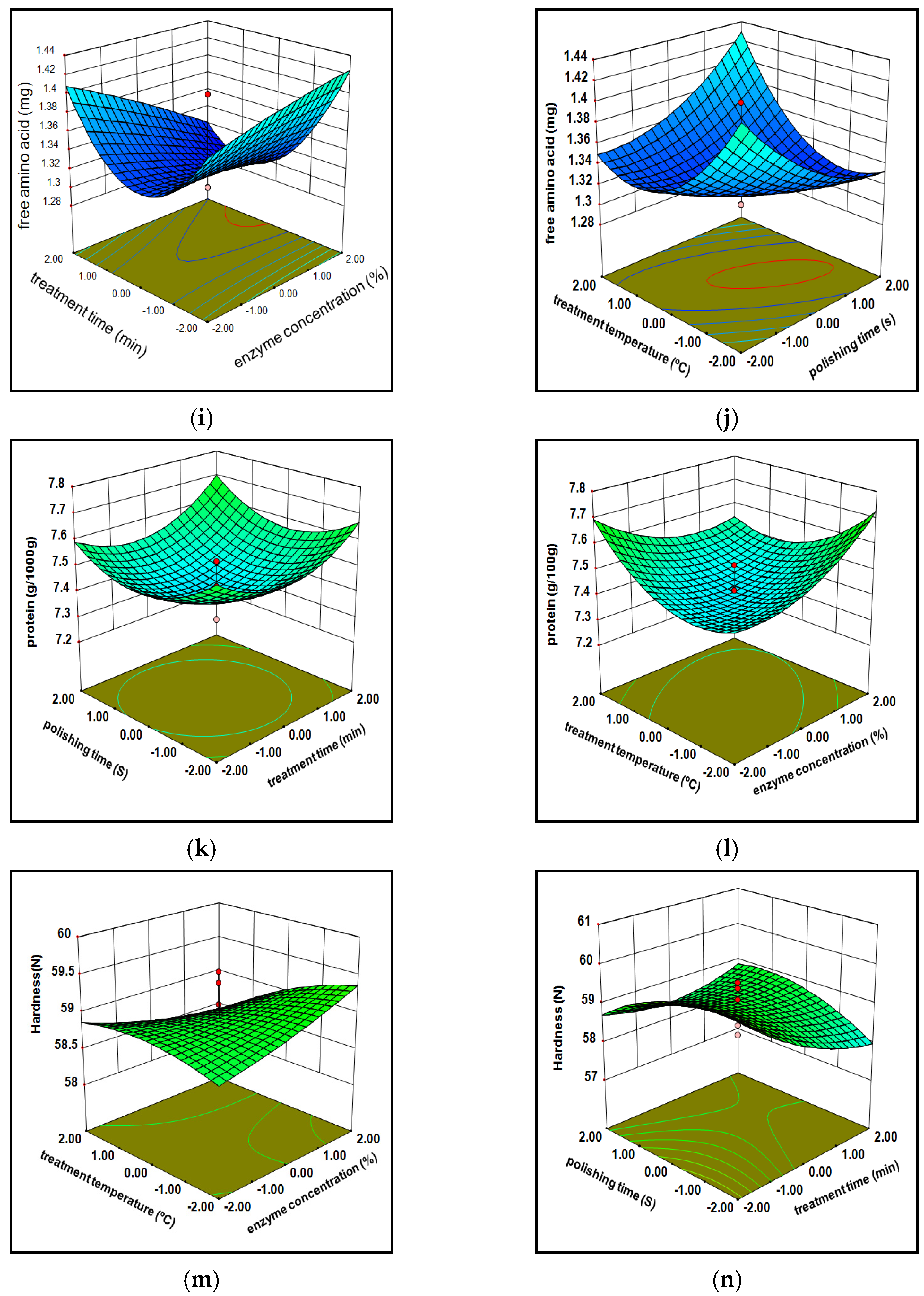
3.1.3. Free Amino Acids
3.1.4. Protein Composition
3.1.5. Hardness
3.1.6. Scanning Electron Microscopy Structural Analysis
3.1.7. Model Optimization
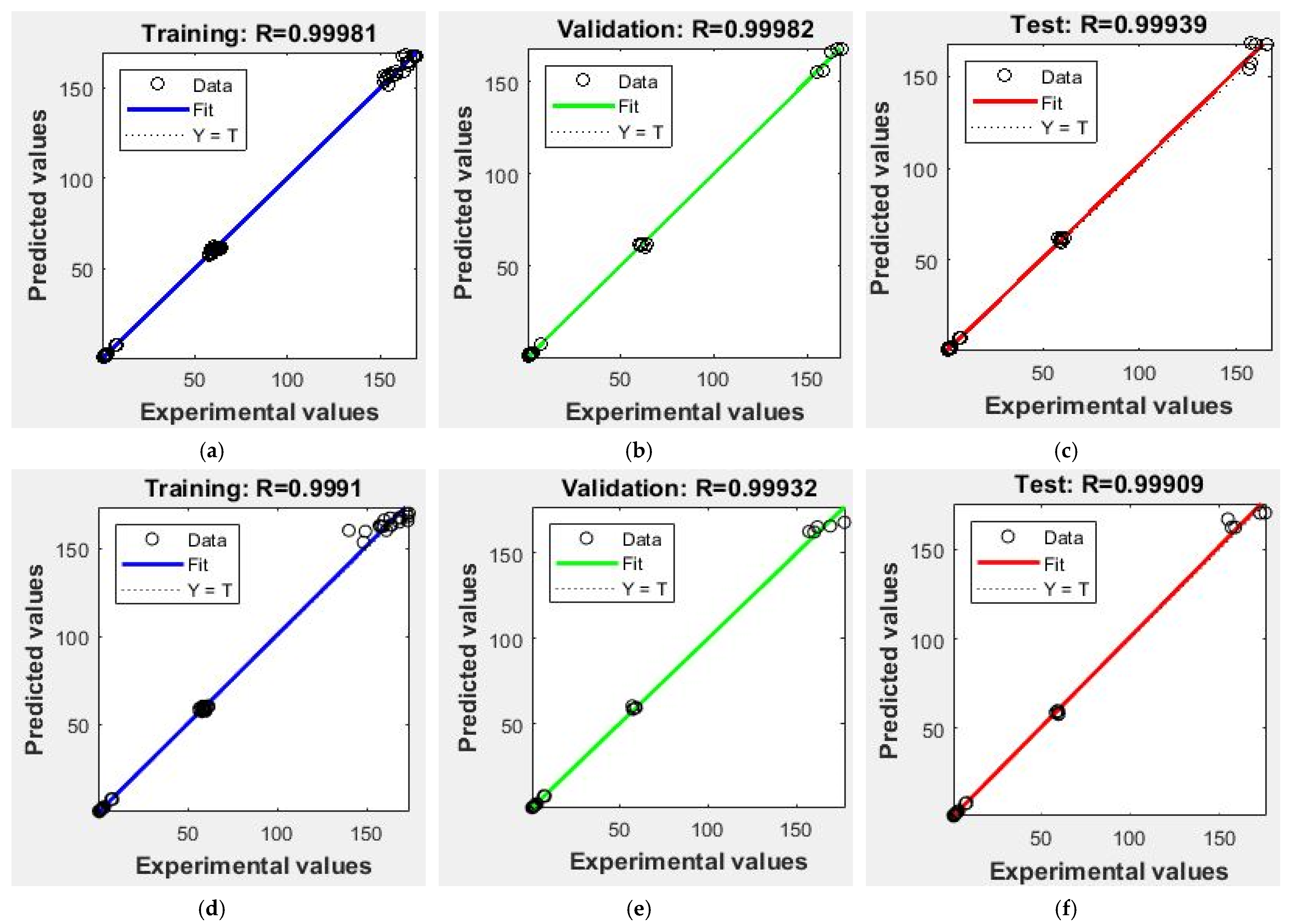
3.2. Artificial Neural Network
Process Optimization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernandes Monks, J.L.; Vanier, N.L.; Casaril, J.; Manica Berto, R.; de Oliveira, M.; Gomes, C.B.; de Carvalho, M.P.; Guerra Dias, A.R.; Elias, M.C. Effect of milling on proximate composition, folic acid, fatty acids and technological properties of rice. J. Food Compos. Anal. 2013, 30, 73–79. [Google Scholar] [CrossRef]
- Chen, H.H.; Chen, Y.K.; Chang, H.C. Evaluation of physicochemical properties of plasma treated brown rice. Food Chem. 2012, 135, 74–79. [Google Scholar] [CrossRef]
- Kennedy, G.; Burlingame, B.; Nguyen, V. Nutritional Contribution of Rice and Impact of Biotechnology and Biodiversity in Rice-Consuming Countries. In Proceedings of the 20th Session of the International Rice Commission, Bangkok, Thailand, 23–26 July 2002; pp. 59–70. Available online: http://www.fao.org/3/Y4751E/y4751e05.htm%0Ahttp://www.fao.org/docrep/006/Y4751E/y4751e05.htm (accessed on 15 October 2021).
- Lamberts, L.; De Bie, E.; Vandeputte, G.E.; Veraverbeke, W.S.; Derycke, V.; De Man, W.; Delcour, J.A. Effect of milling on colour and nutritional properties of rice. Food Chem. 2007, 100, 1496–1503. [Google Scholar] [CrossRef]
- Thirumdas, R.; Deshmukh, R.R.; Annapure, U.S. Effect of low temperature plasma processing on physicochemical properties and cooking quality of basmati rice. Innov. Food Sci. Emerg. Technol. 2015, 31, 83–90. [Google Scholar] [CrossRef]
- Boluda-Aguilar, M.; Taboada-Rodríguez, A.; López-Gómez, A.; Marín-Iniesta, F.; Barbosa-Cánovas, G.V. Quick cooking rice by hydrostatic pressure processing. LWT Food Sci. Technol. 2013, 51, 196–204. [Google Scholar] [CrossRef]
- Ding, J.; Ulanov, A.V.; Dong, M.; Yang, T.; Nemzer, B.V.; Xiong, S.; Zhao, S.; Feng, H. Enhancement of gamma-aminobutyric acid (GABA) and other health-related metabolities in germinated red rice (Oryza sativa L.) by ultrasonication. Ultrason. Sonochem. 2018, 40, 791–797. [Google Scholar] [CrossRef]
- Qian, J.-Y.; Gu, Y.-P.; Jiang, W.; Chen, W. Inactivating effect of pulsed electric field on lipase in brown rice. Innov. Food Sci. Emerg. Technol. 2014, 22, 89–94. [Google Scholar] [CrossRef]
- Das, M.; Banerjee, R.; Bal, S. Evaluation of physicochemical properties of enzyme treated brown rice (Part B). LWT 2008, 41, 2092–2096. [Google Scholar] [CrossRef]
- Sarao, L.K.; Arora, M.; Sehgal, V.K.; Bhatia, S. The Use of Fungal Enzymes viz Protease, Cellulase, And Xylanase for Polishing Rice. Int. J. Food Saf. 2011, 13, 26–37. [Google Scholar]
- Spagna, G.; Barbagallo, R.; Palmeri, R.; Restuccia, C.; Giudici, P. Properties of endogenous β-glucosidase of a Pichia anomala strain isolated from Sicilian musts and wines. Enzym. Microb. Technol. 2002, 31, 1036–1041. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; ElMasry, G.; Sun, D.-W.; Allen, P. Non-destructive prediction and visualization of chemical composition in lamb meat using NIR hyperspectral imaging and multivariate regression. Innov. Food Sci. Emerg. Technol. 2012, 16, 218–226. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Manikantan, M.R.; Sunoj, S.; Sreejith, S.; Beegum, S. Modeling of coconut milk residue incorporated rice-corn extrudates properties using multiple linear regression and artificial neural network. J. Food Process. Eng. 2019, 42, e12981. [Google Scholar] [CrossRef]
- Kothakota, A.; Yadav, K.C.; Panday, J.P. Development of Microwave Baked Potato Chips using Tomato Flavour. Asian J. Agric. Food Sci. 2013, 1, 1571–2321. [Google Scholar]
- Kothakota, A. A study on evaluation and characterization of extruded product by using various by-products. Afr. J. Food Sci. 2013, 7, 485–497. [Google Scholar] [CrossRef]
- Zurera-Cosano, G.; García-Gimeno, R.M.; Rodríguez-Pérez, M.R.; Hervás-Martínez, C. Validating an artificial neural network model of Leuconostoc mesenteroides in vacuum packaged sliced cooked meat products for shelf-life estimation. Eur. Food Res. Technol. 2005, 221, 717–724. [Google Scholar] [CrossRef]
- Sagarika, N.; Prince, M.; Kothakota, A.; Pandiselvam, R.; Sreeja, R.; Mathew, S.M. Characterization and Optimization of Microwave Assisted Process for Extraction of Nutmeg (Myristica fragrans Houtt.) Mace Essential Oil. J. Essent. Oil Bear. Plants 2018, 21, 895–904. [Google Scholar] [CrossRef]
- Pakalapati, H.; Tariq, M.A.; Arumugasamy, S.K. Optimization and modelling of enzymatic polymerization of ε-caprolactone to polycaprolactone using Candida Antartica Lipase B with response surface methodology and artificial neural network. Enzym. Microb. Technol. 2019, 122, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Kothakota, A.; Pandey, J.P.; Ahmad, A.H.; Kumar, A.; Ahmad, W. Determination and optimization of Vitamin B complex in xylanase enzyme treated polished rice by response surface methodology. J. Environ. Biol. 2016, 37, 543–550. [Google Scholar] [PubMed]
- Kothakota, A.; Pandiselvam, R.; Rajesh, G.; Gowthami, K. Determination and Optimization of Vitamin B Complex (B1, B2, B3 and B6) in Cellulase Treated Polished Rice by HPLC with UV Detector. Asian J. Chem. 2017, 29, 385–392. [Google Scholar] [CrossRef]
- Bora, P.; Ragaee, S.; Marcone, M. Effect of parboiling on decortication yield of millet grains and phenolic acids and in vitro digestibility of selected millet products. Food Chem. 2019, 274, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J. Post Column Amino Acid Analysis—Protein Sequencing Protocols; Humana Press: Totowa, NJ, USA, 2003; Volume 211. [Google Scholar]
- Park, J.K.; Kim, S.S.; Kim, K.O. Effect of milling ratio on sensory properties of cooked rice and on physicochemical properties of milled and cooked rice. Cereal Chem. 2001, 78, 151–156. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Analytical Chemists. In AOAC (1990), 15th ed.; A.A.C.C.: Washington, DC, USA, 1990. [Google Scholar]
- Nourbakhsh, H.; Emam-Djomeh, Z.; Omid, M.; Mirsaeedghai, H.; Moini, S. Prediction of red plum juice permeate flux during membrane processing with ANN optimized using RSM. Comput. Electron. Agric. 2014, 102, 1–9. [Google Scholar] [CrossRef]
- Srikanth, V.; Rajesh, G.K.; Kothakota, A.; Pandiselvam, R.; Sagarika, N.; Manikantan, M.R.; Sudheer, K.P. Modeling and optimization of developed cocoa beans extractor parameters using box behnken design and artificial neural network. Comput. Electron. Agric. 2020, 177, 105715. [Google Scholar] [CrossRef]
- Itani, T.; Tamaki, M.; Arai, E.; Horino, T. Distribution of Amylose, Nitrogen, and Minerals in Rice Kernels with Various Characters. J. Agric. Food Chem. 2002, 50, 5326–5332. [Google Scholar] [CrossRef]
- Mo, H.; Qiu, J.; Yang, C.; Zang, L.; Sakai, E.; Chen, J. Porous biochar/chitosan composite for high performance cellulase immobilization by g;utaraldehyde. Enzyme Microb. Technol. 2020, 138, 109561. [Google Scholar] [CrossRef]
- Somerville, C.; Bauer, S.; Brininstool, G.; Facette, M.; Hamann, T.; Milne, J.; Osborne, E.; Paredez, A.; Persson, S.; Raab, T.; et al. Toward a Systems Approach to Understanding Plant Cell Walls. Science 2004, 306, 2206–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohapatra, D.; Bal, S. Cooking quality and instrumental textural attributes of cooked rice for different milling fractions. J. Food Eng. 2006, 73, 253–259. [Google Scholar] [CrossRef]
- Arora, G.; Sehgal, V.; Arora, M. Optimization of process parameters for milling of enzymatically presented Basmati rice. J. Food Eng. 2007, 82, 153–159. [Google Scholar] [CrossRef]




| Source | Y1 | Y2 | Y3 | Y4 | Y5 | Y6 | Y7 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | |
| Model | 9.01 | <0.0001 | 8.50 | <0.0001 | 8.63 | <0.0001 | 9.20 | <0.0001 | 3.64260 | <0.0001 | 8.90 | <0.0001 | 7.84 | 0.0001 |
| X1 | 0.07041 | 0.0075 | 0.01260 | 0.2267 | 0.00770 | 0.2088 | 3.64260 | 0.1694 | 4.10853 | 0.0505 | 0.09375 | 0.0089 | 0.00135 | 0.7220 |
| X2 | 0.00426 | 0.4588 | 0.00453 | 0.4611 | 0.05133 | 0.0040 | 4.10853 | 0.1613 | 1.52510 | 0.0393 | 0.05041 | 0.0436 | 0.35526 | <0.0001 |
| X3 | 0.06406 | 0.0100 | 0.06100 | 0.0142 | 0.02870 | 0.0229 | 1.52510 | 0.0002 | 21.6030 | 0.1891 | 0.03375 | 0.0916 | 0.16666 | 0.0011 |
| X4 | 0.01401 | 0.1884 | 0.00050 | 0.8043 | 0.00633 | 0.2521 | 21.6030 | 0.5067 | 17.1226 | 0.0001 | 0.35041 | <0.0001 | 0.02801 | 0.1195 |
| X1X2 | 0.13043 | 0.0740 | 0.24591 | 0.3154 | 0.01845 | 0.0053 | 17.1226 | 0.1079 | 19.5895 | 0.1063 | 0.38003 | 0.8096 | 0.05200 | 0.2365 |
| X1X3 | 0.15003 | 0.4610 | 0.22682 | 0.3689 | 0.10607 | 0.0005 | 19.5895 | 0.0580 | 38.8484 | 0.2748 | 0.02503 | 0.1066 | 0.02234 | 0.0736 |
| X1X4 | 0.07985 | 0.8637 | 0.04052 | 0.3978 | 0.02027 | 0.6338 | 38.8484 | 0.1733 | 9.23028 | 0.7490 | 0.12574 | 0.0433 | 0.38543 | 0.0880 |
| X2X3 | 0.19914 | <0.0001 | 0.28641 | 0.3978 | 0.14792 | 0.0281 | 9.23028 | 0.9077 | 2.37930 | 0.2093 | 0.08360 | 0.0061 | 0.00600 | 0.2950 |
| X2X4 | 0.02722 | 0.0172 | 0.00855 | 0.0014 | 0.04730 | 0.5830 | 2.37930 | 0.7404 | 1.03530 | 0.5439 | 0.00062 | 0.0433 | 0.01562 | 0.0961 |
| X3X4 | 0.00422 | 0.0822 | 0.00680 | 0.0013 | 0.08555 | 0.0029 | 1.03530 | 0.1137 | 0.08555 | 0.7326 | 0.03062 | 0.8096 | 0.03802 | 0.0805 |
| X12 | 0.00022 | 0.0008 | 0.00600 | <0.0001 | 0.00105 | 0.0602 | 0.08555 | 0.0006 | 1.38650 | 0.0003 | 0.05062 | <0.0001 | 0.03422 | 0.0399 |
| X22 | 0.2704 | 0.0004 | 0.00600 | <0.0001 | 0.02640 | 0.0002 | 1.38650 | <0.0001 | 0.31080 | 0.0002 | 0.10562 | 0.0977 | 0.0121 | 0.1610 |
| X32 | 0.0529 | 0.0050 | 0.12075 | 0.0391 | 0.00140 | 0.0501 | 0.31080 | 0.0336 | 0.09765 | <0.0001 | 0.05062 | 0.0022 | 0.0324 | <0.0001 |
| X42 | 0.0256 | 0.0001 | 0.12425 | <0.0001 | 0.05640 | <0.0001 | 0.09765 | <0.0001 | 3.64260 | <0.0001 | 0.00062 | 0.0081 | 0.0361 | 0.4566 |
| R2 | 0.8938 | 0.8880 | 0.8896 | 0.8957 | 0.8902 | 0.8925 | 0.8798 | |||||||
| Adj. R2 | 0.7946 | 0.7835 | 0.7865 | 0.7883 | 0.7877 | 0.7946 | 0.7676 | |||||||
| Pred. R2 | 0.4490 | 0.4158 | 0.4418 | 0.4392 | 0.5148 | 0.4490 | 0.3695 | |||||||
| SSE | 10.31 | 8.94 | 9.52 | 92.35 | 12.09 | 0.16 | 0.0183 | |||||||
| LOF | NS | NS | NS | NS | NS | NS | NS | |||||||
| Source | Y8 | Y9 | Y10 | Y11 | Y12 | Y13 | Y14 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | |
| Model | 10.49 | <0.0001 | 10.52 | <0.0001 | 8.78 | <0.0001 | 7.21 | 1.316667 | 8.39 | <0.0001 | 7.21 | 0.0002 | 6.17 | 0.0005 |
| X1 | 0.03450 | 0.0718 | 0.00015 | 0.8351 | 0.00041 | 0.8261 | 349.988 | −0.0125 | 0.01550 | 0.8452 | 0.00375 | 0.3243 | 0.01353 | 0.3871 |
| X2 | 0.01870 | 0.1743 | 0.01306 | 0.0667 | 0.05226 | 0.0243 | 0.81770 | −0.02083 | 4.51533 | 0.0040 | 0.01041 | 0.1101 | 0.03010 | 0.2039 |
| X3 | 0.04593 | 0.0411 | 0.03081 | 0.0083 | 0.02041 | 0.1385 | 2.74050 | −0.00417 | 0.09003 | 0.6390 | 0.00041 | 0.7388 | 3.75E-0 | 0.9632 |
| X4 | 0.00700 | 0.3966 | 0.01126 | 0.0863 | 0.10401 | 0.0030 | 6.25260 | 0.004167 | 1.19260 | 0.1018 | 0.00041 | 0.7388 | 0.00220 | 0.7243 |
| X1X2 | 0.18905 | 0.0426 | 0.02714 | 0.0765 | 0.15087 | 0.0212 | 283.930 | −0.03125 | 0.07832 | 0.3036 | 0.01574 | 0.0551 | 0.17508 | 0.0056 |
| X1X3 | 0.00771 | 0.8980 | 0.20503 | 0.2447 | 0.42287 | 0.8296 | 4.09425 | 0.04375 | 3.16491 | 0.1202 | 0.07145 | 0.0107 | 0.37000 | 0.0082 |
| X1X4 | 0.31268 | 0.0028 | 0.12574 | 0.9322 | 0.10430 | 0.3400 | 32.8812 | −0.01875 | 4.36802 | 0.1017 | 2.98E-0 | 0.2311 | 0.37000 | 0.0082 |
| X2X3 | 0.07710 | <0.0001 | 1.19E-0 | 0.0085 | 0.12190 | 0.0909 | 93.2305 | −0.06875 | 0.54966 | 0.0046 | 0.01860 | 0.0004 | 0.36208 | 0.6128 |
| X2X4 | 0.04515 | 0.0005 | 0.0121 | 0.4007 | 0.05522 | 0.2272 | 267.567 | −0.00625 | 0.44555 | 0.0244 | 0.01562 | 0.6833 | 0.17850 | 0.2191 |
| X3X4 | 0.00015 | 0.5921 | 0.0049 | 0.6114 | 0.0004 | 0.0007 | 13.9689 | 0.04375 | 1.06605 | <0.0001 | 0.03062 | 0.0107 | 0.15800 | 0.0831 |
| X12 | 0.11730 | 0.0004 | 2.5E-05 | 0.0019 | 0.0081 | 0.0007 | 312.317 | −0.00312 | 1.19355 | 0.6615 | 0.00562 | 0.7891 | 0.15800 | 0.0059 |
| X22 | 0.43230 | 0.3742 | 0.03062 | <0.0001 | 0.02722 | <0.0001 | 8.65830 | 0.071875 | 4.35765 | 0.0124 | 0.07562 | <0.0001 | 0.00455 | 0.0003 |
| X32 | 0.17850 | <0.0001 | 0.0025 | <0.0001 | 0.01322 | 0.0030 | 579.726 | 0.021875 | 2.45705 | 0.0045 | 0.00062 | 0.0759 | 0.02805 | 0.0003 |
| X42 | 0.00275 | 0.0111 | 0.0009 | 0.3697 | 0.1521 | 0.0017 | 99.9500 | 0.046875 | 21.3213 | 0.2552 | 0.03062 | 0.0010 | 0.05880 | 0.0003 |
| R2 | 0.9073 | 0.9076 | 0.8913 | 0.8706 | 0.8867 | 0.8706 | 0.8574 | |||||||
| Adj. R2 | 0.8209 | 0.8214 | 0.7898 | 0.7499 | 0.7810 | 0.7499 | 0.7243 | |||||||
| Pred. R2 | 0.5896 | 0.5421 | 0.4708 | 0.3438 | 0.4650 | 0.3438 | 0.2429 | |||||||
| SSE | 0.14 | 0.050 | 0.13 | 203.85 | 5.89 | 8.69 | 6.44 | |||||||
| LOF | NS | NS | NS | NS | NS | NS | NS | |||||||
| Y8 | Y9 | Y10 | Y11 | Y12 | Y13 | Y14 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. |
| 2.84 | 3.09 | 2.06 | 2.00 | 0.94 | 0.98 | 160.67 | 160.03 | 61.12 | 60.66 | 1.50 | 1.41 | 7.65 | 7.51 |
| 2.94 | 3.18 | 1.93 | 2.05 | 0.77 | 0.92 | 162.78 | 167.10 | 61.78 | 60.45 | 1.40 | 1.45 | 7.91 | 7.75 |
| 3.47 | 3.20 | 1.97 | 2.06 | 0.86 | 0.79 | 172.89 | 170.53 | 58.11 | 58.68 | 1.60 | 1.41 | 7.61 | 7.66 |
| 3.62 | 3.21 | 2.05 | 2.13 | 1.09 | 0.83 | 168.95 | 166.97 | 59.98 | 58.73 | 1.50 | 1.43 | 7.92 | 7.87 |
| 3.22 | 3.07 | 2.08 | 2.08 | 0.59 | 0.95 | 147.92 | 153.31 | 58.09 | 59.44 | 1.30 | 1.38 | 7.91 | 7.63 |
| 3.14 | 3.04 | 1.98 | 2.12 | 0.62 | 1.11 | 157.97 | 162.59 | 58.14 | 60.40 | 1.60 | 1.44 | 7.83 | 7.60 |
| 3.00 | 3.19 | 1.99 | 2.08 | 0.58 | 0.80 | 176.89 | 167.76 | 57.43 | 58.53 | 1.40 | 1.40 | 7.64 | 7.68 |
| 3.18 | 3.10 | 2.16 | 2.22 | 0.63 | 0.95 | 162.89 | 163.10 | 56.55 | 57.94 | 1.30 | 1.41 | 8.16 | 7.80 |
| 3.43 | 3.31 | 2.09 | 2.08 | 0.78 | 0.73 | 173.24 | 169.78 | 58.57 | 58.00 | 1.40 | 1.55 | 7.77 | 8.00 |
| 2.98 | 3.21 | 2.06 | 2.12 | 0.68 | 0.86 | 159.47 | 165.93 | 57.99 | 58.37 | 1.30 | 1.53 | 7.9 | 7.92 |
| 3.43 | 3.16 | 2.02 | 2.16 | 1.03 | 0.86 | 172.78 | 167.95 | 56.82 | 57.93 | 1.60 | 1.43 | 7.81 | 7.78 |
| 3.27 | 3.00 | 1.97 | 2.20 | 0.99 | 1.13 | 139.78 | 160.10 | 56.45 | 59.27 | 1.40 | 1.43 | 8.12 | 7.66 |
| 3.52 | 3.32 | 1.97 | 2.08 | 1.05 | 0.69 | 175.78 | 170.64 | 59.73 | 57.73 | 1.60 | 1.55 | 8.13 | 8.02 |
| 3.19 | 3.20 | 2.02 | 2.12 | 0.78 | 0.88 | 168.98 | 165.59 | 57.78 | 58.50 | 1.60 | 1.52 | 7.19 | 7.90 |
| 2.97 | 3.18 | 2.22 | 2.14 | 0.89 | 0.84 | 171.89 | 169.60 | 59.89 | 58.14 | 1.40 | 1.43 | 7.92 | 7.75 |
| 2.89 | 3.03 | 2.25 | 2.27 | 1.08 | 1.04 | 148.99 | 159.52 | 60.12 | 57.78 | 1.40 | 1.41 | 7.84 | 7.79 |
| 3.29 | 3.18 | 2.09 | 2.09 | 0.79 | 0.80 | 172.59 | 165.58 | 58.99 | 58.37 | 1.30 | 1.39 | 7.72 | 7.70 |
| 3.17 | 3.12 | 2.11 | 2.08 | 0.88 | 1.01 | 167.89 | 164.75 | 59.78 | 60.39 | 1.30 | 1.45 | 7.79 | 7.69 |
| 2.94 | 3.06 | 1.85 | 2.13 | 0.59 | 1.08 | 156.78 | 162.69 | 61.17 | 59.76 | 1.70 | 1.47 | 7.87 | 7.67 |
| 2.99 | 3.12 | 1.91 | 2.17 | 0.68 | 0.92 | 154.87 | 167.09 | 59.89 | 58.29 | 1.50 | 1.41 | 7.93 | 7.71 |
| 3.37 | 3.00 | 1.87 | 2.13 | 0.83 | 1.16 | 159.91 | 162.07 | 57.17 | 60.43 | 1.40 | 1.44 | 7.89 | 7.54 |
| 3.28 | 3.07 | 2.04 | 2.22 | 0.94 | 0.99 | 163.59 | 162.91 | 57.98 | 58.05 | 1.40 | 1.41 | 7.91 | 7.75 |
| 3.28 | 3.21 | 2.19 | 2.03 | 0.77 | 0.77 | 161.67 | 165.10 | 58.98 | 59.15 | 1.50 | 1.40 | 7.85 | 7.71 |
| 2.94 | 3.29 | 2.26 | 2.13 | 0.96 | 0.74 | 167.82 | 167.39 | 58.23 | 57.41 | 1.50 | 1.55 | 7.94 | 8.06 |
| 3.05 | 3.03 | 2.3 | 2.16 | 1.12 | 1.09 | 159.23 | 162.47 | 58.44 | 59.54 | 1.40 | 1.43 | 7.52 | 7.62 |
| 2.99 | 3.03 | 2.32 | 2.16 | 1.06 | 1.09 | 156.9 | 162.47 | 59.11 | 59.54 | 1.30 | 1.43 | 7.42 | 7.62 |
| 2.87 | 3.03 | 2.22 | 2.16 | 1.21 | 1.09 | 158.92 | 162.47 | 59.54 | 59.54 | 1.60 | 1.43 | 7.42 | 7.62 |
| 2.94 | 3.03 | 2.28 | 2.16 | 1.19 | 1.09 | 157.01 | 162.47 | 59.40 | 59.54 | 1.40 | 1.43 | 7.42 | 7.62 |
| 2.89 | 3.03 | 2.3 | 2.16 | 1.24 | 1.09 | 158.15 | 162.47 | 58.19 | 59.54 | 1.50 | 1.43 | 7.42 | 7.62 |
| 2.79 | 3.03 | 2.22 | 2.16 | 1.09 | 1.09 | 156.92 | 162.47 | 58.89 | 59.54 | 1.20 | 1.43 | 7.29 | 7.62 |
| Response | Equation |
|---|---|
| CellulaseY1 | Y1 = 3.05 + 0.0547X1 + 0.0246X4 + 0.041X1X2 |
| Y2 | Y2 = 0.92 + 0.054X1X2 + 0.073X1X3 + 0.041X2X3 + 9.375 × 10−3 X2X4 |
| Y3 | Y3 = 2.49 + 0.0237X1 + 0.014X2 + 0.050X3 + 4.583 × 1036 X4 + 0.019X1X4 + 0.019X2X3 + 0.087X2X4 |
| Y4 | Y4 = 168.24 + 1.27X1X3 + 0.89X1X4 + 1.04X3X4 |
| Y5 | Y5 = 63.14 + 0.39X1 + 0.25X3 + 0.95X4 + 0.25X1X3 + 0.073X1X4 |
| Y6 | Y6 = 1.23 − 0.063X1 + 0.044X1X3 + 0.056X1X4 + 0.081X2X3 + 0.056X2X4 + 0.12X12 + 0.034X22 + 0.072X32 + 0.059X42 |
| Y7 | Y7 = +7.77 + 0.12X2 + 0.083X3 + 0.031X1X2 + 0.027 X2X3 + 0.047 X3X4 |
| Xylanase Y8 | Y8 = 2.92 + 0.028X2 + 0.053X1X2 + 3.125 × 10−0.086 X1X3 + 0.083X12 + 0.017X22 + 0.11X32 + 0.053X42 |
| Y9 | Y9 = 2.27 + 2.500 × 10−3 X1 + 0.023X2 + 0.036X3 + 0.022X4 + 0.028X1X2 + 0.018X1X3 + 0.044X2X3 + 0.013X2X4 + 7.500 × 10−3 X3X4 |
| Y10 | Y10 = 1.15 + 4.167 × 10−3 X1 + 0.047X2 + 0.066X4 + 0.059X1X2 + 5.000 × 10−3 X1X3 + 0.029X2X4 + 0.098X3X4 |
| Y11 | Y11 = 168.24 + 1.27X1X3 + 0.89X1X4 + 1.04X3X4 |
| Y12 | Y12 = 63.14 + 0.39X1 + 0.25X3 + 0.95X4 + 0.073X1X4 |
| Y13 | Y13 = 1.23 + 0.044X1X3 + 0.056X1X4 + 0.081X2X3 + 0.12X12 + 0.034X22 + 0.072X32 + 0.059X42 |
| Y14 | Y14 = 7.77 − 7.500 × 10−3 X1 + 0.12X2 + 0.083X3 − 0.034X4 − 0.044 X12 + 0.031 X1X2 + 0.027 X2X3 + 0.047 X3 X4 |
| Y1 | Y2 | Y3 | Y4 | Y5 | Y6 | Y7 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. |
| 2.51 | 2.73 | 2.04 | 2.05 | 0.95 | 0.79 | 165.33 | 163.84 | 58.14 | 58.55 | 1.90 | 1.72 | 7.45 | 7.61 |
| 2.65 | 2.72 | 2.12 | 2.06 | 0.58 | 0.75 | 162.88 | 159.21 | 59.73 | 58.53 | 1.70 | 1.71 | 7.31 | 7.62 |
| 2.82 | 2.73 | 1.93 | 2.05 | 0.76 | 0.78 | 165.67 | 162.60 | 57.72 | 58.52 | 1.80 | 1.72 | 7.48 | 7.61 |
| 2.96 | 2.75 | 1.82 | 2.11 | 0.71 | 0.74 | 158.9 | 157.41 | 59.19 | 58.99 | 1.40 | 1.67 | 7.82 | 7.63 |
| 2.88 | 2.82 | 2.29 | 2.19 | 0.64 | 0.74 | 154.91 | 156.28 | 58.94 | 59.32 | 1.60 | 1.62 | 7.46 | 7.67 |
| 2.66 | 2.87 | 2.33 | 2.27 | 0.67 | 0.72 | 156.74 | 153.89 | 59.91 | 59.81 | 1.50 | 1.56 | 7.39 | 7.70 |
| 2.45 | 2.88 | 2.25 | 2.26 | 0.71 | 0.75 | 154.23 | 157.42 | 59.19 | 59.39 | 1.80 | 1.59 | 7.73 | 7.70 |
| 2.76 | 2.86 | 2.15 | 2.25 | 0.97 | 0.68 | 152.21 | 153.14 | 58.97 | 58.67 | 1.60 | 1.56 | 7.91 | 7.74 |
| 2.79 | 2.91 | 1.99 | 2.35 | 0.91 | 0.86 | 160.11 | 167.67 | 59.22 | 62.11 | 1.80 | 1.41 | 7.24 | 7.65 |
| 2.89 | 2.85 | 2.22 | 2.31 | 0.71 | 0.80 | 164.21 | 163.78 | 61.15 | 61.68 | 1.60 | 1.38 | 7.42 | 7.67 |
| 2.92 | 2.89 | 2.25 | 2.33 | 0.95 | 0.83 | 162.7 | 166.00 | 59.74 | 61.92 | 1.30 | 1.40 | 7.46 | 7.66 |
| 3.01 | 2.73 | 2.28 | 2.21 | 0.79 | 0.66 | 155.32 | 154.87 | 61.18 | 60.69 | 1.30 | 1.30 | 7.41 | 7.71 |
| 2.92 | 2.78 | 2.05 | 2.24 | 0.56 | 0.71 | 156.21 | 158.58 | 60.18 | 60.82 | 1.40 | 1.33 | 7.72 | 7.71 |
| 2.91 | 2.74 | 1.95 | 2.21 | 0.47 | 0.66 | 158.9 | 156.05 | 63.17 | 60.37 | 1.50 | 1.31 | 7.44 | 7.72 |
| 2.41 | 2.75 | 2.19 | 2.21 | 0.59 | 0.67 | 151.92 | 156.63 | 60.15 | 60.27 | 1.30 | 1.32 | 7.92 | 7.73 |
| 2.54 | 2.76 | 2.25 | 2.22 | 0.78 | 0.67 | 157.29 | 157.67 | 58.77 | 60.06 | 1.40 | 1.33 | 7.64 | 7.73 |
| 2.65 | 2.91 | 2.08 | 2.35 | 0.81 | 0.86 | 163.67 | 168.05 | 59.89 | 62.04 | 1.80 | 1.42 | 7.65 | 7.65 |
| 2.96 | 2.75 | 2.29 | 2.23 | 0.79 | 0.68 | 157.21 | 156.95 | 60.17 | 60.67 | 1.50 | 1.32 | 7.62 | 7.71 |
| 2.78 | 2.91 | 2.15 | 2.35 | 0.57 | 0.86 | 157.21 | 167.93 | 60.91 | 62.06 | 1.30 | 1.42 | 7.45 | 7.65 |
| 2.79 | 2.79 | 2.25 | 2.25 | 0.74 | 0.72 | 158.78 | 159.42 | 58.71 | 60.84 | 1.30 | 1.34 | 7.94 | 7.71 |
| 2.92 | 2.90 | 2.31 | 2.34 | 0.76 | 0.86 | 165.87 | 167.79 | 57.72 | 62.00 | 1.50 | 1.42 | 7.24 | 7.65 |
| 2.81 | 2.87 | 2.51 | 2.25 | 0.83 | 0.74 | 162.44 | 167.24 | 59.14 | 58.27 | 1.40 | 1.44 | 7.43 | 7.79 |
| 2.77 | 2.68 | 2.19 | 2.00 | 0.65 | 0.68 | 154.92 | 151.73 | 58.12 | 57.22 | 1.70 | 1.80 | 7.88 | 7.63 |
| 2.71 | 2.72 | 2.12 | 2.21 | 0.57 | 0.65 | 152.89 | 154.64 | 63.62 | 60.64 | 1.10 | 1.30 | 7.62 | 7.72 |
| 3.1 | 2.91 | 2.54 | 2.35 | 0.96 | 0.86 | 165.96 | 167.90 | 62.14 | 61.96 | 1.30 | 1.42 | 7.89 | 7.66 |
| 2.96 | 2.91 | 2.55 | 2.35 | 0.85 | 0.86 | 169.01 | 167.90 | 63.34 | 61.96 | 1.20 | 1.42 | 7.79 | 7.66 |
| 3.11 | 2.91 | 2.41 | 2.35 | 0.97 | 0.86 | 167.89 | 167.90 | 63.1 | 61.96 | 1.30 | 1.42 | 7.74 | 7.66 |
| 3.05 | 2.91 | 2.49 | 2.35 | 0.94 | 0.86 | 168.11 | 167.90 | 63.98 | 61.96 | 1.40 | 1.42 | 7.74 | 7.66 |
| 3.03 | 2.91 | 2.52 | 2.35 | 0.91 | 0.86 | 169.66 | 167.90 | 64.14 | 61.96 | 1.20 | 1.42 | 7.74 | 7.66 |
| 3.06 | 2.91 | 2.45 | 2.35 | 0.87 | 0.86 | 168.79 | 167.90 | 62.14 | 61.96 | 1.10 | 1.42 | 7.74 | 7.66 |
| Variables | Experimental Range | Predicated Range | R2 | MSE |
|---|---|---|---|---|
| Cellulase Y1 | 2.41–3.11 | 2.68–2.91 | 0.91 | 1.1641 |
| Y2 | 1.82–2.51 | 2.00–2.35 | 0.94 | 1.1622 |
| Y3 | 0.47–0.97 | 0.65–0.87 | 0.96 | 6.1344 |
| Y4 | 152.21–169.66 | 151.92–168.05 | 0.92 | 0.971 |
| Y5 | 57.72–64.14 | 57.72–64.14 | 0.94 | 2.734 |
| Y6 | 1.10–1.90 | 1.30–1.80 | 0.93 | 4.924 |
| Y7 | 7.24–7.88 | 7.62–7.72 | 0.96 | 0.229 |
| Xylanase Y8 | 2.84–3.62 | 3.00–3.32 | 0.91 | 0.677 |
| Y9 | 1.93–2.32 | 2.00–2.27 | 0.94 | 0.354 |
| Y10 | 0.58–1.24 | 0.69–1.16 | 0.93 | 0.005 |
| Y11 | 147.92–176.89 | 159.52–170.64 | 0.90 | 1.667 |
| Y12 | 57.45–61.78 | 57.41–60.66 | 0.95 | 2.871 |
| Y13 | 1.20–1.70 | 1.39–1.55 | 0.92 | 0.195 |
| Y14 | 7.29–8.16 | 7.51–8.02 | 0.97 | 0.2044 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kothakota, A.; Pandiselvam, R.; Siliveru, K.; Pandey, J.P.; Sagarika, N.; Srinivas, C.H.S.; Kumar, A.; Singh, A.; Prakash, S.D. Modeling and Optimization of Process Parameters for Nutritional Enhancement in Enzymatic Milled Rice by Multiple Linear Regression (MLR) and Artificial Neural Network (ANN). Foods 2021, 10, 2975. https://doi.org/10.3390/foods10122975
Kothakota A, Pandiselvam R, Siliveru K, Pandey JP, Sagarika N, Srinivas CHS, Kumar A, Singh A, Prakash SD. Modeling and Optimization of Process Parameters for Nutritional Enhancement in Enzymatic Milled Rice by Multiple Linear Regression (MLR) and Artificial Neural Network (ANN). Foods. 2021; 10(12):2975. https://doi.org/10.3390/foods10122975
Chicago/Turabian StyleKothakota, Anjineyulu, Ravi Pandiselvam, Kaliramesh Siliveru, Jai Prakash Pandey, Nukasani Sagarika, Chintada H. Sai Srinivas, Anil Kumar, Anupama Singh, and Shivaprasad D. Prakash. 2021. "Modeling and Optimization of Process Parameters for Nutritional Enhancement in Enzymatic Milled Rice by Multiple Linear Regression (MLR) and Artificial Neural Network (ANN)" Foods 10, no. 12: 2975. https://doi.org/10.3390/foods10122975
APA StyleKothakota, A., Pandiselvam, R., Siliveru, K., Pandey, J. P., Sagarika, N., Srinivas, C. H. S., Kumar, A., Singh, A., & Prakash, S. D. (2021). Modeling and Optimization of Process Parameters for Nutritional Enhancement in Enzymatic Milled Rice by Multiple Linear Regression (MLR) and Artificial Neural Network (ANN). Foods, 10(12), 2975. https://doi.org/10.3390/foods10122975







