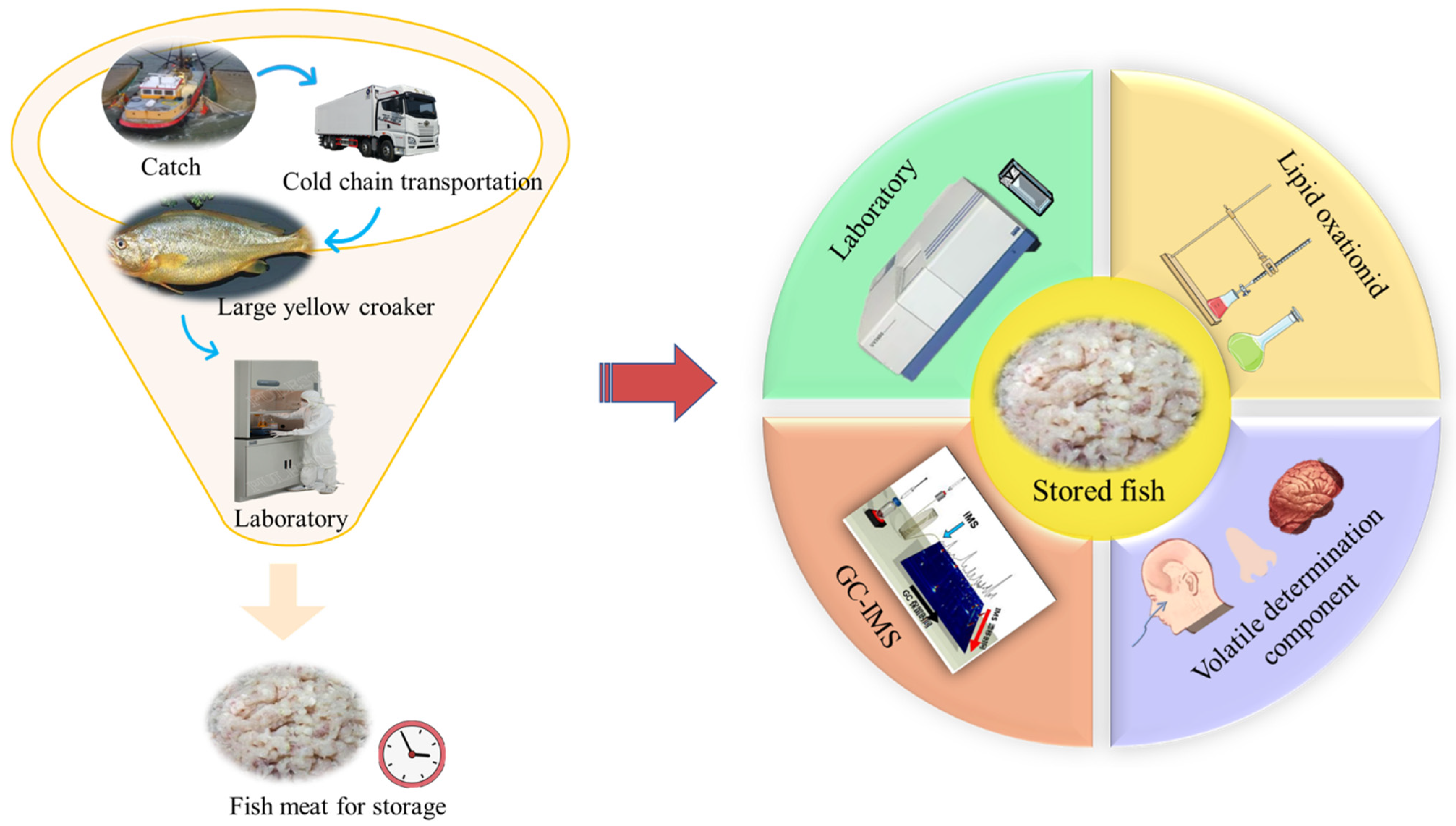
Changes of Volatile Flavor Compounds in Large Yellow Croaker (Larimichthys crocea) during Storage, as Evaluated by Headspace Gas Chromatography–Ion Mobility Spectrometry and Principal Component Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Samples
2.2. Reagents and Chemicals
2.3. Sample Processing
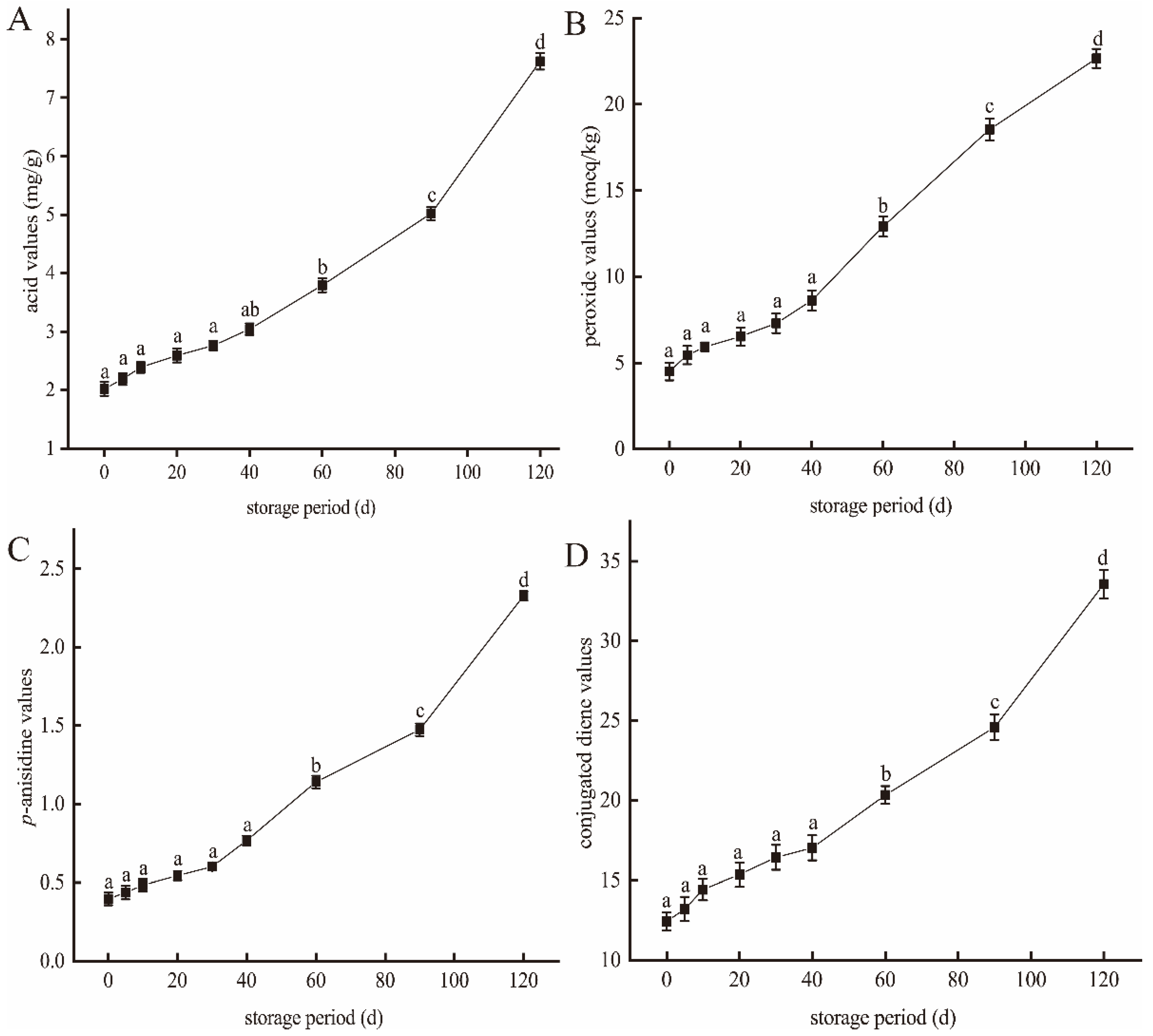
2.4. Determination of the Lipid Profiles
2.4.1. Acid Values
2.4.2. Peroxide Values
2.4.3. p-Anisidine Values
2.4.4. Conjugated Diene Values
2.5. Aroma Detection at Different Points during Storage
2.6. Volatile Compound Analysis at Different Points during Storage
2.6.1. Sample Processing
2.6.2. Headspace Gas Chromatography–Ion Mobility Spectrometry (GC-IMS)
2.7. Data Processing
3. Results and Analysis
3.1. Effect of the Storage Time on Lipid Oxidation in Large Yellow Croaker
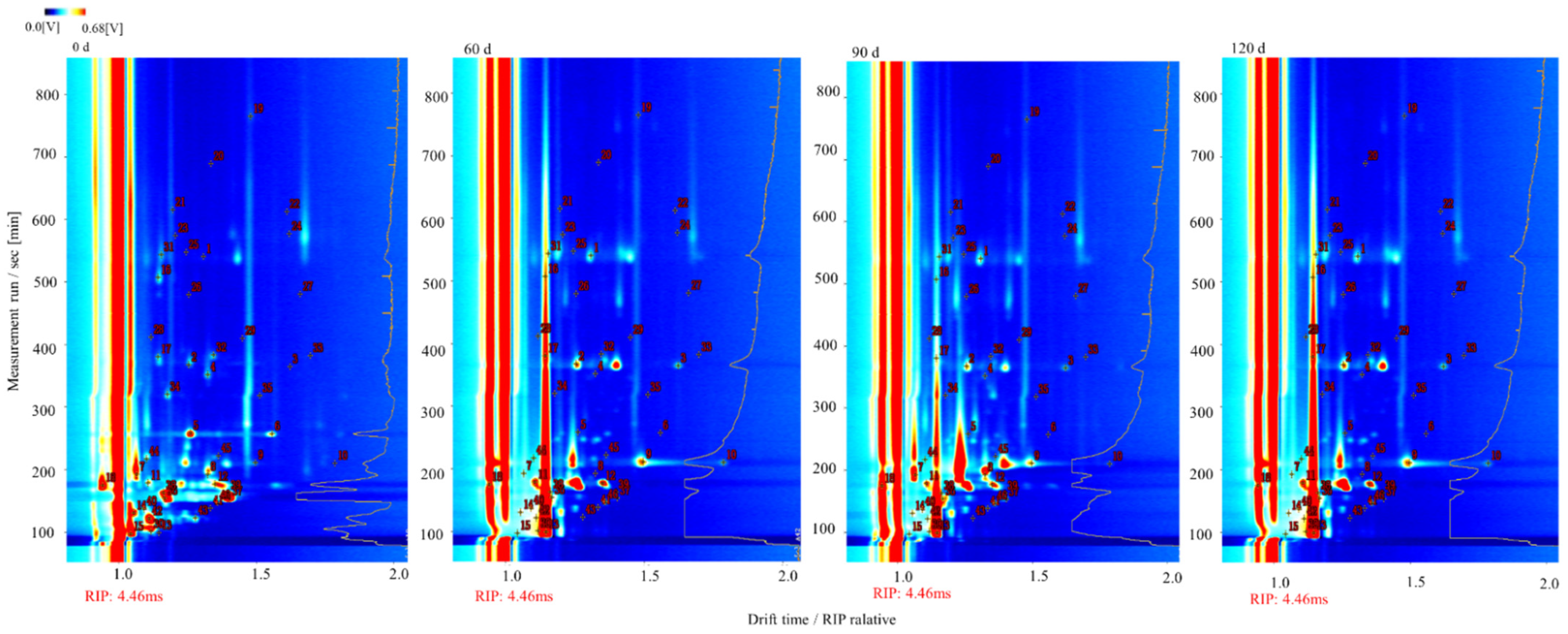
3.2. Identification of Volatile Compounds in Large Yellow Croaker during Storage
3.3. Topographic Map of the Volatile Components Present in Large Yellow Croaker at Different Storage Times
3.4. Variations in the Volatile Flavor Compounds Present in Large Yellow Croaker after Different Storage Periods
3.5. PCA of the Characteristic Flavor Compounds Present in Large Yellow Croaker
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AV | acid value |
| CDV | conjugated diene value |
| GC | gas chromatography |
| HS | headspace |
| IMS | ion mobility spectrometry |
| MS | mass spectrometry |
| p-AV | p-anisidine value |
| POV | peroxidation value |
| PCA | principal component analysis |
| RIP | reaction ion peak |
| SPME | solid-phase microextraction |
References
- Xuan, M.; Jun, M.; Jing, X. Effects of multi-frequency ultrasound on the freezing rates, quality properties and structural characteristics of cultured large yellow croaker (Larimichthys crocea). Ultrason Sonochem. 2021, 76, 105657–105669. [Google Scholar] [CrossRef]
- Wang, S.N.; Das, A.K.; Pang, J.; Liang, P. Artificial Intelligence Empowered Multispectral Vision Based System for Non-Contact Monitoring of Large Yellow Croaker (Larimichthys crocea) Fillets. Foods 2021, 10, 1161. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, W.; Shan, X.; Chen, J.; Weng, H.; Yang, T.; Wang, H. Growth characteristics of cage-cultured large yellow croaker Larimichthys crocea. Aquacult. Rep. 2020, 16, 100242–100248. [Google Scholar] [CrossRef]
- Du, Y.N.; Xue, S.; Han, J.R.; Yan, J.N.; Shang, W.H.; Hong, J.N.; Wu, H.T. Simultaneous extraction by acidic and saline solutions and characteristics of the lipids and proteins from large yellow croaker (Pseudosciaena crocea) roes. Food Chem. 2020, 310, 125928–125938. [Google Scholar] [CrossRef] [PubMed]
- Grazina, L.; Rodrigues, P.J.; Nunes, M.A.; Mafra, I.; Arlorio, M.; Oliveira, M.; Beatriz, P.P.; Amaral, J.S. Machine Learning Approaches Applied to GC-FID Fatty Acid Profiles to Discriminate Wild from Farmed Salmon. Foods 2020, 9, 1622. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Jiawen, D.; Jiwen, Z.; Hehe, L.; Jinyuan, S.; Mingquan, H.; Baoguo, S. Different distillation stages Baijiu classification by temperature-programmed headspace-gas chromatography-ion mobility spectrometry and gas chromatography-olfactometry-mass spectrometry combined with chemometric strategies. Food Chem. 2021, 365, 130430–130439. [Google Scholar] [CrossRef]
- Fang, W.; Yaqin, G.; Hongbo, W.; Bin, X.; Xiaona, H.; Xiaoling, Y.; Weihong, L. Analysis of volatile compounds and flavor fingerprint in Jingyuan lamb of different ages using gas chromatography-ion mobility spectrometry (GC-IMS). Meat Sci. 2021, 175, 108449–108458. [Google Scholar] [CrossRef]
- Mingjie, C.; Tong, C.; Xingpu, Q.; Daoli, L.; Bin, C. Analyzing changes of volatile components in dried pork slice by gas chromatography-ion mobility spectroscopy. CyTA-J. Food. 2020, 18, 328–335. [Google Scholar] [CrossRef]
- Yi, C.; Pao, L.; Luyan, L.; Yeyou, Q.; Liwen, J.; Yang, L. Characteristic fingerprints and volatile flavor compound variations in Liuyang Douchi during fermentation via HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2021, 361, 13005–13015. [Google Scholar] [CrossRef]
- del Mar Contreras, M.; Jurado, N.; Arce, L.; Arroyo, N. A robustness study of calibration models for olive oil classification: Targeted and non-targeted fingerprint approaches based on GC-IMS. Food Chem. 2019, 288, 315–324. [Google Scholar] [CrossRef]
- Juan, Y.; Siliang, W.; Ruijie, M.; Li, L.; Wenhong, Z.; Weidong, B. Formation of amino acid-derived volatile compounds in dry-cured mackerel (Scomberomorus niphonius): Metabolic pathways involving microorganisms, precursors, and intermediates. Food Chem. 2021, 364, 130163–130173. [Google Scholar] [CrossRef]
- Siliang, W.; Juan, Y.; Hao, D.; Qiaoyu, L.; Xiangluan, L.; Xiaofang, Z.; Weidong, B. Key aroma compounds of Chinese dry-cured Spanish mackerel (Scomberomorus niphonius) and their potential metabolic mechanisms. Food Chem. 2021, 342, 128381–128391. [Google Scholar] [CrossRef]
- Siyang, D.; Yunhe, L.; Feng, H.; Jiqian, L.; Dong, H.; Chunhui, Z.; Christophe, B. Evaluation of volatile flavor compounds in bacon made by different pig breeds during storage time. Food Chem. 2021, 357, 29765–129775. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography–ion mobility spectrometry (GC–IMS). Food Chem. 2020, 315, 126158–126168. [Google Scholar] [CrossRef]
- Aheto, J.H.; Huang, X.; Tian, X.; Lv, R.; Dai, C.; Bonah, E.; Chang, X. Evaluation of lipid oxidation and volatile compounds of traditional dry-cured pork belly: The hyperspectral imaging and multi-gas-sensory approaches. J. Food Process Eng. 2020, 43, 13092–13101. [Google Scholar] [CrossRef]
- Kunyaboon, S.; Thumanu, K.; Park, J.W.; Khongla, C.; Yongsawatdigul, J. Evaluation of Lipid Oxidation, Volatile Compounds and Vibrational Spectroscopy of Silver Carp (Hypophthalmichthys molitrix) during Ice Storage as Related to the Quality of Its Washed Mince. Foods 2021, 10, 495. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.K.; Zór, K.; Kostesha, N.; Alstrøm, T.S.; Heiskanen, A.; ElTanahi, H.; Sharoba, A.; Papkovsky, D.; Larsen, J.; Khalaf, H.; et al. Development and validation of a colorimetric sensor array for fish spoilage monitoring. Food Cont. 2016, 60, 346–352. [Google Scholar] [CrossRef]
- Khan, I.T.; Nadeem, M.; Imran, M.; Khalique, A. Impact of post fermentation cooling patterns on fatty acid profile, lipid oxidation and antioxidant features of cow and buffalo milk set yoghurt. Lipids Health Dis. 2020, 19, 62–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Liu, G.; Cheng, W.; Liu, X.; Brennan, C.; Brennan, M.A.; Liu, H.; Wang, Q. The effect of heating on the formation of 4-hydroxy-2-hexenal and 4-hydroxy-2-nonenal in unsaturated vegetable oils: Evaluation of oxidation indicators. Food Chem. 2020, 321, 126603–126613. [Google Scholar] [CrossRef]
- Emre, Y. Determination of fish quality parameters with low costs electronic nose. Food Biosci. 2021, 41, 100948. [Google Scholar] [CrossRef]
- Tu, C.H.; Qi, X.E.; Shui, S.S.; Lin, H.M.; Benjakul, S.; Zhang, B. Investigation of the changes in lipid profiles induced by hydroxyl radicals in whiteleg shrimp (Litopenaeus vannamei) muscle using LC/MS-based lipidomics analysis. Food Chem. 2021, 369, 130925–130934. [Google Scholar] [CrossRef]
- Elavarasan, K.; Shamasundar, B.A. Antioxidant properties of papain mediated protein hydrolysates from fresh water carps (Catla catla, Labeo rohita and Cirrhinus mrigala) and its application on inhibition of lipid oxidation in oil sardine mince during ice storage. J. Food Sci. Technol. 2021, 1–10. [Google Scholar] [CrossRef]
- Thimmappa, M.H.; Reddy, A.M.; Prabhu, R.M.; Elavarasan, K. Quality Changes in Deep-Sea Shrimp (Aristeus alcocki) During Ice Storage: Biochemical and Organoleptic Changes. Aquacult. Rep. 2019, 8, 497–504. [Google Scholar] [CrossRef]
- Lin, H.X.; Yang, Q.H.; Wang, A.J.; Wang, J.X.; Tan, B.P.; Ray, G.W.; Dong, X.H.; Chi, S.Y.; Liu, H.Y.; Zhang, S. Effects of fish meal under different storage conditions on growth, serum biochemical indices and antioxidant capacity for juvenile grouper Epinephelus coioides. Aquacult. Nutr. 2021, 27, 723–733. [Google Scholar] [CrossRef]
- Phung, A.S.; Bannenberg, G.; Vigor, C.; Reversat, G.; Oger, C.; Roumain, M.; Galano, J.M.; Durand, T.; Muccioli, G.G.; Ismail, A.; et al. Chemical Compositional Changes in Over-Oxidized Fish Oils. Foods 2020, 9, 1501. [Google Scholar] [CrossRef] [PubMed]
- Malik, I.A.; Elgasim, E.A.; Adiamo, O.Q.; Ali, A.A.; Mohamed, A.I.A. Effect of frozen storage on the biochemical composition of five commercial freshwater fish species from River Nile, Sudan. Food Sci. Nutr. 2021, 9, 3758–3767. [Google Scholar] [CrossRef] [PubMed]
- Crexi, V.T.; Monte, M.L.; de Souza Soares, L.A.; Pinto, L.A.A. Production and refinement of oil from carp (Cyprinus carpio) viscera. Food Chem. 2009, 119, 945–950. [Google Scholar] [CrossRef]
- Dian, G.Z.; Tao, Z.; Christer, H.; Han, M.Y.; Xiao, J.X.; Zhi, L. Oxidized fish oils increased lipid deposition via oxidative stress-mediated mitochondrial dysfunction and the CREB1-Bcl2-Beclin1 pathway in the liver tissues and hepatocytes of yellow catfish. Food Chem. 2021, 360, 129814–129822. [Google Scholar] [CrossRef]
- Sullivan, J.C.; Budge, S.M. Fish oil sensory properties can be predicted using key oxidative volatiles. Eur. J. Lipid Sci. Technol. 2012, 114, 496–503. [Google Scholar] [CrossRef]
- Ye, L.Y.; Harris, E.; Budge, S.M.; Sullivan, R.J. Flavors’ Decreasing Contribution to p-Anisidine Value over Shelf Life May Invalidate the Current Recommended Protocol for Flavored Fish Oils. J. Am. Oil Chem. Soc. 2020, 97, 1335–1341. [Google Scholar] [CrossRef]
- Iberahim, N.I.; Tan, B.C. Hexane-isopropanol Extraction and Quality Assessment of Omega-3 Fish Oil From Atlantic Salmon (Salmo salar). IOP Conf. Ser. Mater. Sci. Eng. 2020, 932, 012038–012048. [Google Scholar] [CrossRef]
- Jia, S.; Li, Y.; Zhuang, S.; Sun, X.; Zhang, L.; Shi, J.; Hong, H.; Luo, Y. Biochemical changes induced by dominant bacteria in chill-stored silver carp (Hypophthalmichthys molitrix) and GC-IMS identification of volatile organic compounds. Food Microbiol. 2019, 84, 103284–103293. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Songyi, L.; Ruichun, W.; Duo, G.; Zhi, J.B.; Dong, C.; Yue, T.; Na, S.; Simin, Z. Inhibitory effect and mechanism of various fruit extracts on the formation of heterocyclic aromatic amines and flavor changes in roast large yellow croaker (Pseudosciaena crocea). Food Cont. 2022, 131, 108410. [Google Scholar] [CrossRef]
- Sofia, S.A.; Miguel, R.A.L.; Relvas, J.B.; Estevez, P.M. Fatty acid role on obesity induced hypothalamus inflammation: From problem to solution—A review. Trends Food Sci. Technol. 2021, 112, 592–607. [Google Scholar] [CrossRef]
- Zhenkun, C.; Han, Y.; Tatiana, M.; Haizhen, M.; Hongbo, L.; Hao, Z. Changes in the volatile components of squid (Illex argentinus) for different cooking methods via headspace–gas chromatography–ion mobility spectrometry. Food Sci. Nutr. 2020, 8, 5748–5762. [Google Scholar] [CrossRef]
- Zelin, D.; Shuanglin, D.; Yunwei, D.; Qinfeng, G. Geographical origin identification of two salmonid species via flavor compound analysis using headspace-gas chromatography-ion mobility spectrometry combined with electronic nose and tongue. Food Res. Int. 2021, 145, 110385–110395. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, H.B.; Su, L.J.; Chen, X.N. Kappa-carrageenan oligosaccharides retard the progression of protein and lipid oxidation in mackerel (Scomber japonicus) fillets during frozen storage. RSC Adv. 2020, 10, 20827–20836. [Google Scholar] [CrossRef]
- Wensheng, Y.; Yingxuan, C.; Dengyong, L.; Zhinan, Z.; Zhenghao, Z.; Shuangyu, M.; Mingcheng, Z.; Hao, Z. Comparative analysis of characteristic volatile compounds in Chinese traditional smoked chicken (specialty poultry products) from different regions by headspace–gas chromatography−ion mobility spectrometry. Poult. Sci. 2020, 99, 7192–7201. [Google Scholar] [CrossRef]
- Hua, Q.; Gao, P.; Xu, Y.; Xia, W.; Sun, Y.; Jiang, Q. Effect of commercial starter cultures on the quality characteristics of fermented fish-chili paste. LWT-Food Sci. Technol. 2020, 122, 109016–109026. [Google Scholar] [CrossRef]
- Chen, Y.P.; Cai, D.; Li, W.; Blank, I.; Liu, Y. Application of gas chromatography-ion mobility spectrometry (GC-IMS) and ultrafast gas chromatography electronic-nose (uf-GC E-nose) to distinguish four Chinese freshwater fishes at both raw and cooked status. J. Food Biochem. 2021, 6, e13840–e13849. [Google Scholar] [CrossRef]
- Yu, L.; Wang, Y.; Wen, H.; Jiang, M.; Wu, F.; Tian, J. Synthesis and evaluation of acetylferulic paeonol ester and ferulic paeonol ester as potential antioxidants to inhibit fish oil oxidation. Food Chem. 2021, 365, 130384–130394. [Google Scholar] [CrossRef]
- Duan, Z.; Dong, S.; Sun, Y.; Dong, Y.; Gao, Q. Response of Atlantic salmon (Salmo salar) flavor to environmental salinity while culturing between freshwater and seawater. Aquaculture 2021, 530, 735953–735962. [Google Scholar] [CrossRef]
- Bartual, A.; Hernanz-Torrijos, M.; Sala, I.; Ortega, M.J.; González-García, C.; Bolado-Penagos, M.; López-Urrutia, A.; Romero-Martínez, L.; Lubián, L.M.; Bruno, M.; et al. Types and Distribution of Bioactive Polyunsaturated Aldehydes in a Gradient from Mesotrophic to Oligotrophic Waters in the Alborán Sea (Western Mediterranean). Mar. Drugs. 2020, 18, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Luo, J.; Lou, A.; Wang, Y.; Yang, D.; Shen, Q.W. Duck breast muscle proteins, free fatty acids and volatile compounds as affected by curing methods. Food Chem. 2021, 338, 128138–128148. [Google Scholar] [CrossRef]
- Huang, L.; Zeng, X.; Sun, Z.; Wu, A.; He, J.; Dang, Y.; Pan, D. Production of a safe cured meat with low residual nitrite using nitrite substitutes. Meat Sci. 2020, 1629, 108027–108036. [Google Scholar] [CrossRef]
- Qi, Z.; Yicheng, D.; Saiqi, G.; Shichen, Z.; Xuxia, Z.; Yuting, D. Identification of changes in volatile compounds in dry-cured fish during storage using HS-GC-IMS. Food Res. Int. 2020, 137, 109339–109349. [Google Scholar] [CrossRef]
- Tan, M.T.; Xie, J. Exploring the Effect of Dehydration on Water Migratng Property and Protein Changes of Large Yellow Croaker (Pseudosciaena crocea) during Frozen Storage. Foods 2021, 10, 784. [Google Scholar] [CrossRef]
- Nguyen, N.T.P.; Nguyen, L.V.H.; Tran, N.M.P.; Nguyen, D.T.; Nguyen, T.N.T.; Tran, H.A.; Dang, N.N.T.; Vo, T.V.; Nguyen, T.H. The effect of oxidation degree and volume ratio of components on properties and applications of in situ cross-linking hydrogels based on chitosan and hyaluronic acid. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109670–109679. [Google Scholar] [CrossRef]
- Peipei, D.; Xianchao, F.; Xingguang, C.; Qinhao, G.; Junlan, W.; Shan, Q.; Xinglian, X.; Guanghong, Z.; Niamat, U.; Beiwei, Z.; et al. Binding of aldehyde flavour compounds to beef myofibrillar proteins and the effect of nonenzymatic glycation with glucose and glucosamine. LWT-Food Sci. Technol. 2021, 144, 111198. [Google Scholar] [CrossRef]
- Yokouchi, C.; Nishimura, Y.; Goto, H.; Sato, M.; Hidoh, Y.; Takeuchi, K.; Ishii, Y. Reduction of fatty liver in rats by nicotinamide via the regeneration of the methionine cycle and the inhibition of aldehyde oxidase: Original Article. J. Toxicol. Sci. 2021, 46, 31–42. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, X.; Cong, P.; Liu, Y.; Wang, Y.; Xu, J.; Xue, C. Mechanism of Phospholipid Hydrolysis for Oyster Crassostrea plicatula Phospholipids During Storage Using Shotgun Lipidomics. Lipids 2017, 52, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Shi, C.; Wang, Y.; Yang, X.; Zhang, J.; Ji, Z. Nondestructive determination of salmon fillet freshness during storage at different temperatures by electronic nose system combined with radial basis function neural networks. Int. J. Food Sci. Technol. 2020, 55, 2080–2091. [Google Scholar] [CrossRef]
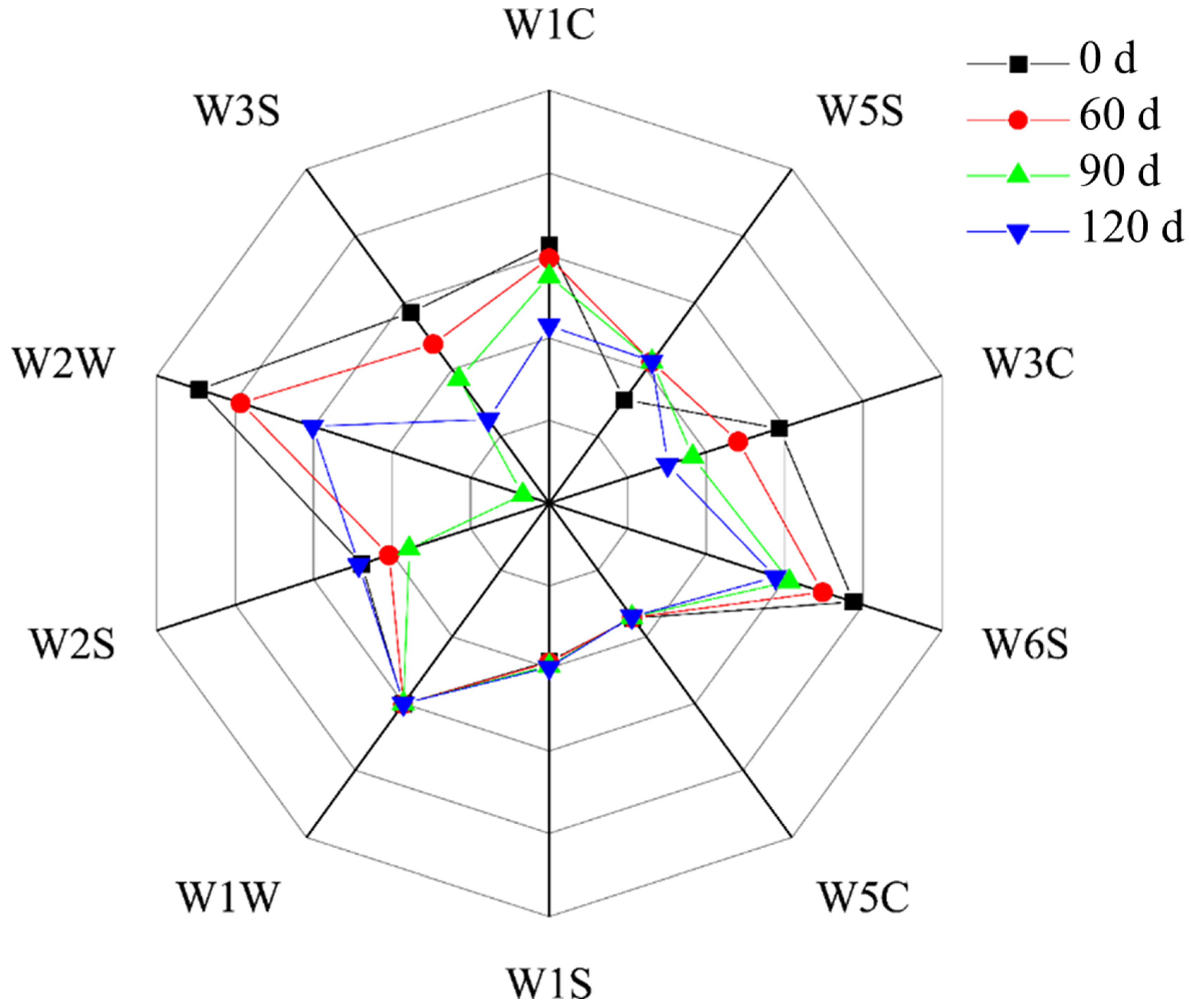
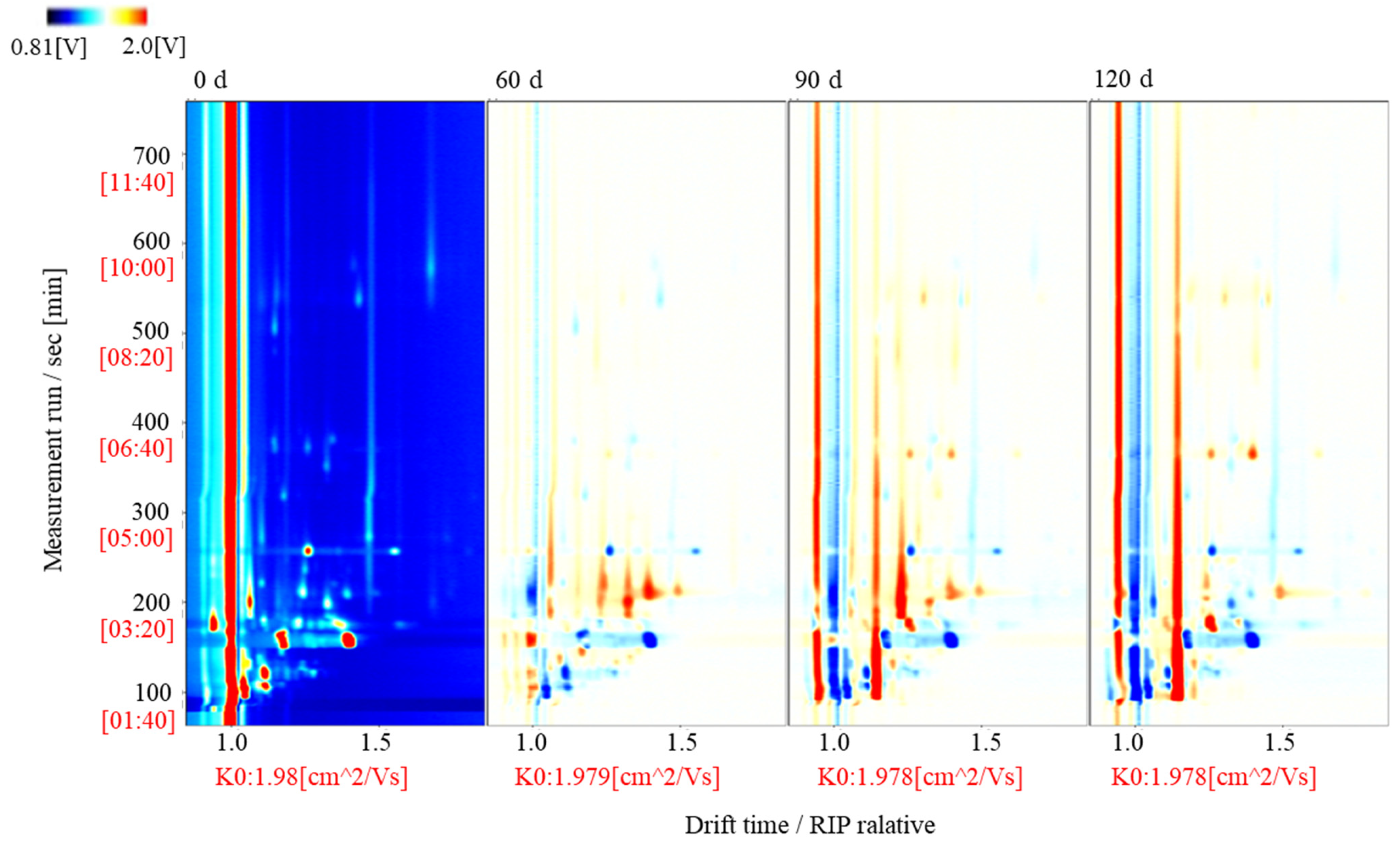
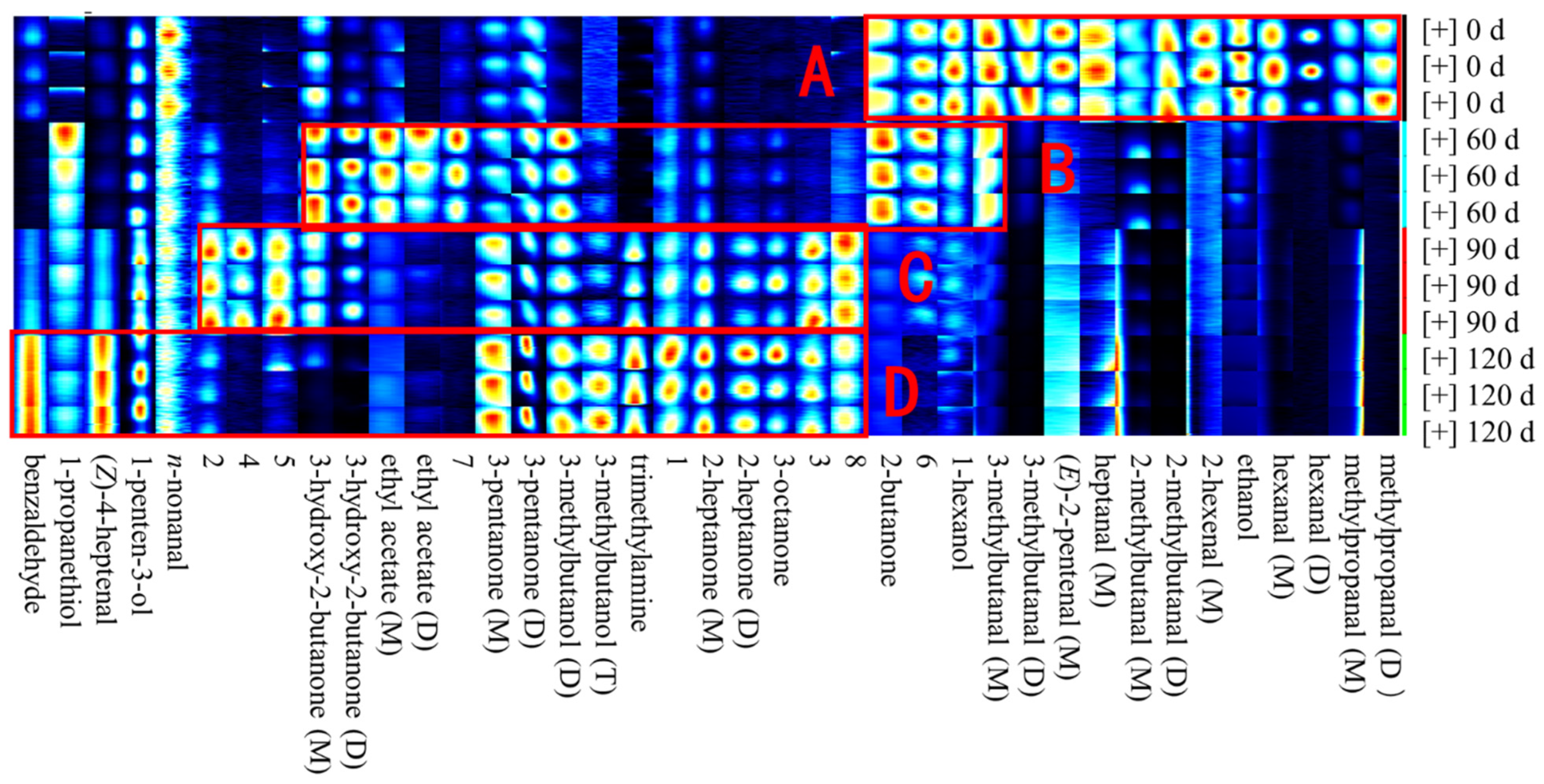
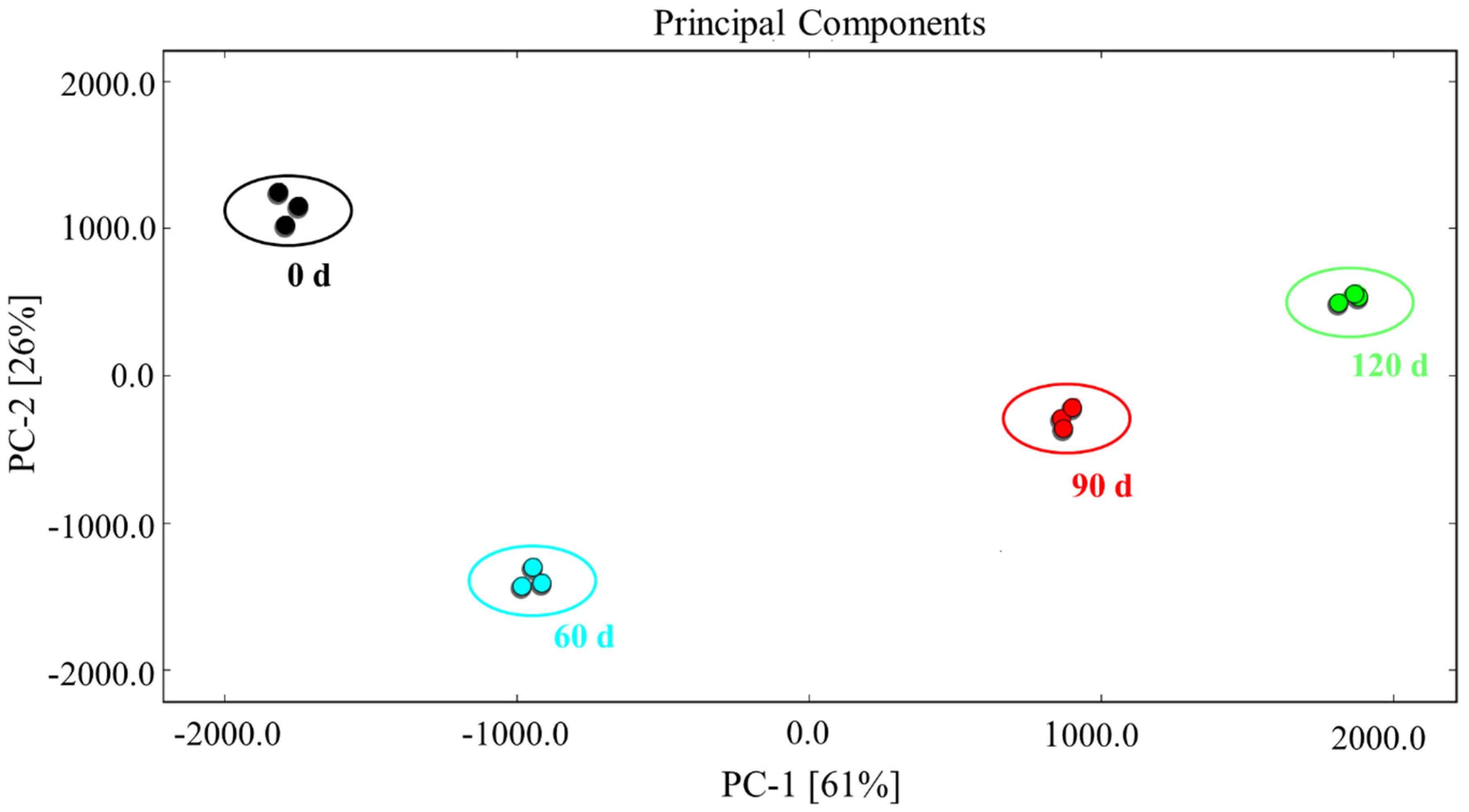
| No. | Compound | CAS# | Molecule Formula | MW | RI1 | RT2 | DT3 | Storage Period | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 d | 60 d | 90 d | 120 d | ||||||||
| 1 | 3-Octanone | C106683 | C8H16O | 128.2 | 992.4 | 540.9 | 1.31 | 197.55 | 232.44 | 439.69 | 549.19 |
| 2 | 2-Heptanone (M) | C110430 | C7H14O | 114.2 | 892 | 367.6 | 1.256 | 226 | 367.96 | 572.06 | 770.82 |
| 3 | 2-Heptanone (D) | C110430 | C7H14O | 114.2 | 889.2 | 364 | 1.625 | 33.98 | 46.23 | 99.86 | 129.45 |
| 4 | 3-Hydroxy-2-butanone (M) | C513860 | C4H8O2 | 88.1 | 716.1 | 192.3 | 1.068 | 1336.8 | 1350.1 | 1362.8 | 1376.56 |
| 5 | 3-Hydroxy-2-butanone (D) | C513860 | C4H8O2 | 88.1 | 715.8 | 192 | 1.326 | 884.81 | 914.77 | 953.59 | 987.56 |
| 6 | 3-Pentanone (M) | C96220 | C5H10O | 86.1 | 696.6 | 178.5 | 1.108 | 260.51 | 339.22 | 389.51 | 518.62 |
| 7 | 3-Pentanone (D) | C96220 | C5H10O | 86.1 | 694.1 | 176.8 | 1.351 | 1270.9 | 1472 | 1588.4 | 1631.99 |
| 8 | 2-Butanone | C78933 | C4H8O | 72.1 | 590.9 | 129.7 | 1.057 | 624.73 | 561.24 | 483.93 | 321.34 |
| 9 | 1-Hexanol | C111273 | C6H14O | 102.2 | 879.8 | 351.9 | 1.326 | 225.16 | 122.06 | 83.02 | 94.84 |
| 10 | 3-Methylbutanol (D) | C123513 | C5H12O | 88.1 | 739.9 | 210.7 | 1.499 | 202.46 | 505.84 | 820 | 1088.71 |
| 11 | 1-Penten-3-ol | C616251 | C5H10O | 86.1 | 690.1 | 174.1 | 0.944 | 226.6 | 248.48 | 305.92 | 339.2 |
| 12 | 1-Propanethiol | C107039 | C3H8S | 76.2 | 633.3 | 147 | 1.36 | 109.48 | 82.32 | 50.17 | 47.2 |
| 13 | 3-Methylbutanol (T) | C123513 | C5H12O | 88.1 | 738.8 | 209.8 | 1.788 | 56.03 | 67.77 | 97.83 | 126.9 |
| 14 | Ethanol | C64175 | C2H6O | 46.1 | 491.5 | 96.79 | 1.046 | 975.52 | 911.11 | 711.76 | 518.53 |
| 15 | Hexanal (M) | C66251 | C6H12O | 100.2 | 793 | 257.8 | 1.264 | 453.89 | 534.21 | 643.29 | 765.02 |
| 16 | Hexanal (D) | C66251 | C6H12O | 100.2 | 792.5 | 257.3 | 1.56 | 237.69 | 256.85 | 298.56 | 316.81 |
| 17 | Benzaldehyde | C100527 | C7H6O | 106.1 | 975.6 | 507 | 1.145 | 232.42 | 302.87 | 356.59 | 400.98 |
| 18 | n-Nonanal | C124196 | C9H18O | 142.2 | 1103 | 765.3 | 1.481 | 147.74 | 124.97 | 123.93 | 110 |
| 19 | 3-Methylbutanal (M) | C590863 | C5H10O | 86.1 | 648.9 | 153.9 | 1.169 | 498.2 | 419.74 | 397.01 | 260.11 |
| 20 | 3-Methylbutanal (D) | C590863 | C5H10O | 86.1 | 646.2 | 152.7 | 1.406 | 157.91 | 111.17 | 92.31 | 56.65 |
| 21 | 2-Methylbutanal (M) | C96173 | C5H10O | 86.1 | 667.7 | 162.7 | 1.164 | 172.2 | 163.62 | 154.96 | 143.92 |
| 22 | 2-Methylbutanal (D) | C96173 | C5H10O | 86.1 | 667.7 | 162.7 | 1.4 | 263.97 | 231.09 | 210.64 | 186.84 |
| 23 | (E)-2-Pentenal (M) | C1576870 | C5H8O | 84.1 | 747.8 | 217.2 | 1.104 | 146.57 | 132.75 | 129.54 | 116.53 |
| 24 | 2-Hexenal (M) | C505577 | C6H10O | 98.1 | 853.6 | 320.4 | 1.179 | 166.87 | 156.54 | 146.83 | 136.59 |
| 25 | Methylpropanal (M) | C78842 | C4H8O | 72.1 | 568 | 121.3 | 1.113 | 317.71 | 289.15 | 254.57 | 229.72 |
| 26 | Methylpropanal (D) | C78842 | C4H8O | 72.1 | 570.3 | 122.1 | 1.282 | 214.97 | 201.43 | 198.34 | 187.5 |
| 27 | Ethyl acetate (M) | C141786 | C4H8O2 | 88.1 | 609.6 | 137.1 | 1.094 | 184.81 | 143.91 | 100.19 | 58.11 |
| 28 | Ethyl acetate (D) | C141786 | C4H8O2 | 88.1 | 611.6 | 137.9 | 1.336 | 120.57 | 109.78 | 82.49 | 53.92 |
| 29 | Heptanal (M) | C111717 | C7H14O | 114.2 | 902.5 | 382.7 | 1.347 | 55.08 | 51.23 | 46.75 | 39.04 |
| 30 | (Z)-4-Heptenal | C6728310 | C7H12O | 112.2 | 900.6 | 380.1 | 1.146 | 130.31 | 159.75 | 189.86 | 223.49 |
| 31 | Trimethylamine | C75503 | C3H9N | 59.1 | 496.6 | 98.26 | 1.148 | 1543.4 | 1665.65 | 1862.3 | 1950.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, T.; Benjakul, S.; Sanmartin, C.; Ying, X.; Ma, L.; Xiao, G.; Yu, J.; Liu, G.; Deng, S. Changes of Volatile Flavor Compounds in Large Yellow Croaker (Larimichthys crocea) during Storage, as Evaluated by Headspace Gas Chromatography–Ion Mobility Spectrometry and Principal Component Analysis. Foods 2021, 10, 2917. https://doi.org/10.3390/foods10122917
Zhao T, Benjakul S, Sanmartin C, Ying X, Ma L, Xiao G, Yu J, Liu G, Deng S. Changes of Volatile Flavor Compounds in Large Yellow Croaker (Larimichthys crocea) during Storage, as Evaluated by Headspace Gas Chromatography–Ion Mobility Spectrometry and Principal Component Analysis. Foods. 2021; 10(12):2917. https://doi.org/10.3390/foods10122917
Chicago/Turabian StyleZhao, Tengfei, Soottawat Benjakul, Chiara Sanmartin, Xiaoguo Ying, Lukai Ma, Gengsheng Xiao, Jin Yu, Guoqin Liu, and Shanggui Deng. 2021. "Changes of Volatile Flavor Compounds in Large Yellow Croaker (Larimichthys crocea) during Storage, as Evaluated by Headspace Gas Chromatography–Ion Mobility Spectrometry and Principal Component Analysis" Foods 10, no. 12: 2917. https://doi.org/10.3390/foods10122917
APA StyleZhao, T., Benjakul, S., Sanmartin, C., Ying, X., Ma, L., Xiao, G., Yu, J., Liu, G., & Deng, S. (2021). Changes of Volatile Flavor Compounds in Large Yellow Croaker (Larimichthys crocea) during Storage, as Evaluated by Headspace Gas Chromatography–Ion Mobility Spectrometry and Principal Component Analysis. Foods, 10(12), 2917. https://doi.org/10.3390/foods10122917