Selection of Autochthonous LAB Strains of Unripe Green Tomato towards the Production of Highly Nutritious Lacto-Fermented Ingredients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Isolation, Characterisation, and Identification of Autochthonous LAB
2.3. DNA Extraction for Molecular Identification
2.4. In Vitro Probiotic Potential of Selected Lab Strains
2.5. Selected LAB Strains’ Tolerance to Solanine
2.6. Statistical Analysis
3. Results and Discussion
3.1. Isolation, Characterisation, and Identification of Autochthonous LAB
3.2. Molecular Identification of Selected LAB Strains
3.3. In Vitro Probiotic Potential of Selected LAB Strains
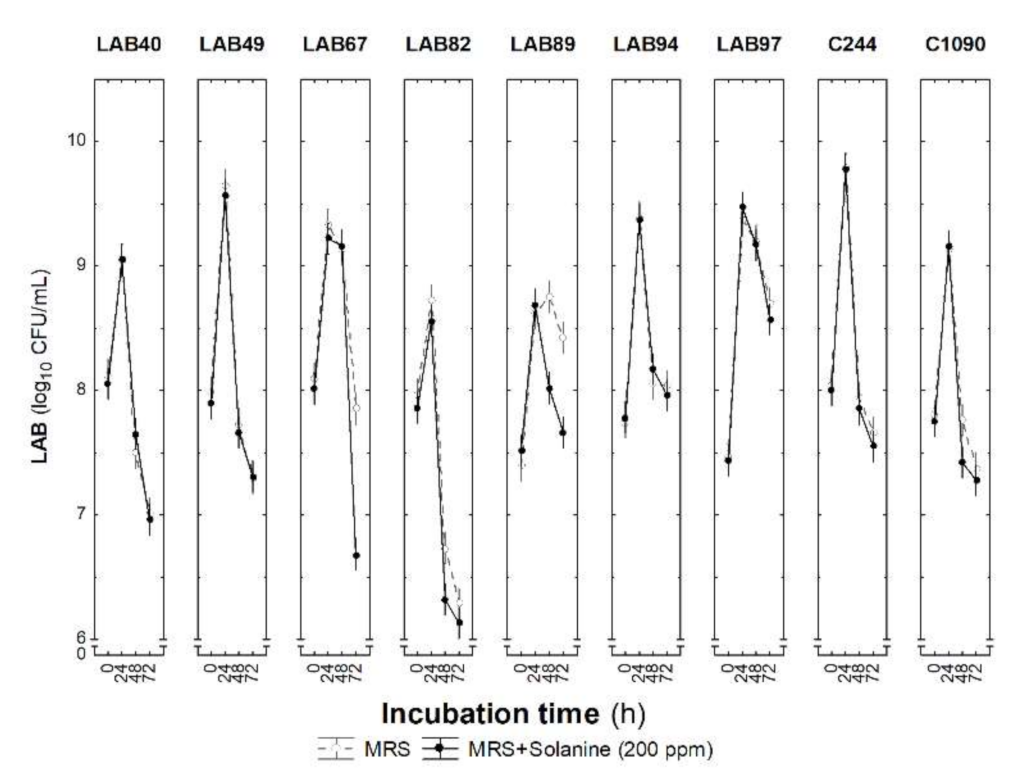
3.4. Selected LAB Strains’ Tolerance to Solanine
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hadj Saadoun, J.; Bertani, G.; Levante, A.; Vezzosi, F.; Ricci, A.; Bernini, V.; Lazzi, C. Fermentation of agri-food waste: A promising route for the production of aroma compounds. Foods 2021, 10, 707. [Google Scholar] [CrossRef]
- Hadj Saadoun, J.; Montevecchi, G.; Zanasi, L.; Bortolini, S.; Macavei, L.I.; Masino, F.; Maistrello, L.; Antonelli, A. Lipid profile and growth of black soldier flies (Hermetia illucens, Stratiomyidae) reared on by-products from different food chains. J. Sci. Food Agric. 2020, 100, 3648–3657. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J.; et al. Food Waste and Byproducts: An Opportunity to Minimize Malnutrition and Hunger in Developing Countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Gao, R.; Ye, F.; Zhao, G. Sustainable valorisation of tomato pomace: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 172–187. [Google Scholar] [CrossRef]
- FAOSTAT Database. Agriculture Holdings Cultivated for the Production of Crops. Statistics Division (ESS). 2020. Available online: https://www.fao.org/faostat/en/#data/QV/metadata (accessed on 29 September 2021).
- Løvdal, T.; Van Droogenbroeck, B.; Eroglu, E.C.; Kaniszewski, S.; Agati, G.; Verheul, M.; Skipnes, D. Valorization of tomato surplus and waste fractions: A case study using Norway, Belgium, Poland, and Turkey as examples. Foods 2019, 8, 229. [Google Scholar] [CrossRef] [Green Version]
- Simões, S.; Santos, R.; Bento-Silva, A.; Santos, M.V.; Mota, M.; Duarte, N.; Sousa, I.; Raymundo, A.; Prista, C. Improving nutritional quality of unripe tomato through fermentation by a consortium of yeast and lactic acid bacteria. J. Sci. Food Agric. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Zhang, Y.; Martin, C. Can the world’s favorite fruit, tomato, provide an effective biosynthetic chassis for high-value metabolites? Plant Cell Rep. 2018, 37, 1443–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, M.W.; Lara, I.; Ilahy, R.; Tlili, I.; Ali, A.; Homa, F.; Prasad, K.; Deshi, V.; Lenucci, M.S.; Hdider, C. Dynamic Changes in Health-Promoting Properties and Eating Quality During Off-Vine Ripening of Tomatoes. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1540–1560. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry and Anticarcinogenic Mechanisms of Glycoalkaloids Produced by Eggplants, Potatoes, and Tomatoes. J. Agric. Food Chem. 2015, 63, 3323–3337. [Google Scholar] [CrossRef] [PubMed]
- Tamasi, G.; Pardini, A.; Bonechi, C.; Donati, A.; Pessina, F.; Marcolongo, P.; Gamberucci, A.; Leone, G.; Consumi, M.; Magnani, A.; et al. Characterization of nutraceutical components in tomato pulp, skin and locular gel. Eur. Food Res. Technol. 2019, 245, 907–918. [Google Scholar] [CrossRef]
- Kozukue, N.; Han, J.-S.; Lee, K.-R.; Friedman, M. Dehydrotomatine and α-Tomatine Content in Tomato Fruits and Vegetative Plant Tissues. J. Agric. Food Chem. 2004, 52, 2079–2083. [Google Scholar] [CrossRef]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; Nebbia, C.S.; Nielsen, E.; et al. Risk assessment of glycoalkaloids in feed and food, in particular in potatoes and potato-derived products. EFSA J. 2020, 18, e06222. [Google Scholar] [CrossRef] [PubMed]
- Sabater, C.; Ruiz, L.; Delgado, S.; Ruas-Madiedo, P.; Margolles, A. Valorization of Vegetable Food Waste and By-Products Through Fermentation Processes. Front. Microbiol. 2020, 11, 581997. [Google Scholar] [CrossRef]
- Santos, R.; Sousa, I.; Raymundo, A.; Silva, J.S.; Prista, C.; Mota, M. Development of a fermented green tomato base for dressings and sauces with high nutritional value. Acta Hortic. 2019, 1233, 239–246. [Google Scholar] [CrossRef]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Surico, R.F.; Paradiso, A.; De Angelis, M.; Salmon, J.C.; Buchin, S.; De Gara, L.; Gobbetti, M. Effect of autochthonous lactic acid bacteria starters on health-promoting and sensory properties of tomato juices. Int. J. Food Microbiol. 2009, 128, 473–483. [Google Scholar] [CrossRef]
- Mechmeche, M.; Kachouri, F.; Ksontini, H.; Hamdi, M. Production of bioactive peptides from tomato seed isolate by Lactobacillus plantarum fermentation and enhancement of antioxidant activity. Food Biotechnol. 2017, 31, 94–113. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Gobbetti, M. Lactic acid fermentation drives the optimal volatile flavor-aroma profile of pomegranate juice. Int. J. Food Microbiol. 2017, 248, 56–62. [Google Scholar] [CrossRef]
- Jampaphaeng, K.; Ferrocino, I.; Giordano, M.; Rantsiou, K.; Maneerat, S.; Cocolin, L. Microbiota dynamics and volatilome profile during stink bean fermentation (Sataw-Dong) with Lactobacillus plantarum KJ03 as a starter culture. Food Microbiol. 2018, 76, 91–102. [Google Scholar] [CrossRef]
- Zheng, X.; Wei, W.; Rao, S.; Gao, L.; Li, H.; Yang, Z. Degradation of patulin in fruit juice by a lactic acid bacteria strain Lactobacillus casei YZU01. Food Control 2020, 112, 107147. [Google Scholar] [CrossRef]
- Veselá, M.; Drdák, M.; Šimon, P.; Veselý, M. Utilization of Lactobacillus sp. for steroid glycoalkaloids degradation by lactic acid fermentation. Nahrung 2002, 46, 251–255. [Google Scholar] [CrossRef]
- Cao, G.; Ma, F.; Xu, J.; Zhang, Y. Microbial community succession and toxic alkaloids change during fermentation of Huafeng Dan Yaomu. Lett. Appl. Microbiol. 2020, 70, 318–325. [Google Scholar] [CrossRef]
- Li, C.; Kong, Q.; Mou, H.; Jiang, Y.; Du, Y.; Zhang, F. Biotransformation of alkylamides and alkaloids by lactic acid bacteria strains isolated from Zanthoxylum bungeanum meal. Bioresour. Technol. 2021, 330, 124944. [Google Scholar] [CrossRef]
- Friedman, M. Tomato glycoalkaloids: Role in the plant and in the diet. J. Agric. Food Chem. 2002, 50, 5751–5780. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. BioMed Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef] [Green Version]
- Guan, Q.; Xiong, T.; Xie, M. Influence of Probiotic Fermented Fruit and Vegetables on Human Health and the Related Industrial Development Trend. Engineering 2021, 7, 212–218. [Google Scholar] [CrossRef]
- Pereira, N.; Martins, P.; Gonçalves, E.M.; Ramos, A.C.; Abreu, M. Inoculated fermentation of immature-tomato with potential probiotic Lactiplantibacillus plantarum and Weissella paramesenteroides starter cultures. In Livro de Resumos do XV Encontro de Química dos Alimentos: Estratégias para a Excelência, Auntenticidade, Segurança e Sustentabilidade Alimentar, Funchal, Madeira, Portugal, 5–8 September 2021; Câmara, J.S., Pereira, J.A.M., Gouveia, R.P., Eds.; Universidade da Madeira, Centro de Química da Madeira: Funchal, Portugal, 2021. [Google Scholar]
- EN ISO 15214:1998. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria—Colony-Count Technique at 30 °C; ISO: Geneva, Switzerland, 1998. [Google Scholar]
- StatSoft, Inc. STATISTICA (Data Analysis Software System), Version 8.0.
- Bah, A.; Ferjani, R.; Fhoula, I.; Gharbi, Y.; Najjari, A.; Boudabous, A.; Ouzari, H.I. Microbial community dynamic in tomato fruit during spontaneous fermentation and biotechnological characterisation of indigenous lactic acid bacteria. Ann. Microbiol. 2018, 69, 41–49. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Kouretas, K.; Drosinos, E.H. Effect of ripening stage on the development of the microbial community during spontaneous fermentation of green tomatoes. J. Sci. Food Agric. 2014, 94, 1600–1606. [Google Scholar] [CrossRef]
- Ajayi, A.O. Nature of Tomatoes Microflora under Storage. Am. J. Exp. Agric. 2012, 3, 89–101. [Google Scholar] [CrossRef] [Green Version]
- Østlie, H.M.; Eliassen, L.; Florvaag, A.; Skeie, S. Phenotypic and PCR-based characterisation of the microflora in Norvegia cheese during ripening. Int. J. Food Microbiol. 2004, 94, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Ahrné, S.; Molin, G.; Ståhl, S. Plasmids in Lactobacillus Strains Isolated from Meat and Meat Products. Syst. Appl. Microbiol. 1989, 11, 320–325. [Google Scholar] [CrossRef]
- Domingos-Lopes, M.F.P.; Stanton, C.; Ross, P.R.; Dapkevicius, M.L.E.; Silva, C.C.G. Genetic diversity, safety and technological characterisation of lactic acid bacteria isolated from artisanal Pico cheese. Food Microbiol. 2016, 63, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Angmo, K.; Kumari, A.; Savitri; Bhalla, T.C. Probiotic characterisation of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. LWT 2016, 66, 428–435. [Google Scholar] [CrossRef]
- Lakra, A.K.; Domdi, L.; Hanjon, G.; Tilwani, Y.M.; Arul, V. Some probiotic potential of Weissella confusa MD1 and Weissella cibaria MD2 isolated from fermented batter. LWT 2020, 109261, 1–37. [Google Scholar] [CrossRef]
- Sathyapriya, A.; Anitha, A. Assessment of Probiotic Potential Leuconostoc mesenteroides from Natural Microbiota. Int. J. Res. Biol. Sci. 2019, 5, 26–33. [Google Scholar] [CrossRef] [Green Version]
- de Paula, A.T.; Jeronymo-Ceneviva, A.B.; Silva, L.F.; Todorov, S.D.; Franco, B.D.G.M.; Penna, A.L.B. Leuconostoc mesenteroides SJRP55: A potential probiotic strain isolated from Brazilian water buffalo mozzarella cheese. Ann. Microbiol. 2014, 65, 899–910. [Google Scholar] [CrossRef]
- Argyri, A.A.; Zoumpopoulou, G.; Karatzas, K.A.G.; Tsakalidou, E.; Nychas, G.J.E.; Panagou, E.Z.; Tassou, C.C. Selection of potential probiotic lactic acid bacteria from fermented olives by in vitro tests. Food Microbiol. 2013, 33, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Vitali, B.; Minervini, G.; Rizzello, C.G.; Spisni, E.; Maccaferri, S.; Brigidi, P.; Gobbetti, M.; Di Cagno, R. Novel probiotic candidates for humans isolated from raw fruits and vegetables. Food Microbiol. 2012, 31, 116–125. [Google Scholar] [CrossRef]
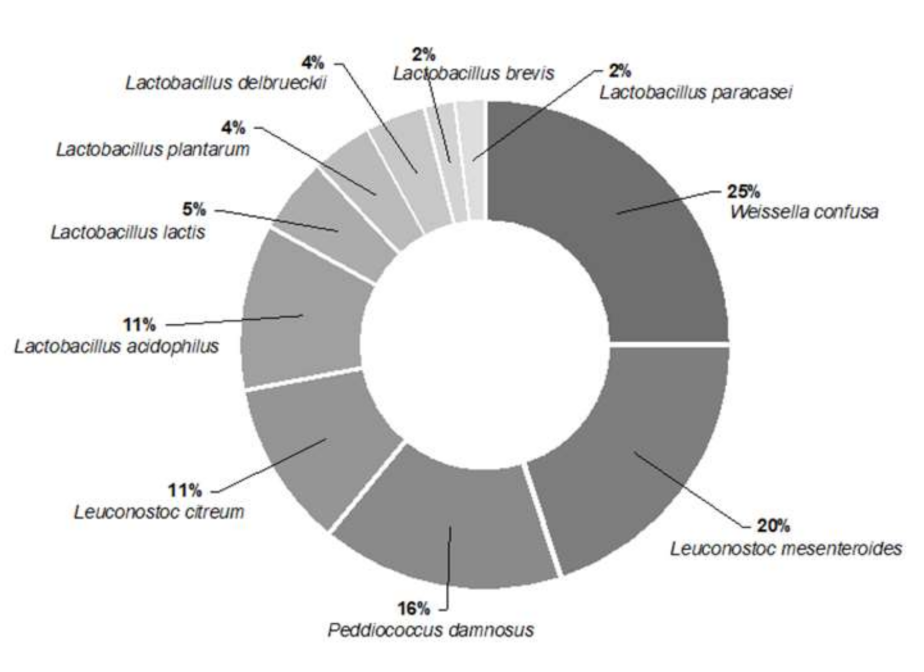
| Isolates | Species | ID% | Isolates | Species | ID% | Isolates | Species | ID% |
|---|---|---|---|---|---|---|---|---|
| LAB39 | Weissella confusa | 59.9 | LAB58 | Pediococcus damnosus | 97.5 | LAB81 | Lactobacillus acidophilus | 68.4 |
| LAB40 | Leuconostoc mesenteroides | 99.9 | LAB59 | Pediococcus damnosus | 97.5 | LAB82 | Leuconostoc citreum | 99.9 |
| LAB41 | Leuconostoc mesenteroides | 99.9 | LAB60 | Leuconostoc mesenteroides | 90.4 | LAB83 | Weissella confusa | 98.1 |
| LAB42 | Weissella confusa | 99.6 | LAB61 | Lacticaseibacillus paracasei | 52.9 | LAB84 | Leuconostoc mesenteroides | 99.9 |
| LAB43 | Weissella confusa | 96.6 | LAB62 | Weissella confusa | 96.6 | LAB85 | Leuconostoc citreum | 63.3 |
| LAB44 | Lactobacillus brevis | 62.6 | LAB63 | Weissella confusa | 96.6 | LAB86 | Leuconostoc mesenteroides | 90.4 |
| LAB45 | Lactococcus lactis | 73.9 | LAB64 | Weissella confusa | 96.6 | LAB87 | Weissella confusa | 99.6 |
| LAB46 | Weissella confusa | 62.5 | LAB65 | Weissella confusa | 96.6 | LAB88 | Leuconostoc mesenteroides | 99.9 |
| LAB47 | Weissella confusa | 83.4 | LAB66 | Pediococcus damnosus | 97.5 | LAB89 | Weissella confusa | 99.9 |
| LAB48 | Weissella confusa | 83.4 | LAB67 | Lactococcus lactis | 99.9 | LAB90 | Lactobacillus acidophilus | 86.7 |
| LAB49 | Pediococcus damnosus | 97.5 | LAB68 | Lactococcus lactis | 99.9 | LAB91 | Leuconostoc citreum | 87.7 |
| LAB50 | Weissella confusa | 95 | LAB69 | Leuconostoc mesenteroides | 97.5 | LAB92 | Lactobacillus delbrueckii | 58.7 |
| LAB51 | Leuconostoc mesenteroides | 97.8 | LAB70 | Leuconostoc mesenteroides | 93.6 | LAB93 | Lactobacillus acidophilus | 74.1 |
| LAB52 | Pediococcus damnosus | 97.5 | LAB75 | Leuconostoc citreum | 63.3 | LAB94 | Lactobacillus acidophilus | 74.7 |
| LAB53 | Leuconostoc mesenteroides | 99.9 | LAB76 | Leuconostoc citreum | 99.9 | LAB95 | Lactobacillus acidophilus | 74.1 |
| LAB54 | Pediococcus damnosus | 97.5 | LAB77 | Leuconostoc citreum | 99.9 | LAB96 | Lactobacillus plantarum | 99.9 |
| LAB55 | Pediococcus damnosus | 97.5 | LAB78 | Lactobacillus delbrueckii | 58.7 | LAB97 | Lactobacillus plantarum | 99.9 |
| LAB56 | Pediococcus damnosus | 97.5 | LAB79 | Leuconostoc mesenteroides | 99.9 | |||
| LAB57 | Pediococcus damnosus | 97.5 | LAB80 | Lactobacillus acidophilus | 58.2 |
| Isolate Id. | Maximum Score | E-Value | Identity | Species (16S rRNA Gene Analysis) | Accession |
|---|---|---|---|---|---|
| LAB40 | 28378 | 0 | 100 | Weissella cibaria | OL405452 |
| LAB49 | 12941 | 0 | 100 | Lactiplantibacillus plantarum | OL405451 |
| LAB67 | 15019 | 0 | 100 | Lactococcus lactis | OL405455 |
| LAB82 | 10197 | 0 | 100 | Leuconostoc citreum | OL405449 |
| LAB89 | 41511 | 0 | 100 | Weissella cibaria | OL405450 |
| LAB94 | 2593 | 0 | 100 | Weissella confusa | OL405453 |
| LAB97 | 12849 | 0 | 100 | Lactiplantibacillus plantarum | OL405454 |
| C244 * | 12858 | 0 | 99.93 | Lactiplantibacillus plantarum | OL405447 |
| C1090 * | 20248 | 0 | 100 | Weissella paramesenteroides | OL405448 |
| Strain | Species | Origin | Condition | Initial Count (Mean ± SD) | Final Count (Mean ± SD) | Survival Rate (%) | Probiotic Potential * |
|---|---|---|---|---|---|---|---|
| LAB40 | Weissella cibaria | Autochthonous | pH 2.5 | 1.9 ± 0.3 | 0.0 ± 0.0 | 0 | - |
| 0.3% bile salts | 7.7 ± 0.1 | 7.7 ± 0.1 | 101 | + | |||
| LAB49 | Lactiplantibacillus plantarum | Autochthonous | pH 2.5 | 9.6 ± 0.0 | 0.0 ± 0.0 | 0 | - |
| 0.3% bile salts | 9.5 ± 0.0 * | 8.8 ± 0.1 | 93 | + | |||
| LAB67 | Lactococcus lactis | Autochthonous | pH 2.5 | 7.2 ± 0.1 | 0.0 ± 0.0 | 0 | - |
| 0.3% bile salts | 7.0 ± 0.0 | 9.5 ± 0.0 | 135 | + | |||
| LAB82 | Leuconostoc citreum | Autochthonous | pH 2.5 | 5.9 ± 0.2 | 0.0 ± 0.0 | 0 | - |
| 0.3% bile salts | 9.4 ± 0.3 | 7.9 ± 0.1 | 84 | + | |||
| LAB89 | Weisella cibaria | Autochthonous | pH 2.5 | 6.6 ± 0.2 | 2.2 ± 0.0 | 34 | - |
| 0.3% bile salts | 7.7 ± 0.1 | 9.5 ± 0.0 | 123 | + | |||
| LAB94 | Weissella confusa | Autochthonous | pH 2.5 | 7.3 ± 0.0 | 0.0 ± 0.0 | 0 | - |
| 0.3% bile salts | 7.7 ± 0.1 | 8.2 ± 0.2 | 106 | + | |||
| LAB97 | Lactiplantibacillus plantarum | Autochthonous | pH 2.5 | 7.3 ± 0.0 | 0.0 ± 0.0 | 0 | - |
| 0.3% bile salts | 7.7 ± 0.0 | 8.3 ± 0.0 | 109 | + | |||
| C244 | Lactiplantibacillus plantarum | INIAV’s collection | pH 2.5 | 7.0 ± 0.3 | 0.0 ± 0.0 | 0 | - |
| 0.3% bile salts | 8.0 ± 0.2 | 7.2 ± 0.4 | 91 | + | |||
| C1090 | Weissella paramesenteroides | INIAV’s collection | pH 2.5 | 7.3 ± 0.5 | 0.0 ± 0.0 | 0 | - |
| 0.3% bile salts | 7.9 ± 0.0 | 8.7 ± 0.0 | 110 | + |
| Strain | Species | Origin | Condition | Initial Count (Mean ± SD) | Final Count (Mean ± SD) | Survival Rate (%) | Probiotic Potential * |
|---|---|---|---|---|---|---|---|
| LAB40 | Weissella cibaria | Autochthonous | pH 2.5 | 3.5 ± 0.1 | 3.3 ± 0.7 | 94 | - |
| 0.3% bile salts | 0.0 ± 0.0 | 0.0 ± 0.0 | 0 | - | |||
| LAB49 | Lactiplantibacillus plantarum | Autochthonous | pH 2.5 | 9.8 ± 0.1 | 3.9 ± 0.1 | 40 | - |
| 0.3% bile salts | 9.5 ± 0.1 | 9.5 ± 0.0 | 99 | + | |||
| LAB67 | Lactococcus lactis | Autochthonous | pH 2.5 | 7.0 ± 0.0 | 0.0 ± 0.0 | 0 | - |
| 0.3% bile salts | 6.6 ± 0.1 | 0.0 ± 0.0 | 0 | - | |||
| LAB82 | Leuconostoc citreum | Autochthonous | pH 2.5 | 9.5 ± 0.0 | 0.0 ± 0.0 | 0 | - |
| 0.3% bile salts | 0.0 ± 0.0 | 0.0 ± 0.0 | 0 | - | |||
| LAB89 | Weisella cibaria | Autochthonous | pH 2.5 | 6.2 ± 0.1 | 0.0 ± 0.0 | 0 | - |
| 0.3% bile salts | 7.7 ± 0.0 | 4.0 ± 0.0 | 53 | - | |||
| LAB94 | Weissella confusa | Autochthonous | pH 2.5 | 6.2 ± 0.4 | 1.4 ± 0.2 | 22 | - |
| 0.3% bile salts | 0.0 ± 0.0 | 0.0 ± 0.0 | 0 | - | |||
| LAB97 | Lactiplantibacillus plantarum | Autochthonous | pH 2.5 | 7.4 ± 0.0 | 7.5 ± 0.1 | 101 | + |
| 0.3% bile salts | 6.1 ± 0.3 | 6.9 ± 0.3 | 113 | + | |||
| C244 | Lactiplantibacillus plantarum | INIAV’s collection | pH 2.5 | 7.9 ± 0.1 | 4.2 ± 0.2 | 54 | - |
| 0.3% bile salts | 7.9 ± 0.0 | 6.1 ± 0.1 | 77 | + | |||
| C1090 | Weissella paramesenteroides | INIAV’s collection | pH 2.5 | 7.8 ± 0.1 | 9.5 ± 0.0 | 122 | + |
| 0.3% bile salts | 7.2 ± 0.2 | 6.8 ± 0.2 | 95 | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, N.; Alegria, C.; Aleixo, C.; Martins, P.; Gonçalves, E.M.; Abreu, M. Selection of Autochthonous LAB Strains of Unripe Green Tomato towards the Production of Highly Nutritious Lacto-Fermented Ingredients. Foods 2021, 10, 2916. https://doi.org/10.3390/foods10122916
Pereira N, Alegria C, Aleixo C, Martins P, Gonçalves EM, Abreu M. Selection of Autochthonous LAB Strains of Unripe Green Tomato towards the Production of Highly Nutritious Lacto-Fermented Ingredients. Foods. 2021; 10(12):2916. https://doi.org/10.3390/foods10122916
Chicago/Turabian StylePereira, Nelson, Carla Alegria, Cristina Aleixo, Paula Martins, Elsa M. Gonçalves, and Marta Abreu. 2021. "Selection of Autochthonous LAB Strains of Unripe Green Tomato towards the Production of Highly Nutritious Lacto-Fermented Ingredients" Foods 10, no. 12: 2916. https://doi.org/10.3390/foods10122916
APA StylePereira, N., Alegria, C., Aleixo, C., Martins, P., Gonçalves, E. M., & Abreu, M. (2021). Selection of Autochthonous LAB Strains of Unripe Green Tomato towards the Production of Highly Nutritious Lacto-Fermented Ingredients. Foods, 10(12), 2916. https://doi.org/10.3390/foods10122916







