Evaluation of Marrubium vulgare Growing Wild in Tunisia for Its Potential as a Dietary Supplement
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents and Equipment
2.1.1. Reagents
2.1.2. Equipment
2.2. Plant Material
2.3. Extraction
2.4. Distillation of Essential Oil and GC-MS Analysis Conditions
2.5. Toxicity to the Aquatic Microcrustacean Artemia Salina
2.6. Determination of Total Phenolic Compounds
2.7. Antioxidant Activity
2.7.1. Fe3+−Fe2+ Reduction
2.7.2. DPPH Radical Scavenging Activity
2.7.3. β-Carotene/Linoleic Acid Assay
2.8. Isolation of Marrubiin and Quantification of Marrubiin Labdanoids
2.9. Determination of Mineral Contents
2.10. Statistical Analysis
3. Results and Discussion
3.1. Plant Material and Sampling
3.2. Chemical Composition and Toxicity of Essential Oils
3.2.1. Chemical Composition of Essential Oils
3.2.2. Toxicity against Artemia Salina
3.3. Total Phenolic Compounds
3.4. Antioxidant Activity
3.4.1. Ferric Reducing Power
3.4.2. DPPH Radical Scavenging Activity
3.4.3. Antioxidant Activity in the β-Carotene–Linoleate Model System
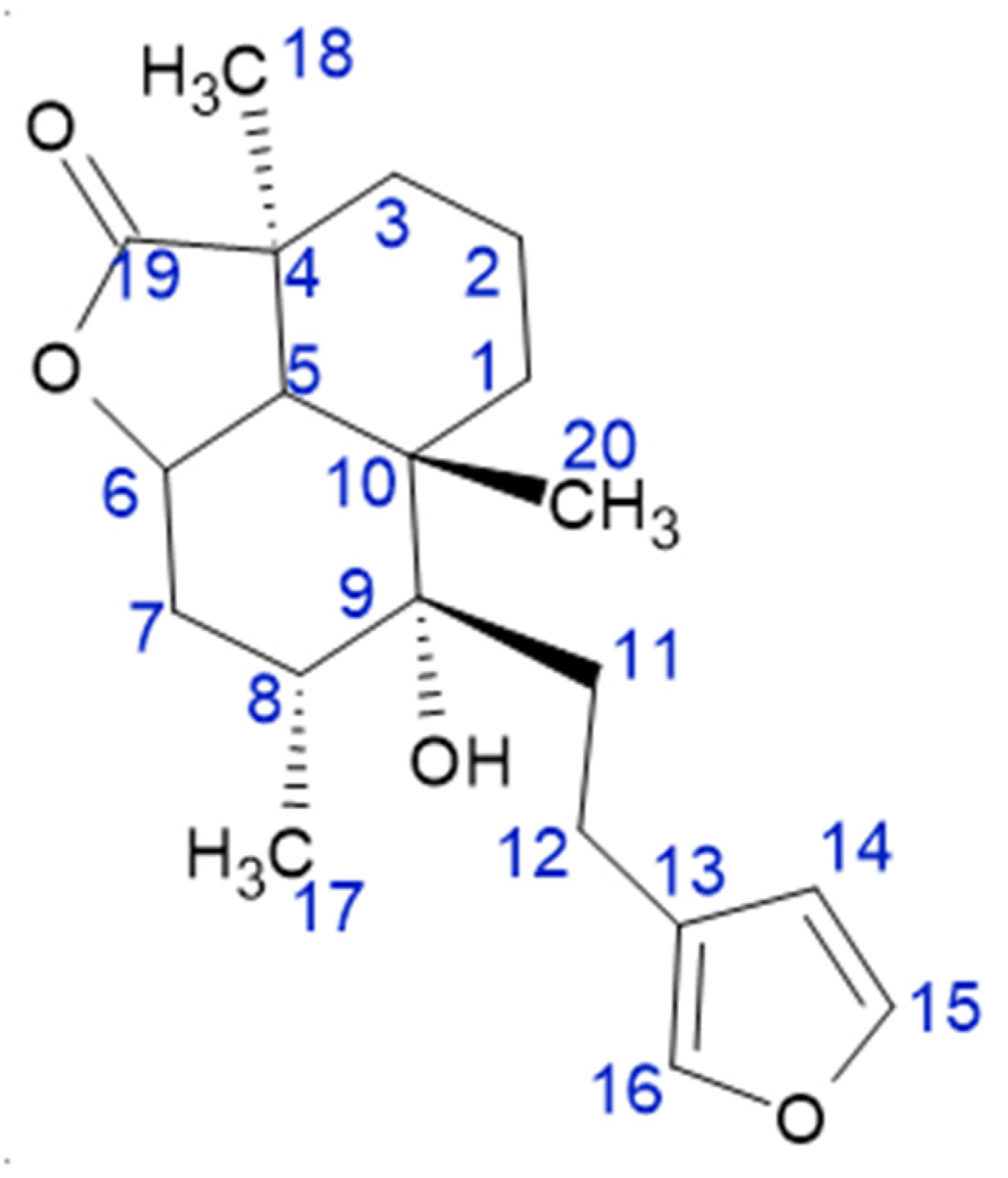
3.5. Marrubiin Labdanoids Content
3.6. Correlation between Polyphenols Content, Marrubiin, and Antioxidant Activities
3.7. Metal Content
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zaabat, N.; Hay, A.; Michalet, S.; Darbour, N.; Bayet, C.; Skandrani, I.; Chekir-Ghedira, L.; Akkal, S.; Dijoux-Franca, M. Antioxidant and antigenotoxic properties of compounds isolated from Marrubium deserti de Noé. Food Chem. Toxicol. 2011, 49, 3328–3335. [Google Scholar] [CrossRef]
- Akther, N.; Shawl, A.S.; Sultana, S.; Chandan, B.K.; Akhter, M. Hepatoprotective activity of Marrubium vulgare against paracetamol induced toxicity. J. Pharm. Res. 2013, 7, 565. [Google Scholar] [CrossRef]
- Paula de Olivera, A.; Santin, J.R.; Lemos, M.; Klein, L.C.J.; Couto, A.G.; Bittencourt, C.M.S.; Cechinel, F.; Valdir, F.A. Gastroprotective activity of methanol extract and marrubiin obtained from leaves of Marrubium vulgare L. (Lamiaceae). J. Pharm. Pharmacol. 2011, 63, 1230–1237. [Google Scholar] [CrossRef]
- Hussain, J.; Rehman, N.; Al-Harrasi, A.; Ali, L.; Ullah, R.; Mabood, F.; Hussain, H.; Ismail, M. Nutritional prospects and mineral compositions of selected vegetables from Dhoda Sharif-Kohat. J. Med. Plants Res. 2011, 5, 6509–6514. [Google Scholar]
- Mnonopi, N.; Levendal, R.A.; Mzilikezi, N.; Frost, C.L. Marrubiin, a constituent of Leonotus leonurus, alleviates diabetic symptoms. Phytomedicine 2012, 19, 488–493. [Google Scholar] [CrossRef]
- Marrelli, M.; Conforti, F.; Rigano, D.; Formisano, C.; Bruno, M.; Senatore, F.; Menichini, F. Cytotoxic properties of Marrubium globosum ssp. libanoticum and its bioactive components. Nat. Prod. Commun. 2013, 8, 567–569. [Google Scholar]
- Hellen, K.; Stulzer, H.K.; Tagliari, M.P.; Zampirolo, J.A.; Cechinel-Filho, V.; Schlemper, V. Antioedematogenic effect of marrubiin obtained from Marrubium vulgare. J. Ethnopharmacol. 2006, 108, 379–384. [Google Scholar]
- Laonigro, G.; Lanzetta, R.; Parrilli, M.; Adinolfi, M.; Mangoni, L. The configuration of the diterpene spiro ethers from Marrubium vulgare and from Leonotis leonurus. Gazz. Chim. Ital. 1979, 109, 145–150. [Google Scholar]
- Zarai, Z.S.; Kadri, A.; Ben Chobba, I.; Ben Mansour, R.; Bekir, A.; Mejdoub, H.; Gharsallah, N. The in-vitro evaluation of antibacterial, antifungal and cytotoxic properties of Marrubium vulgare L. essential oil grown in Tunisia. Lipids Health Dis. 2011, 10, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadri, A.; Zarai, Z.; Békir, A.; Gharsallah, N.; Damak, M.; Gdoura, R. Chemical composition and antioxidant activity of Marrubium vulgare L. essential oil from Tunisia. Afr. J. Biotechnol. 2011, 10, 3908–3914. [Google Scholar]
- Weel, K.C.G.; Venskutonis, P.R.; Pukalskas, A.; Gruzdiene, D.; Linssen, J.P.H. Antioxidant activity of horehound (Marrubium vulgare) grown in Lithuania. Fett Lipid 1999, 101, 395–400. [Google Scholar] [CrossRef]
- Nagy, M.; Svajdlenka, E. Comparison of Essential Oils from Marrubium vulgare L. and M. peregrinum L. J. Essent. Oil Res. 1998, 10, 585–587. [Google Scholar] [CrossRef]
- Morteza-Semnani, K.; Saeedi, M. The Essential Oil Composition of Marrubium astracanicum Jacq. from Iran. J. Essent. Oil Bear. Plants 2004, 7, 239–242. [Google Scholar] [CrossRef]
- Boulila, A.; Sanaa, A.; Ben Salem, I.; Rokbeni, N.; M’rabet, Y.; Hosni, K.; Fernandez, X. Antioxidant properties and phenolic variation in wild populations of Marrubium vulgare L. (Lamiaceae). Ind. Crops Prod. 2015, 76, 616–622. [Google Scholar] [CrossRef]
- Arceusz, A.; Radecka, I.; Wesolowski, M. Identification of diversity in elements content in medicinal plants belonging to different plant families. Food Chem. 2010, 120, 52–58. [Google Scholar] [CrossRef]
- Alapetite, P.G. Flore de la Tunisie. Angiospermes-Dicotylédones Gamopétales; Imprimerie Officielle de la République Tunisienne: Tunis, Tunisia, 1988; pp. 581–582. [Google Scholar]
- Khadhri, A.; El Mokni, R.; Almeida, C.; Nogueira, J.M.F.; Araújo, M.E.M. Chemical composition of essential oil of Psidium guajava L. growing in Tunisia. Ind. Crop Prod. 2014, 52, 29–31. [Google Scholar] [CrossRef]
- Gouveia, W.; Jorge, T.F.; Martins, S.; Meireles, M.; Carolino, M.; Cruz, C.; Almeida, T.V.; Araújo, M.E.M. Toxicity of ionic liquids prepared from biomaterials. Chemosphere 2014, 104, 51–56. [Google Scholar] [CrossRef]
- Oktay, M.; Gulcin, I.; Kufrevioglu, O.I. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT Food Sci. Technol. 2003, 36, 263–271. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reactions: Antioxidative activities of browning products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Mata, A.T.; Proença, C.; Ferreira, A.R.; Serralheiro, M.L.M.; Nogueira, J.M.F.; Araújo, M.E.M. Antioxidant and antiacetylcholinesterase activities of five plants used as Portuguese food spices. Food Chem. 2007, 103, 778–786. [Google Scholar] [CrossRef]
- Khadri, A.; Neffati, M.; Smiti, S.; Fale, P.; Lino, A.R.L.; Serralheiro, M.L.M.; Araújo, M.E.M. Antioxidant, antiacetylcholinesterase and antimicrobial activities of Cymbopogon schoenanthus L. Spreng (lemon grass) from Tunisia. LWT Food Sci. Technol. 2010, 43, 331–336. [Google Scholar] [CrossRef]
- Rezgui, M.; Majdoub, N.; Ben-Kaab, S.; Marzouk, B.; Gouia, H.; Araújo, M.E.M.; Bettaieb Ben-Kaab, L. How Salt Stress Represses the Biosynthesis of Marrubiin and Disturbs the Antioxidant Activity of Marrubium Vulgare L. Pol. J. Environ. Stud. 2017, 26, 267–277. [Google Scholar] [CrossRef]
- Ben Othman, R.; Ibrahim, H.; Mankai, A.; Abid, N.; Othmani, N.; Jenhani, N.; Tertek, H.; Trabelsi, N.; Trimesh, A.; Ben Mami, F. Use of hypoglycemic plants by Tunisian diabetic patients. Alex. J. Med. 2013, 49, 261–264. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Malca, G.; Glenn, A.; Sharon, D.; Nilsen, B.; Parris, B.; Dubose, D.; Ruiz, D.; Saleda, J.; Martinez, M.; et al. Toxicity of medicinal plants used in traditional medicine in Northern Peru. J. Ethnopharmacol. 2011, 137, 121–140. [Google Scholar] [CrossRef] [Green Version]
- Fawole, O.A.; Ndhlala, A.R.; Amoo, S.O.; Finnie, J.F.; Van Staden, J. Anti-inflammatory and phytochemical properties of twelve medicinal plants used for treating gastro-intestinal ailments in South Africa. J. Ethnopharmacol. 2009, 123, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; Tucci, M.; De Palma, M.; Pepe, R.; Nazzaro, F. Polyphenolic composition in different parts of some cultivars of globe artichoke (Cynara cardunculus L. var. scolymus (L.) Fiori). Food Chem. 2007, 104, 1282–1286. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Falleh, H.; Trabelsi, N.; Boulaaba, M.; Smaoui, A.; Abdelly, C. Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. C. R. Biol. 2008, 331, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Falleh, H.; Ksouri, R.; Boulaaba, M.; Guyot, S.; Abdelly, C.; Magné, C. Phenolic nature, occurrence and polymerization degree as marker of environmental adaptation in the edible halophyte Mesembryanthemum edule. S. Afr. J. Bot. 2012, 79, 117–124. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–17. [Google Scholar]
- Berker, K.I.; Güçlü, K.; Tor, I.; Apak, R. Comparative evaluation of Fe(III) reducing power-based antioxidant capacity assays in the presence of phenanthroline, bathophenanthroline, tripyridyltriazine (FRAP), and ferricyanide reagentes. Talanta 2007, 72, 1157–1165. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J.A. Free radical-scavenging capacity, antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004, 84, 551–562. [Google Scholar] [CrossRef]
- Khaled-Khodja, N.; Boulekbache-Makhlouf, L.; Madani, K. Phytochemical screening of antioxidant and antibacterial activities of methanolic extracts of some Lamiaceae. Ind. Crops Prod. 2014, 61, 41–48. [Google Scholar] [CrossRef]
- Khadri, A.; Serralheiro, M.L.M.; Nogueira, J.M.F.; Neffati, M.; Smiti, S.; Araújo, M.E.M. Antioxidant and antiacetylcholinesterase activities of essential oils from Cymbopogon schoenanthus L. Spreng. Determination of chemical composition by GC–mass spectrometry and 13C NMR. Food Chem. 2008, 109, 630–637. [Google Scholar] [CrossRef]
- Yaniv, Z.; Platevitch, D. Effect of drought on the secondary metabolites of medicinal and aromatic plants. In Cultivation and Utilisation of Medicinal Plants; Atal, C.K., Kapur, B.M., Eds.; CSIR: Jammu Tawi, India, 1982; pp. 1–12. [Google Scholar]
- El-Bardai, S.; Morel, N.; Wibo, M.; Fabre, N.; Llabres, G.; Lyoussi, B. The vasorelaxant activity of marrubenol and marrubiin from Marrubium vulgare. Planta Med. 2003, 69, 75–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jesus, R.A.P.; Cechinel-Filho, V.; Oliveira, A.E.; Schlemper, V. Analysis of the antinociceptive properties of marrubiin isolated from Marrubium vulgare. Phytomedicine 2000, 7, 111–115. [Google Scholar] [CrossRef]
- Aćimović, M.; Jeremić, K.; Salaj, N.; Gavarić, N.; Kiprovski, B.; Sikora, V.; Zeremski, T. Marrubium vulgare L.: A Phytochemical and Pharmacological Overview. Molecules 2020, 25, 2898. [Google Scholar] [CrossRef]
- Izzo, A.A.; Hoon-Kim, S.; Radhakrishnan, R.; Williamson, E.M. A critical approach to evaluating clinical efficacy, adverse events and drug interactions of herbal remedies. Phytother. Res. 2016, 30, 691–700. [Google Scholar] [CrossRef]
- Ajasa, A.O.; Bello, M.O.; Ibrahim, A.O.; Ogunwande, I.A.; Olawore, N.O. Heavy trace metals and macronutrients status in herbal plants of Nigeria. Food Chem. 2004, 85, 67–71. [Google Scholar] [CrossRef]
- Chizzola, R.; Franz, C.H. Metallic trace elements in medicinal and aromatic plants from Austria. J. Appl. Biol. 1996, 70, 52–56. [Google Scholar]
- Stipanuk, M.H.; Caudill, M.A. Biochemical, Physiological and Molecular Aspects of Human Nutrition, 3rd ed.; Elsevier Saunders: St. Louis, MO, USA, 2012. [Google Scholar]
- McLaughlin, M.M.J.; Parker, D.R.; Clarke, J.M. Metals and micronutrients-food safety 660 issues. Field. Crops. Res. 1999, 60, 143–163. [Google Scholar] [CrossRef]
| Harvest Site | Bioclimatic Stage a | Latitude | Longitude | Altitude (M) | Rainfall (Mm/Year) | Mean Temperature (°C) |
|---|---|---|---|---|---|---|
| Bizerte | Inferior humid | 37°16′00″ N | 9°52′00″ E | 37 | 442 | 19 |
| Boussalem | Inferior humid | 36°36′40″ N | 8°58′11″ E | 141 | 404 | 18.4 |
| Zaghouan | Superior semi-arid | 36°21′07″ N | 10°06′43″ E | 800 | 617 | 17.7 |
| Tunis | Subhumid | 36°47′51″ N | 10°09′57″ E | 40 | 446 | 16 |
| Kasserine | Inferior semi-arid | 35°10′00″ N | 8°50′00″ E | 656 | 239 | 18.1 |
| Harvest Site | TPC (mg GAE/gDW) | Reducing Power Fe3+/Fe2+ (Asc AE) | DPPH Scavenging (EC50 µg.mL−1) | Bleaching of Beta Carotene (%) * |
|---|---|---|---|---|
| Boussalem | 11.44 a ± 0.12 | 149.72 1 b ± 0.00 | 780.54 b ± 1.05 | 2.38 |
| Bizerte | 31.89 c ± 0.35 | 125.86 1 b ± 0.50 | 1292.78 c ± 4.94 | 36.33 |
| Tunis | 16.81 c ± 0.14 | 45.99 1 a ± 0.11 | 2884.66 d ± 0.01 | 37.03 |
| Zaghouan | 13.61 ab ± 0.01 | 225.876 1 d± 0.01 | 30.66 a ± 0.03 | 46.41 |
| Kasserine | 24.56 1 b ± 0.01 | 199.82 1 c ± 2.78 | 319.36 a ±1.05 | 38.99 |
| Harvest Site | RSA (%) 1 | |
|---|---|---|
| Leaves | Stems | |
| Boussalem | 57.74 f ± 0.06 | 35.96 f ± 0.07 |
| Bizerte | 22.93 b ± 0.08 | 32.43 d ± 0.08 |
| Tunis | 70.68 g ± 0.06 | 27.55 c ± 0.07 |
| Zaghouan | 20.02 a ± 0.09 | ND 2 |
| Verbanone | 33.73 e ± 0.02 | |
| Cravacrol | 27.00 d ± 0.04 | |
| BHT 3 | 24.35 c ± 0.35 | |
| Harvest Site | Marrubiin Content (mg/g DW 1) | |
|---|---|---|
| Peak 1 2 | Peak 2 3 | |
| Boussalem | 2.60 b ± 0.28 | 2.73 b ± 0.34 |
| Bizerte | 1.65 a ± 0.33 | 1.82 a ± 0.34 |
| Zaghouan | nd 4 | nd 4 |
| Tunis | 5.98 c ± 0.77 | 6.84 d ± 0.98 |
| Kasserine | 5.14 c ± 0.01 | 5.5 c ± 0.01 |
| Harvest Site | Minerals (ppm) * | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ca | Mg | Fe | Zn | Cu | Mn | K | Pb | Cd | Ni | |
| Boussalem | 38,720 a ± 4 | 9648 a ± 2 | 208 ab ± 1 | 620 a ± 0 | 55 b ± 0 | 7 ab ± 0 | 20,800 b ± 0 | 0 | 11 a ± 0 | 34 ab ± 0 |
| Bizerte | 52,352 a ± 4 | 128,40 a ± 2 | 2848 ab ± 1 | 1124 a ± 0 | 26 a ± 0 | 5 a ± 0 | 112,00 a ± 0 | 0 | 13 a ± 0 | 28 ab ± 0 |
| Tunis | 46,160 a ± 1 | 12,448 a ± 5 | 2664 ab ± 1 | 869 a ± 0 | 28 a ±0 | 33 b ± 0 | 15,600 ab ± 0 | 0 | 12 a ± 0 | 122 b ± 0 |
| Zaghouan | 55,760 a ± 31 | 8176 a ± 0 | 1313 a ± 0 | 531 a ± 0 | 18 a ±0 | 7 ab ± 0 | 8400 a ± 0 | 0 | 12 a ± 0 | 28 ab ± 0 |
| Kasserine | 54,312 a ± 21 | 7672 a ± 0 | 9104 b ± 6 | 1148 a ± 0 | 24 a ±0 | 28 b ± 0 | 14,400 ab ± 0 | 0 | 8 a ± 0 | 22 a ± 0 |
| Compound | Rt (min) | Leaves | Stems | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Bizerte | Boussalem | Zaghouan | Tunis | Bizerte | Boussalem | Zaghouan | Tunis | ||
| Linalool | 4.13 | - | - | 1.05 | 0.49 | - | - | 0.36 | 0.13 |
| Phenylethyl alcohol | 4.30 | - | - | - | 0.16 | - | - | - | 0.10 |
| 1-terpinol | 4.52 | 0.12 | - | - | - | - | - | - | - |
| Ketoisophorone | 4.54 | - | - | - | 0.21 | - | - | - | - |
| Indole | 5.84 | - | 1.17 | 0.25 | 2.11 | 0.20 | 0.27 | - | 0.24 |
| 2-methoxy-4-vinylphenol | 5.94 | - | 1.72 | 1.51 | - | 0.31 | 0.42 | 0.49 | - |
| 3-allyl-6-methoxyphenol | 6.26 | - | - | - | - | 0.69 | - | - | - |
| Eugenol | 6.28 | - | 15.29 | 0.98 | 8.06 | - | 2.18 | 0.27 | 1.31 |
| Trans-caryophyllene | 6.83 | - | - | - | 0.61 | - | - | - | - |
| Geranylacetone | 6.90 | - | 0.75 | - | - | - | - | - | - |
| β-ionone | 7.22 | - | 4.14 | - | - | - | - | - | - |
| Germacrene D | 7.25 | - | - | 1.34 | - | - | - | 0.60 | - |
| β-bisabolene | 7.35 | - | 2.37 | - | 1.60 | 0.42 | 0.4 | - | 3.14 |
| δ-cadinene | 7.49 | - | - | 1.71 | 1.07 | - | - | - | - |
| Dihydroactinidiolide | 7.64 | - | - | - | 1.41 | - | 0.70 | - | - |
| Nerolidol | 7.67 | - | 1.02 | - | - | - | 0.5 | - | - |
| Hexadecame | 7.85 | - | - | - | - | 27.29 | - | - | - |
| Megastigmatrienone 2 | 7.86 | - | 2.45 | - | 1.95 | - | 0.49 | - | - |
| Spathylenol | 7.94 | - | - | 1.48 | - | - | - | - | - |
| Caryophyllene oxide | 7.99 | - | 0.70 | 0.65 | 0.56 | - | - | - | - |
| Megastigmatrienone isomer | 8.19 | - | 1.68 | - | - | - | 0.27 | - | - |
| τ muurolol | 8.31 | - | - | - | - | - | - | 1.24 | - |
| τ cadinol | 8.32 | - | - | 4.40 | - | - | - | - | - |
| α-cadinol | 8.40 | - | - | - | - | - | - | 1.01 | - |
| Miristic acid | 8.88 | - | - | - | 0.84 | 0.42 | - | - | - |
| Hexahydrofarnesyl acetone | 9.36 | - | 1.40 | 0.78 | 1.06 | - | 0.28 | - | - |
| Methyl palmitate | 9.80 | 1.23 | - | - | - | - | - | - | - |
| Palmitic acid | 10.04 | - | - | - | - | 9.99 | 2.03 | 0.57 | 2.00 |
| Methyl linoleate | 10.75 | - | - | - | - | - | 1.35 | - | - |
| Phytol | 10.81 | - | 1.04 | - | - | - | - | - | - |
| Linoleic acid | 11.01 | - | - | - | - | 3.27 | - | - | - |
| Butyl palmitate | 11.14 | 10.95 | 1.09 | - | 0.74 | - | - | - | - |
| Tricosane | 11.68 | - | - | - | - | - | 1.35 | - | - |
| Tetracosane | 12.14 | - | - | - | - | - | 1.95 | - | - |
| Pentacosane | 12.61 | - | - | - | - | - | 4.05 | - | 0.99 |
| Hexacosane | 13.01 | - | - | - | - | - | 4.77 | - | 0.45 |
| Heptacosane | 13.63 | - | - | - | - | - | 5.49 | - | 2.04 |
| Eicosane | 13.63 | 2.63 | - | - | - | - | - | - | - |
| Octacosane | 14.24 | - | - | - | - | - | 5.45 | - | 2.21 |
| Squalene | 14.54 | - | - | - | - | - | - | 1.85 | - |
| Marrubin | 14.64 | - | 4.09 | - | - | - | - | 6.67 | - |
| Nonacosane | 14.95 | - | - | - | - | - | 4.67 | - | 2.48 |
| Triacontane | 15.82 | - | - | - | - | - | 4.27 | - | 2.76 |
| Terpenoids | 0.12 | 18.6 | 11.41 | 8.96 | 0.42 | 2.64 | 11.73 | 3.27 | |
| Other compounds | 14.81 | 20.31 | 2.74 | 11.07 | 41.75 | 38.25 | 1.33 | 15.37 | |
| Harvest Site | Essential Oil (2% v/v) | |
|---|---|---|
| Leaves | Stems | |
| Bizerte | 7.14 a ± 1.00 | 9.52 a ±1.52 |
| Boussalem | 16.66 c ± 0.58 | 14.28 b ± 2.00 |
| Zaghouan | ND | 19.04 c ± 1.00 |
| Tunis | 11.90 b ± 0.57 | 14.28 b ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezgui, M.; Basma, M.; Neng, N.; Nogueira, J.M.; Bettaieb Ben-Kaab, L.; Machado Araújo, M.E. Evaluation of Marrubium vulgare Growing Wild in Tunisia for Its Potential as a Dietary Supplement. Foods 2021, 10, 2864. https://doi.org/10.3390/foods10112864
Rezgui M, Basma M, Neng N, Nogueira JM, Bettaieb Ben-Kaab L, Machado Araújo ME. Evaluation of Marrubium vulgare Growing Wild in Tunisia for Its Potential as a Dietary Supplement. Foods. 2021; 10(11):2864. https://doi.org/10.3390/foods10112864
Chicago/Turabian StyleRezgui, Marwa, Mabrouk Basma, Nuno Neng, José Manuel Nogueira, Leila Bettaieb Ben-Kaab, and Maria Eduarda Machado Araújo. 2021. "Evaluation of Marrubium vulgare Growing Wild in Tunisia for Its Potential as a Dietary Supplement" Foods 10, no. 11: 2864. https://doi.org/10.3390/foods10112864
APA StyleRezgui, M., Basma, M., Neng, N., Nogueira, J. M., Bettaieb Ben-Kaab, L., & Machado Araújo, M. E. (2021). Evaluation of Marrubium vulgare Growing Wild in Tunisia for Its Potential as a Dietary Supplement. Foods, 10(11), 2864. https://doi.org/10.3390/foods10112864







