Physical and Oxidative Stability of Low-Fat Fish Oil-in-Water Emulsions Stabilized with Black Soldier Fly (Hermetia illucens) Larvae Protein Concentrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Sample Preparation
2.2.1. Preparation of Defatted Insect Powder
2.2.2. Ohmic Heating Pre-Treatment
2.2.3. Ultrasound and Ohmic Heating Pre-Treatment
2.2.4. Alkaline Extraction of Protein
2.3. Protein Content
2.4. Protein Characterization
2.4.1. Differential Scanning Calorimetry (DSC)
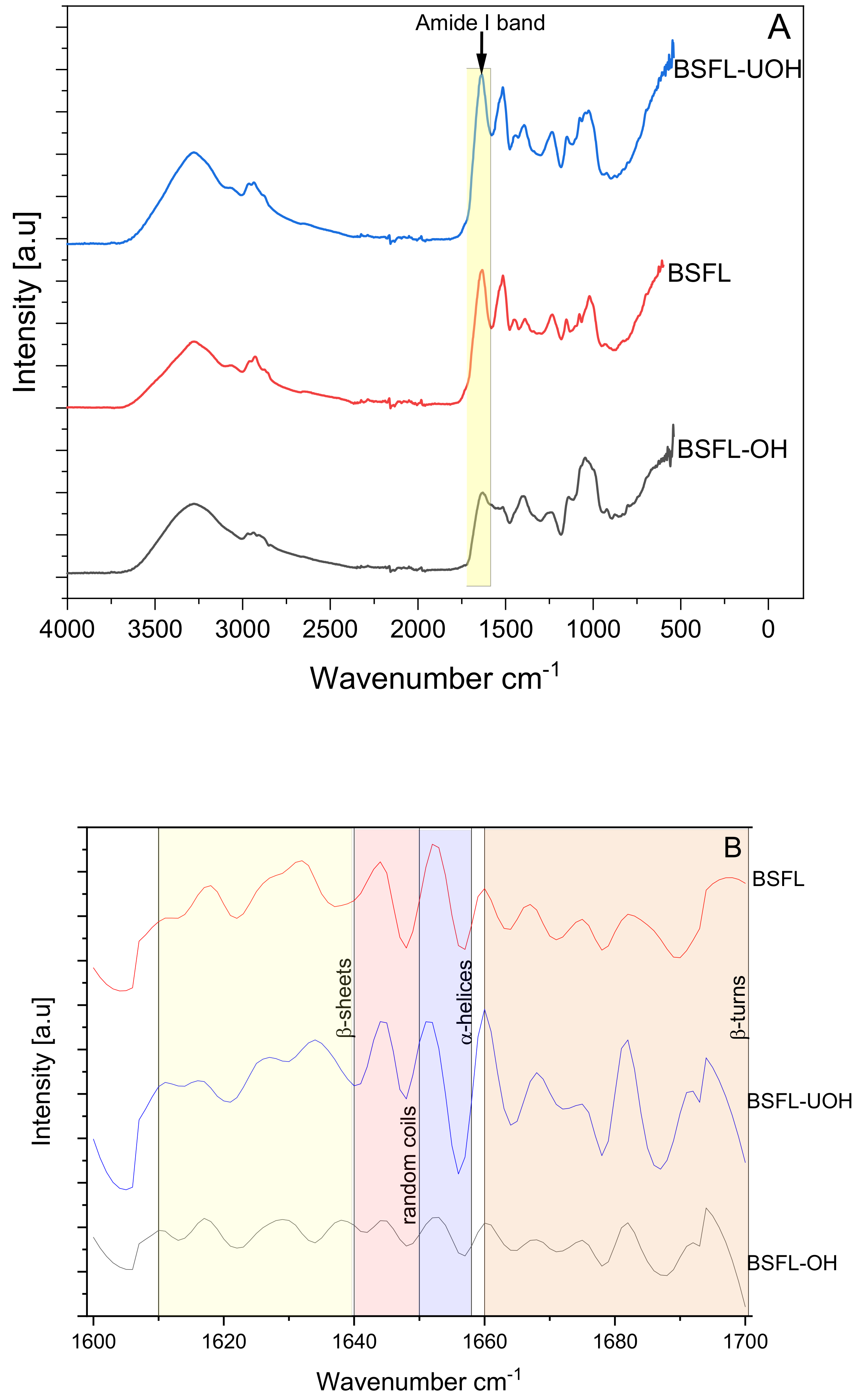
2.4.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.5. Interfacial Tension of BSFL Protein Concentrate at the Oil–Water Interface
2.6. Emulsion Preparation and Storage Experiment
2.7. Physical Characterization of Emulsions
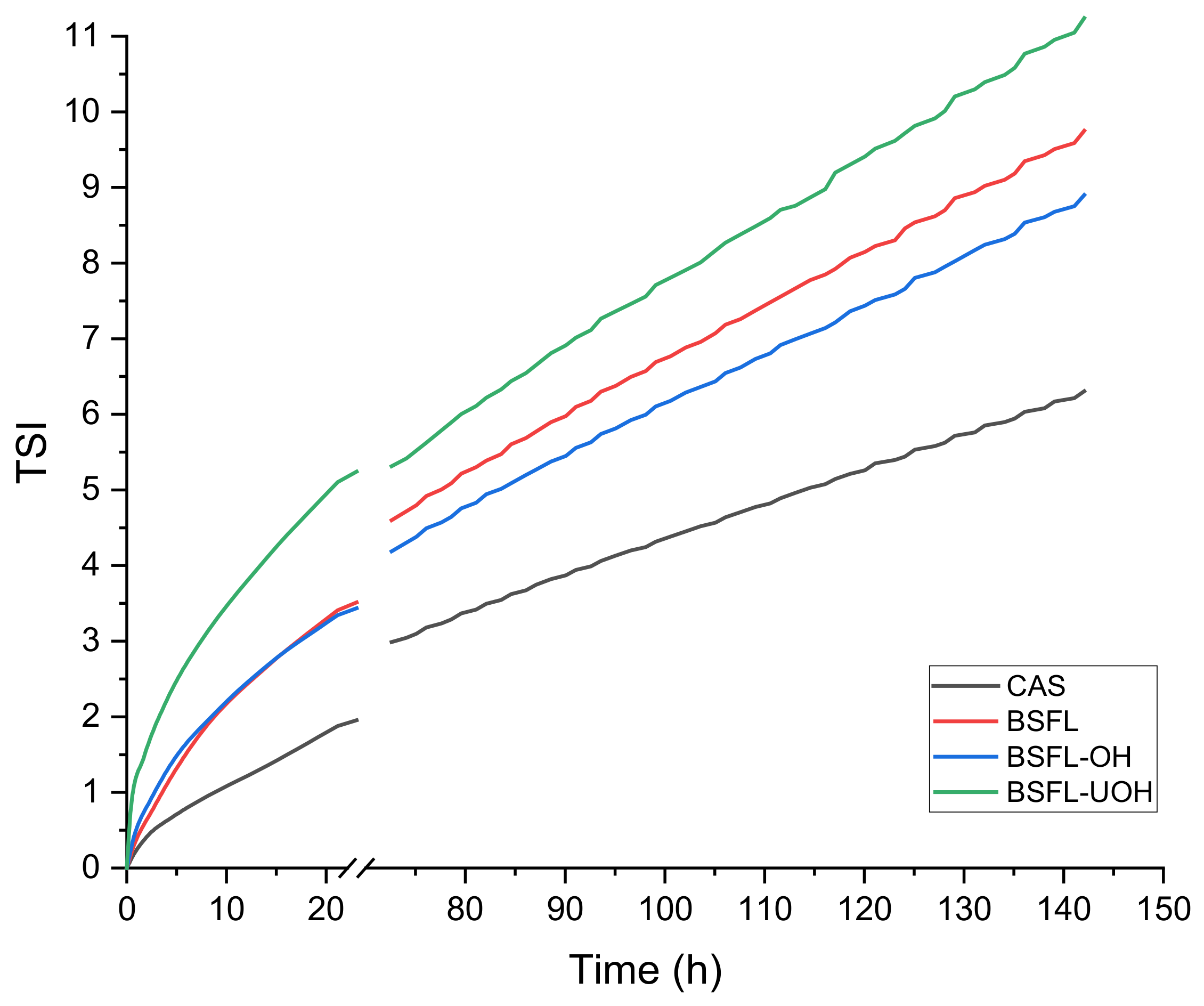
2.7.1. Turbiscan Analysis
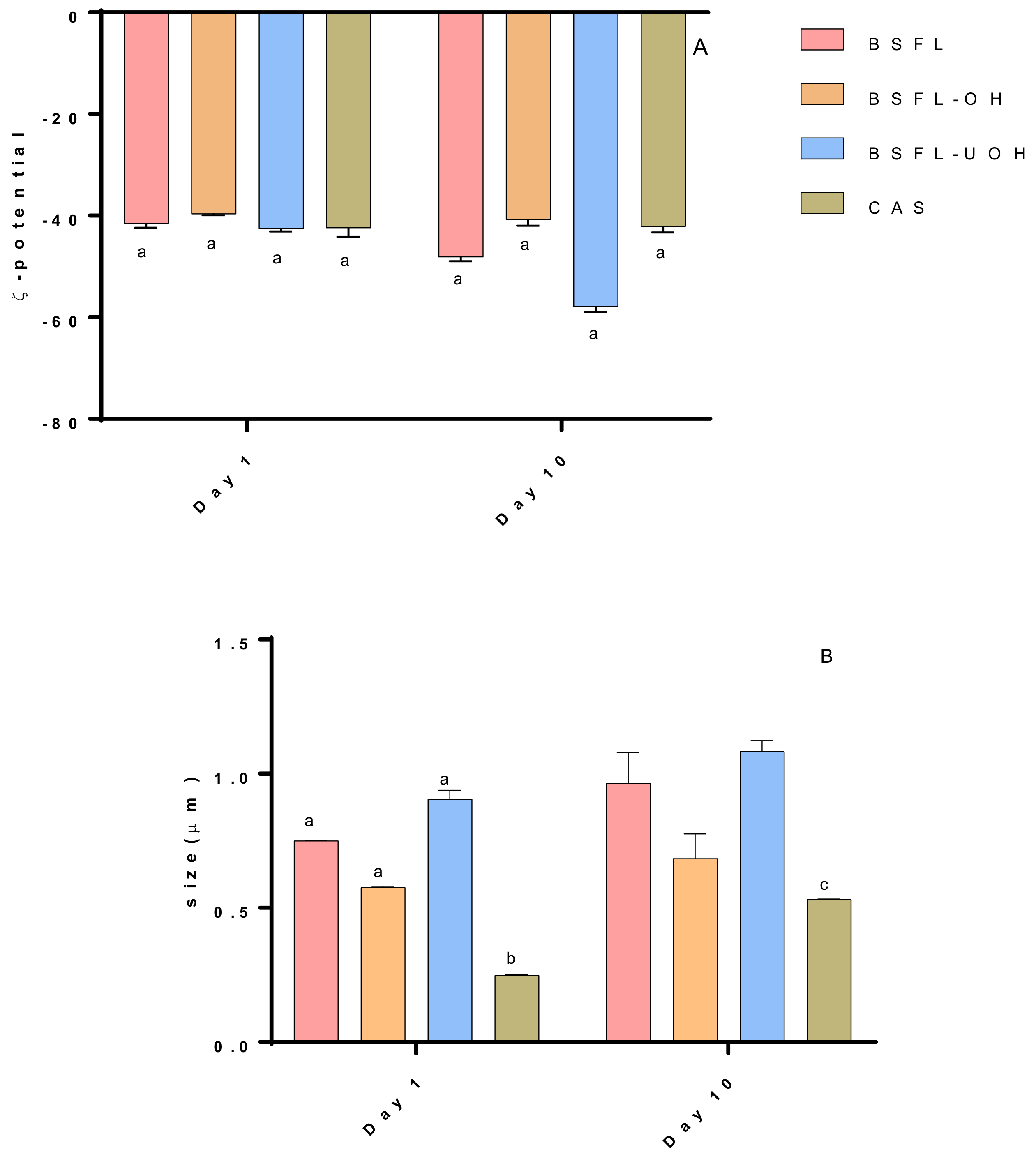
2.7.2. Hydrodynamic Diameter (Dh) and ζ-Potential
2.8. Oxidative Stability of Emulsions
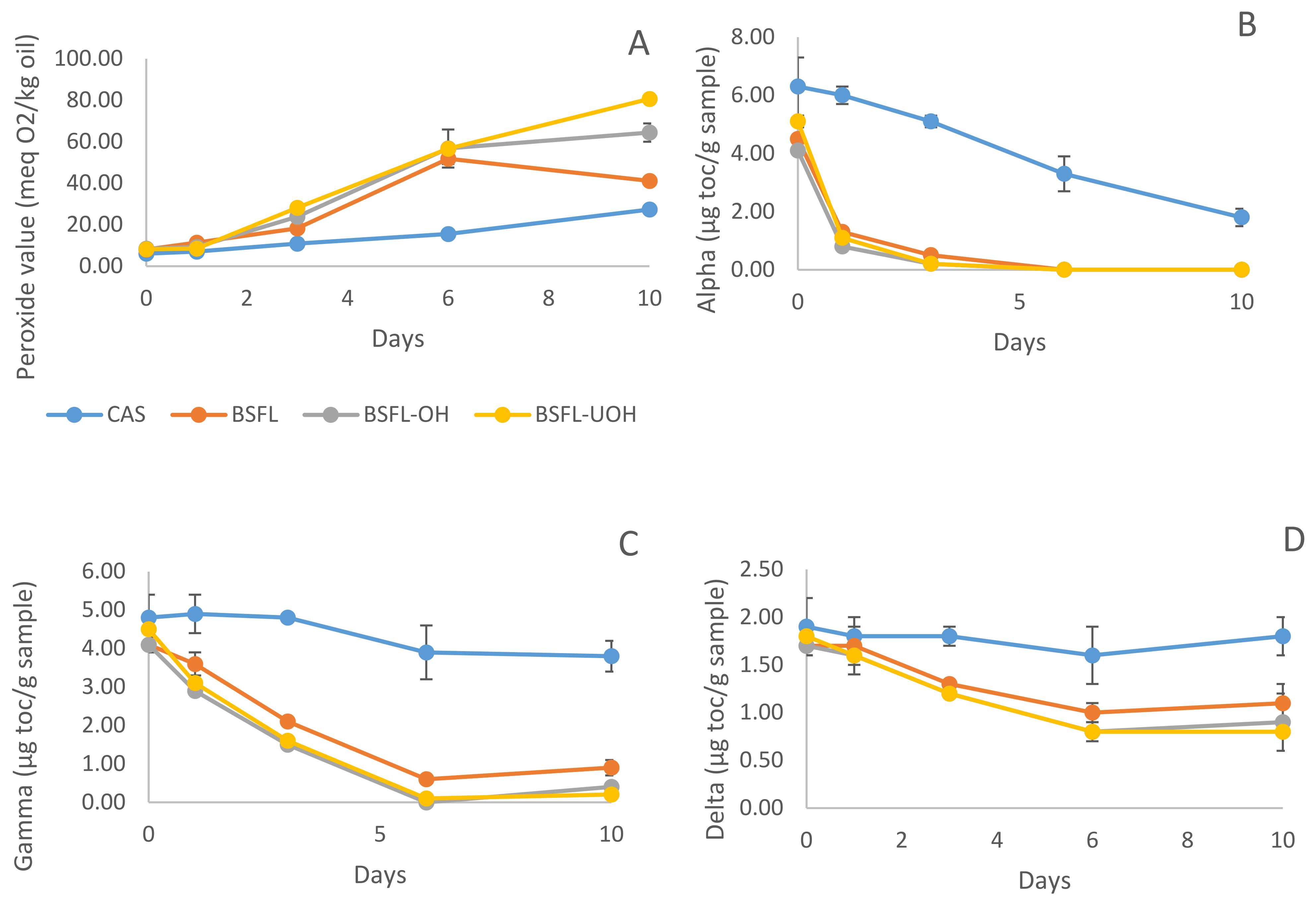
2.8.1. Primary Oxidation Products—Peroxide Value (PV)
2.8.2. Tocopherol Content—HPLC
2.8.3. Secondary Oxidation Products—Dynamic Head Space GC-MS
2.9. Statistical Analysis
3. Results and Discussion
3.1. Protein Content of BSFL before and after Pre-Treatments
3.2. Differential Scanning Calorimetry (DSC)
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.4. Emulsion Characterization
3.4.1. Interfacial Tension
3.4.2. Turbiscan Emulsion Stability Analysis
3.4.3. ζ-Potential and Hydrodynamic Diameter
3.5. Oxidative Stability of Emulsions
3.5.1. Primary Oxidation Products
3.5.2. Changes in Tocopherol Content—HPLC
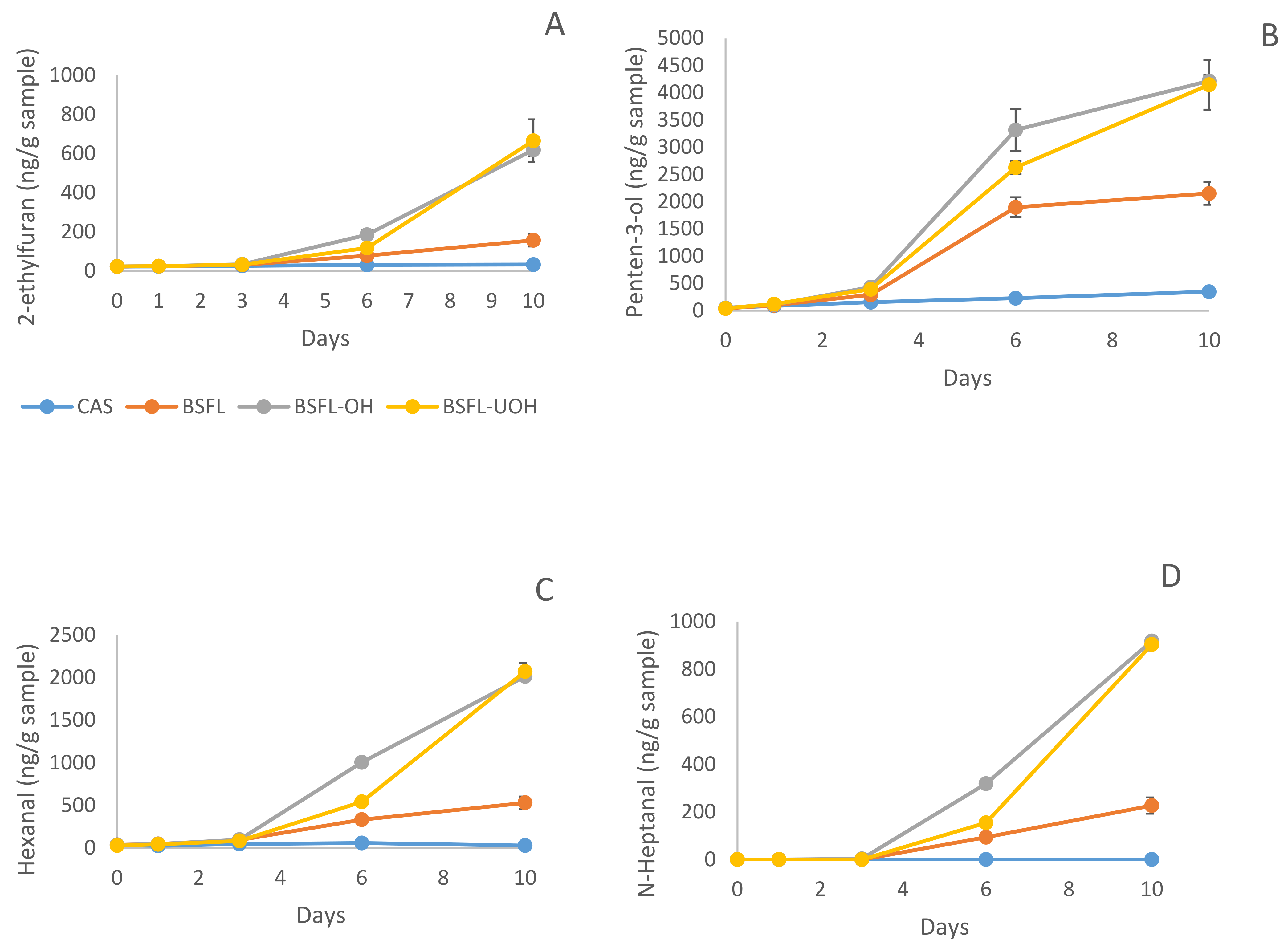
3.5.3. Secondary Volatile Oxidation Products—DHS GC-MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shibabaw, T. Omega-3 polyunsaturated fatty acids: Anti-inflammatory and anti-hypertriglyceridemia mechanisms in cardiovascular disease. Mol. Cell. Biochem. 2021, 476, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Decker, E.A.; Weiss, J. Emulsion-Based Delivery Systems for Lipophilic Bioactive Components. J. Food Sci. 2007, 72, R109–R124. [Google Scholar] [CrossRef] [PubMed]
- Yesiltas, B.; Sørensen, A.-D.M.; García-Moreno, P.J.; Anankanbil, S.; Guo, Z.; Jacobsen, C. Modified phosphatidylcholine with different alkyl chain length and covalently attached caffeic acid affects the physical and oxidative stability of omega-3 delivery 70% oil-in-water emulsions. Food Chem. 2019, 289, 490–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berton, C.; Ropers, M.-H.; Viau, M.; Genot, C. Contribution of the Interfacial Layer to the Protection of Emulsified Lipids against Oxidation. J. Agric. Food Chem. 2011, 59, 5052–5061. [Google Scholar] [CrossRef]
- McClements, D.J.; Bai, L.; Chung, C. Recent Advances in the Utilization of Natural Emulsifiers to Form and Stabilize Emulsions. Annu. Rev. Food Sci. Technol. 2017, 8, 205–236. [Google Scholar] [CrossRef]
- Queiroz, L.S.; Regnard, M.; Jessen, F.; Mohammadifar, M.A.; Sloth, J.J.; Petersen, H.O.; Ajalloueian, F.; Brouzes, C.M.C.; Fraihi, W.; Fallquist, H.; et al. Physico-chemical and colloidal properties of protein extracted from black soldier fly (Hermetia illucens) larvae. Int. J. Biol. Macromol. 2021, 186, 714–723. [Google Scholar] [CrossRef]
- Schösler, H.; de Boer, J.; Boersema, J.J. Can we cut out the meat of the dish? Constructing consumer-oriented pathways towards meat substitution. Appetite 2012, 58, 39–47. [Google Scholar] [CrossRef]
- Balzan, S.; Fasolato, L.; Maniero, S.; Novelli, E. Edible insects and young adults in a north-east Italian city an exploratory study. Br. Food J. 2016, 118, 318–326. [Google Scholar] [CrossRef]
- Mintah, B.K.; He, R.; Agyekum, A.A.; Dabbour, M.; Golly, M.K.; Ma, H. Edible insect protein for food applications: Extraction, composition, and functional properties. J. Food Process Eng. 2020, 43, e13362. [Google Scholar] [CrossRef]
- Moreira, T.C.P.; Pereira, R.N.; Vicente, A.A.; da Cunha, R.L. Effect of Ohmic heating on functionality of sodium caseinate—A relationship with protein gelation. Food Res. Int. 2019, 116, 628–636. [Google Scholar] [CrossRef] [Green Version]
- Xiang, S.; Zou, H.; Liu, Y.; Ruan, R. Effects of microwave heating on the protein structure, digestion properties and Maillard products of gluten. J. Food Sci. Technol. 2020, 57, 2139–2149. [Google Scholar] [CrossRef]
- Arzeni, C.; Martínez, K.; Zema, P.; Arias, A.; Pérez, O.E.; Pilosof, A.M.R. Comparative study of high intensity ultrasound effects on food proteins functionality. J. Food Eng. 2012, 108, 463–472. [Google Scholar] [CrossRef]
- Melchior, S.; Calligaris, S.; Bisson, G.; Manzocco, L. Understanding the impact of moderate-intensity pulsed electric fields (MIPEF) on structural and functional characteristics of pea, rice and gluten concentrates. Food Bioprocess Technol. 2020, 13, 2145–2155. [Google Scholar] [CrossRef]
- Alizadeh, O.; Aliakbarlu, J. Effects of ultrasound and ohmic heating pretreatments on hydrolysis, antioxidant and antibacterial activities of whey protein concentrate and its fractions. LWT 2020, 131, 109913. [Google Scholar] [CrossRef]
- Caruggi, N.; Lucisano, M.; Feyissa, A.H.; Rahimi Yazdi, S.; Mohammadifar, M.A. Effect of Ohmic Heating on the Formation and Texture of Acid Milk Gels. Food Biophys. 2019, 14, 249–259. [Google Scholar] [CrossRef]
- Pereira, R.N.; Souza, B.W.S.; Cerqueira, M.A.; Teixeira, J.A.; Vicente, A.A. Effects of Electric Fields on Protein Unfolding and Aggregation: Influence on Edible Films Formation. Biomacromolecules 2010, 11, 2912–2918. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, C.P.; Tiwari, B.K.; Bourke, P.; Cullen, P.J. Effect of ultrasonic processing on food enzymes of industrial importance. Trends Food Sci. Technol. 2010, 21, 358–367. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, J.; Murray, B.; Flynn, C.; Norton, I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocoll. 2016, 53, 141–154. [Google Scholar] [CrossRef] [Green Version]
- Choi, B.D.; Wong, N.A.; Auh, J.H. Defatting and Sonication Enhances Protein Extraction from Edible Insects. Korean J. Food Sci. Anim. Resour. 2017, 37, 955. [Google Scholar] [CrossRef] [PubMed]
- Vall-llosera, M.; Jessen, F.; Henriet, P.; Marie, R.; Jahromi, M.; Sloth, J.J.; Mohammadifar, M.A.; Petersen, H.O.; Jørgensen, B.M.; Casanova, F. Physical Stability and Interfacial Properties of Oil in Water Emulsion Stabilized with Pea Protein and Fish Skin Gelatin. Food Biophys. 2021, 16, 139–151. [Google Scholar] [CrossRef]
- Nazari, B.; Mohammadifar, M.A.; Shojaee-Aliabadi, S.; Feizollahi, E.; Mirmoghtadaie, L. Effect of ultrasound treatments on functional properties and structure of millet protein concentrate. Ultrason. Sonochem. 2018, 41, 382–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, R.H.; Vincken, J.-P.; van den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-Protein Conversion Factors for Three Edible Insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- Casanova, F.; Mohammadifar, M.A.; Jahromi, M.; Petersen, H.O.; Sloth, J.J.; Eybye, K.L.; Kobbelgaard, S.; Jakobsen, G.; Jessen, F. Physico-chemical, structural and techno-functional properties of gelatin from saithe (Pollachius virens) skin. Int. J. Biol. Macromol. 2020, 156, 918–927. [Google Scholar] [CrossRef]
- Mahdi Jafari, S.; He, Y.; Bhandari, B. Nano-Emulsion Production by Sonication and Microfluidization—A Comparison. Int. J. Food Prop. 2006, 9, 475–485. [Google Scholar] [CrossRef]
- Horn, A.F.; Nielsen, N.S.; Andersen, U.; Søgaard, L.H.; Horsewell, A.; Jacobsen, C. Oxidative stability of 70% fish oil-in-water emulsions: Impact of emulsifiers and pH. Eur. J. Lipid Sci. Technol. 2011, 113, 1243–1257. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Pocklington, W.D.; Dieffenbacher, A. Determination of tocopherols and tocotrienols in vegetable oils and fats by high performance liquid chromatography: Results of a collaborative study and the standardised method. Pure Appl. Chem. 1988, 60, 877–892. [Google Scholar] [CrossRef]
- García Moreno, P.J.; Guadix, A.; Guadix, E.M.; Jacobsen, C. Physical and Oxidative Stability of Fish Oil-In-Water Emulsions Stabilized with Fish Protein Hydrolysates. Food Chem. 2016, 203, 124–135. [Google Scholar] [CrossRef] [Green Version]
- LaClair, C.E.; Etzel, M.R. Turbidity and Protein Aggregation in Whey Protein Beverages. J. Food Sci. 2009, 74, C526–C535. [Google Scholar] [CrossRef]
- Bak, P.; Krinsky, S.; Mukamel, D. First-Order Transitions, Symmetry, and the ε Expansion. Phys. Rev. Lett. 1976, 36, 52–55. [Google Scholar] [CrossRef]
- Aouzelleg, A.; Bull, L.-A. Differential scanning calorimetry study of pressure/temperature processed β-lactoglobulin: The effect of dextran sulphate. Food Res. Int. 2004, 37, 933–940. [Google Scholar] [CrossRef]
- Chandrapala, J.; Zisu, B.; Palmer, M.; Kentish, S.; Ashokkumar, M. Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrason. Sonochem. 2011, 18, 951–957. [Google Scholar] [CrossRef]
- Qian, J.-Y.; Ma, L.-J.; Wang, L.-J.; Jiang, W. Effect of pulsed electric field on structural properties of protein in solid state. LWT 2016, 74, 331–337. [Google Scholar] [CrossRef]
- Tinoco, A.; Rodrigues, R.M.; Machado, R.; Pereira, R.N.; Cavaco-Paulo, A.; Ribeiro, A. Ohmic heating as an innovative approach for the production of keratin films. Int. J. Biol. Macromol. 2020, 150, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Jousse, M.; Jayakumar, J.; Fernández-Arteaga, A.; de Lamo-Castellví, S.; Ferrando, M.; Güell, C. Black Soldier Fly (Hermetia illucens) Protein Concentrates as a Sustainable Source to Stabilize O/W Emulsions Produced by a Low-Energy High-Throughput Emulsification Technology. Foods 2021, 10, 1048. [Google Scholar] [CrossRef] [PubMed]
- Shevkani, K.; Singh, N.; Chen, Y.; Kaur, A.; Yu, L. Pulse proteins: Secondary structure, functionality and applications. J. Food Sci. Technol. 2019, 56, 2787–2798. [Google Scholar] [CrossRef] [PubMed]
- Waśko, A.; Bulak, P.; Polak-Berecka, M.; Nowak, K.; Polakowski, C.; Bieganowski, A. The first report of the physicochemical structure of chitin isolated from Hermetia illucens. Int. J. Biol. Macromol. 2016, 92, 316–320. [Google Scholar] [CrossRef]
- Soetemans, L.; Uyttebroek, M.; Bastiaens, L. Characteristics of chitin extracted from black soldier fly in different life stages. Int. J. Biol. Macromol. 2020, 165, 3206–3214. [Google Scholar] [CrossRef]
- Moberger, L.; Larsson, K. Fat oxidation analysis using a wilhelky surface balance. J. Dispers. Sci. Technol. 1985, 6, 383–389. [Google Scholar] [CrossRef]
- Rodrigues, R.M.; Martins, A.J.; Ramos, O.L.; Malcata, F.X.; Teixeira, J.A.; Vicente, A.A.; Pereira, R.N. Influence of moderate electric fields on gelation of whey protein isolate. Food Hydrocoll. 2015, 43, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Gülseren, İ.; Güzey, D.; Bruce, B.D.; Weiss, J. Structural and functional changes in ultrasonicated bovine serum albumin solutions. Ultrason. Sonochem. 2007, 14, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ye, C.; Tian, Y.; Pan, S.; Wang, L. Effect of ohmic heating on fundamental properties of protein in soybean milk. J. Food Process Eng. 2018, 41, e12660. [Google Scholar] [CrossRef]
- Batish, I.; Brits, D.; Valencia, P.; Miyai, C.; Rafeeq, S.; Xu, Y.; Galanopoulos, M.; Sismour, E.; Ovissipour, R. Effects of Enzymatic Hydrolysis on the Functional Properties, Antioxidant Activity and Protein Structure of Black Soldier Fly (Hermetia illucens) Protein. Insects 2020, 11, 876. [Google Scholar] [CrossRef]
- Barba, A.A.; Bochicchio, S.; Dalmoro, A.; Caccavo, D.; Cascone, S.; Lamberti, G. Polymeric and lipid-based systems for controlled drug release: An engineering point of view. In Nanomaterials for Drug Delivery and Therapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 267–304. [Google Scholar]
- Clayfield, E.J.; Dixon, A.G.; Foulds, A.W.; Miller, R.J.L. The coalescence of secondary dispersions. J. Colloid Interface Sci. 1985, 104, 512–519. [Google Scholar] [CrossRef]
- Subaşı, B.G.; Casanova, F.; Capanoglu, E.; Ajalloueian, F.; Sloth, J.J.; Mohammadifar, M.A. Protein extracts from de-oiled sunflower cake: Structural, physico-chemical and functional properties after removal of phenolics. Food Biosci. 2020, 38, 100749. [Google Scholar] [CrossRef]
- Yesiltas, B.; García-Moreno, P.J.; Sørensen, A.-D.M.; Soria Caindec, A.M.; Hyldig, G.; Anankanbil, S.; Guo, Z.; Jacobsen, C. Enrichment of mayonnaise with a high fat fish oil-in-water emulsion stabilized with modified DATEM C14 enhances oxidative stability. Food Chem. 2021, 341, 128141. [Google Scholar] [CrossRef]
- Tamnak, S.; Mirhosseini, H.; Tan, C.P.; Ghazali, H.M.; Muhammad, K. Physicochemical properties, rheological behavior and morphology of pectin-pea protein isolate mixtures and conjugates in aqueous system and oil in water emulsion. Food Hydrocoll. 2016, 56, 405–416. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.; Qi, B.; Zhang, M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E. Interfacial Antioxidants: A Review of Natural and Synthetic Emulsifiers and Coemulsifiers That Can Inhibit Lipid Oxidation. J. Agric. Food Chem. 2018, 66, 20–35. [Google Scholar] [CrossRef]
- Hu, M.; McClements, D.J.; Decker, E.A. Lipid Oxidation in Corn Oil-in-Water Emulsions Stabilized by Casein, Whey Protein Isolate, and Soy Protein Isolate. J. Agric. Food Chem. 2003, 51, 1696–1700. [Google Scholar] [CrossRef]
- Kellerby, S.S.; McClements, D.J.; Decker, E.A. Role of Proteins in Oil-in-Water Emulsions on the Stability of Lipid Hydroperoxides. J. Agric. Food Chem. 2006, 54, 7879–7884. [Google Scholar] [CrossRef] [PubMed]
- Mouithys-Mickalad, A.; Schmitt, E.; Dalim, M.; Franck, T.; Tome, N.M.; van Spankeren, M.; Serteyn, D.; Paul, A. Black Soldier Fly (Hermetia illucens) Larvae Protein Derivatives: Potential to Promote Animal Health. Animals 2020, 10, 941. [Google Scholar] [CrossRef]
- Gardner, H.W.; Kleiman, R.; Weisleder, D. Homolytic decomposition of linoleic acid hydroperoxide: Identification of fatty acid products. Lipids 1974, 9, 696–706. [Google Scholar] [CrossRef]
- Berton-Carabin, C.C.; Ropers, M.-H.; Genot, C. Lipid Oxidation in Oil-in-Water Emulsions: Involvement of the Interfacial Layer. Compr. Rev. Food Sci. Food Saf. 2014, 13, 945–977. [Google Scholar] [CrossRef]
- Genot, C.; Meynier, A.; Riaublanc, A. Lipid Oxidation in Emulsions. In Lipid Oxidation Pathways; Kamal-Eldin, A., Ed.; Taylor & Francis: Abingdon, UK, 2003. [Google Scholar]
- Díaz, M.; Dunn, C.M.; McClements, D.J.; Decker, E.A. Use of Caseinophosphopeptides as Natural Antioxidants in Oil-in-Water Emulsions. J. Agric. Food Chem. 2003, 51, 2365–2370. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queiroz, L.S.; Casanova, F.; Feyissa, A.H.; Jessen, F.; Ajalloueian, F.; Perrone, I.T.; de Carvalho, A.F.; Mohammadifar, M.A.; Jacobsen, C.; Yesiltas, B. Physical and Oxidative Stability of Low-Fat Fish Oil-in-Water Emulsions Stabilized with Black Soldier Fly (Hermetia illucens) Larvae Protein Concentrate. Foods 2021, 10, 2977. https://doi.org/10.3390/foods10122977
Queiroz LS, Casanova F, Feyissa AH, Jessen F, Ajalloueian F, Perrone IT, de Carvalho AF, Mohammadifar MA, Jacobsen C, Yesiltas B. Physical and Oxidative Stability of Low-Fat Fish Oil-in-Water Emulsions Stabilized with Black Soldier Fly (Hermetia illucens) Larvae Protein Concentrate. Foods. 2021; 10(12):2977. https://doi.org/10.3390/foods10122977
Chicago/Turabian StyleQueiroz, Lucas Sales, Federico Casanova, Aberham Hailu Feyissa, Flemming Jessen, Fatemeh Ajalloueian, Italo Tuler Perrone, Antonio Fernandes de Carvalho, Mohammad Amin Mohammadifar, Charlotte Jacobsen, and Betül Yesiltas. 2021. "Physical and Oxidative Stability of Low-Fat Fish Oil-in-Water Emulsions Stabilized with Black Soldier Fly (Hermetia illucens) Larvae Protein Concentrate" Foods 10, no. 12: 2977. https://doi.org/10.3390/foods10122977
APA StyleQueiroz, L. S., Casanova, F., Feyissa, A. H., Jessen, F., Ajalloueian, F., Perrone, I. T., de Carvalho, A. F., Mohammadifar, M. A., Jacobsen, C., & Yesiltas, B. (2021). Physical and Oxidative Stability of Low-Fat Fish Oil-in-Water Emulsions Stabilized with Black Soldier Fly (Hermetia illucens) Larvae Protein Concentrate. Foods, 10(12), 2977. https://doi.org/10.3390/foods10122977









