Durability Assessment of a Plasma-Polymerized Coating with Anti-Biofilm Activity against L. monocytogenes Subjected to Repeated Sanitization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coating Process
2.2. Sanitization Process
2.3. Biofilm Formation Quantification
2.4. Statistical Analysis
2.5. Characterization of Samples
2.5.1. Atomic Force Microscopy (AFM) Analysis
2.5.2. Scanning Electron Microscopy (SEM) Analysis
2.5.3. Energy-Dispersive X-ray (EDX) Spectroscopy Analysis
2.5.4. X-ray Photoelectron Spectroscopy (XPS) Analysis
2.5.5. Fourier Transform Infrared Spectroscopy with Attenuated Total Reflectance (FTIR-ATR)
2.5.6. Water Contact Angle (WCA) Measurement
3. Results and Discussion
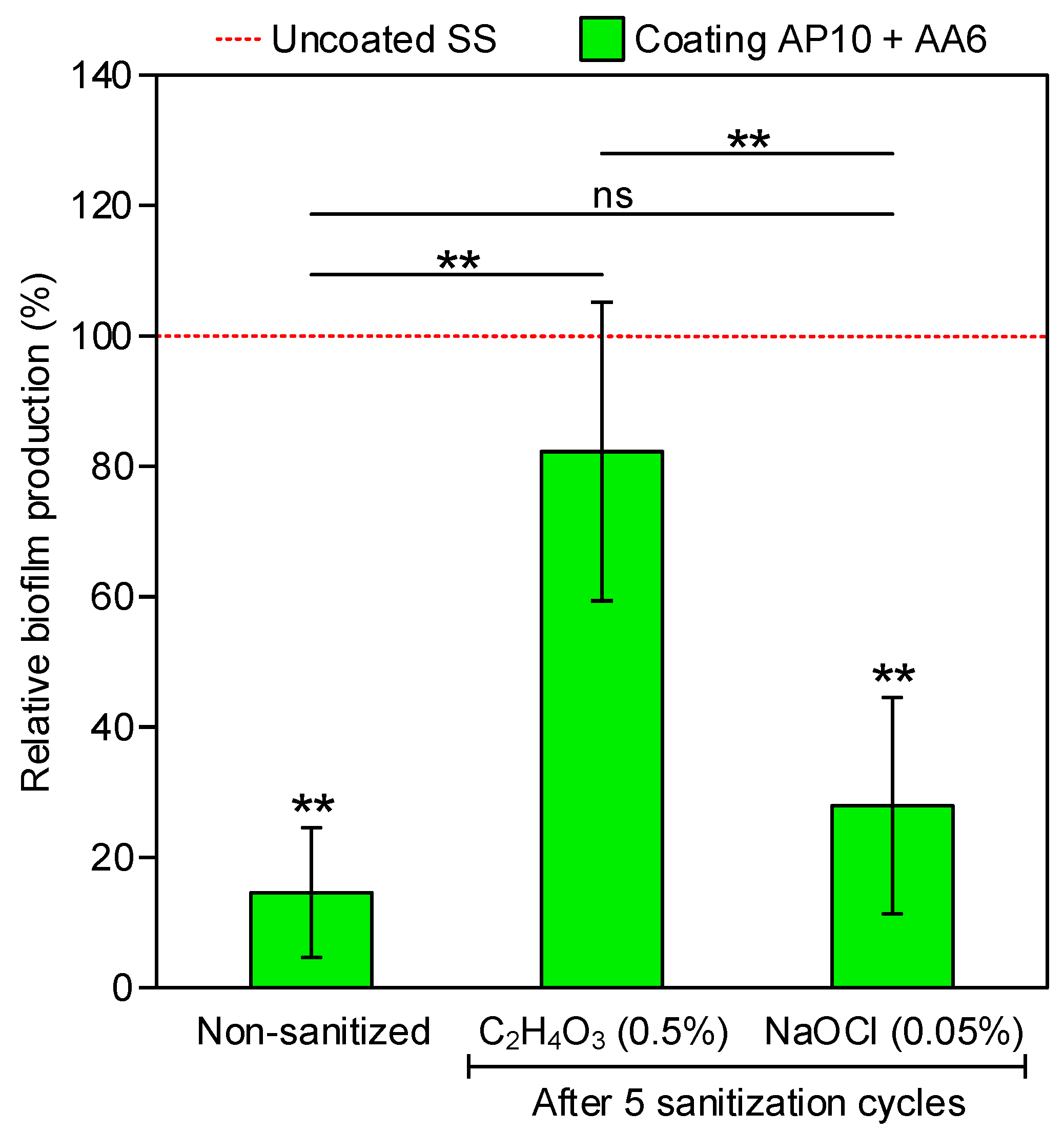
3.1. Biofilm Measurements
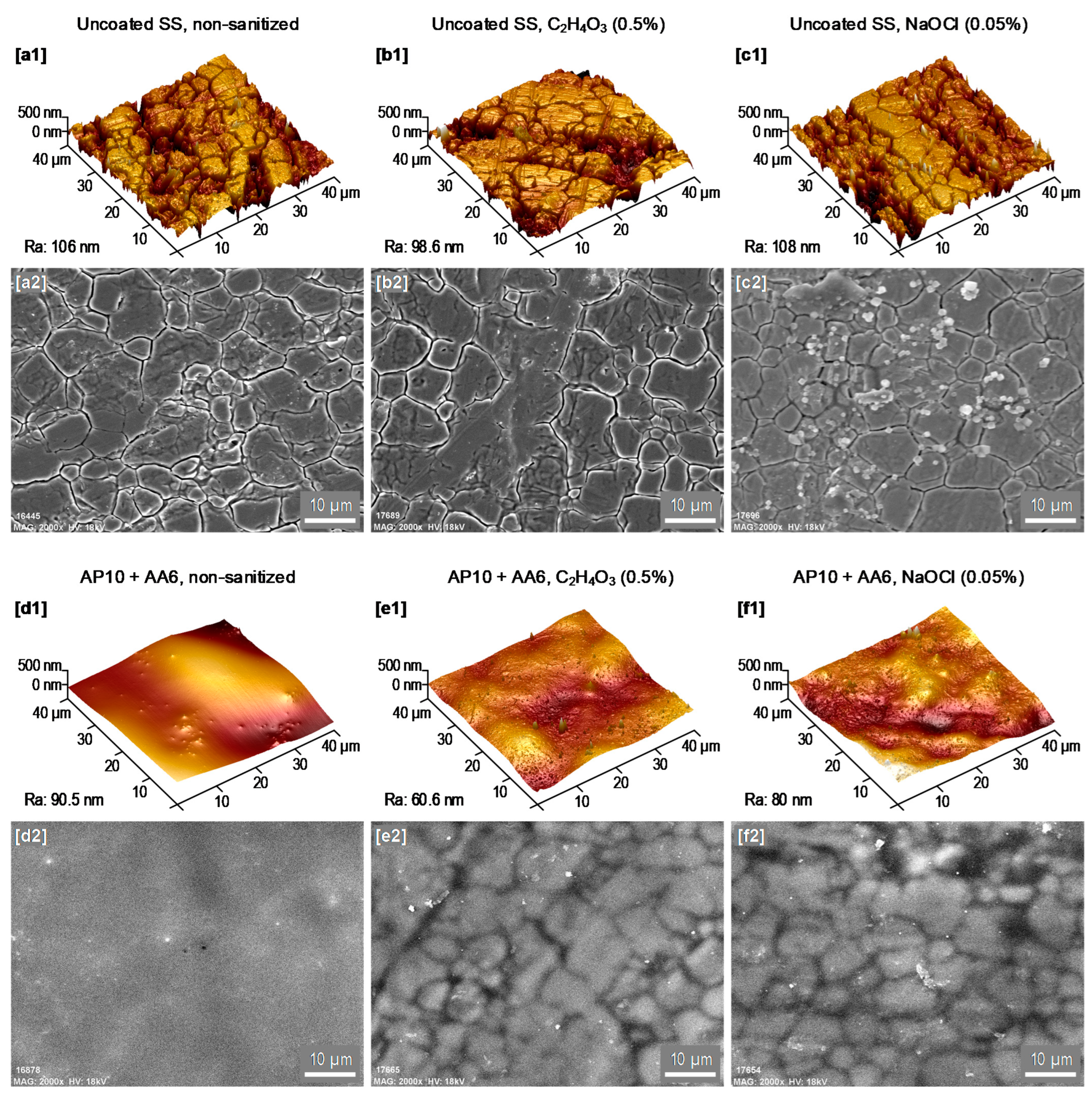
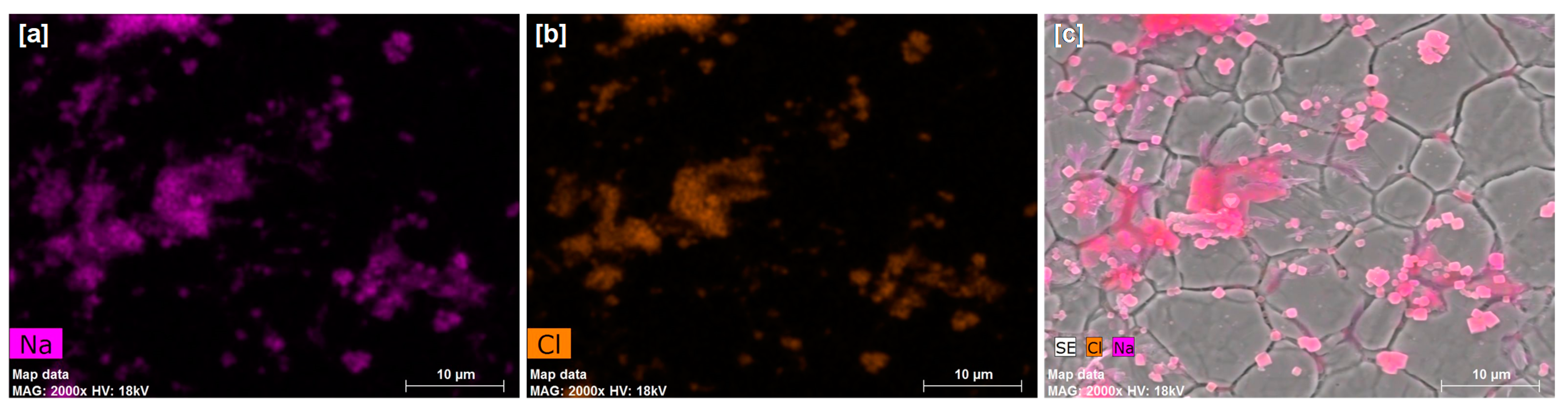
3.2. AFM and SEM-EDX Analyses
3.3. XPS Analysis
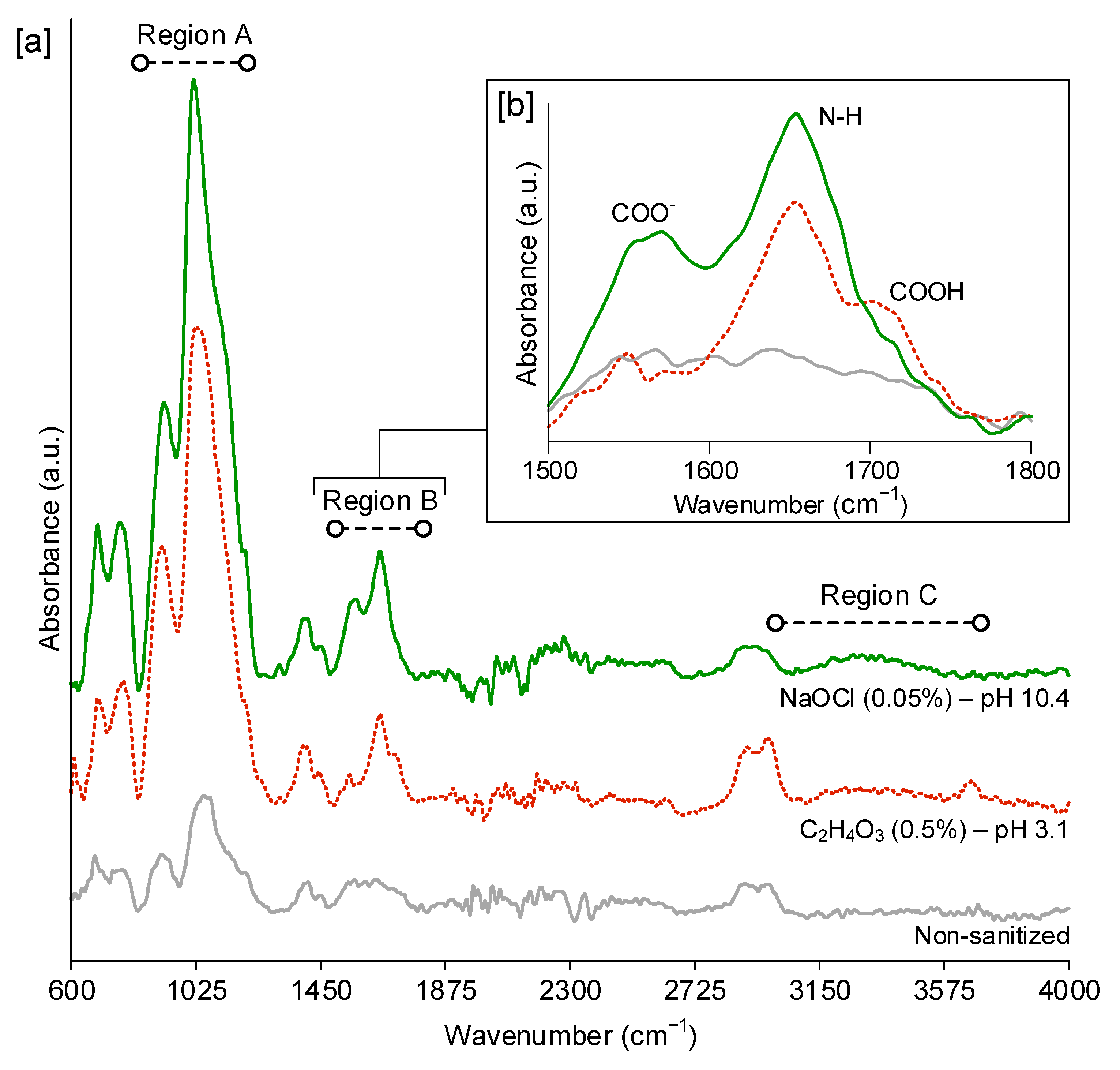
3.4. FTIR-ATR Analysis
3.5. WCA Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez-Melcón, C.; Riesco-Peláez, F.; García-Fernández, C.; Alonso-Calleja, C.; Capita, R. Susceptibility of Listeria monocytogenes planktonic cultures and biofilms to sodium hypochlorite and benzalkonium chloride. Food Microbiol. 2019, 82, 533–540. [Google Scholar] [CrossRef]
- Yuan, L.; Hansen, M.F.; Røder, H.L.; Wang, N.; Burmølle, M.; He, G. Mixed-species biofilms in the food industry: Current knowledge and novel control strategies. Crit. Rev. Food Sci. Nutr. 2020, 60, 2277–2293. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial biofilms in the food industry—A comprehensive review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef]
- Grigore-Gurgu, L.; Bucur, F.I.; Borda, D.; Alexa, E.-A.; Neagu, C.; Nicolau, A.I. Biofilms formed by pathogens in food and food processing environments. In Bacterial Biofilms; Dincer, S., Özdenefe, M.S., Arkut, A., Eds.; Intech Open: London, UK, 2020. [Google Scholar]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Mai, S.; Yang, C.; Wang, X.; An, Y.; Zhou, Z. Research and applications of membrane bioreactors in China: Progress and prospect. Sep. Purif. Technol. 2008, 62, 249–263. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Z.; Wu, Z.; Zhou, Q.; Yang, D. Effect of hypochlorite cleaning on the physiochemical characteristics of polyvinylidene fluoride membranes. Chem. Eng. J. 2010, 162, 1050–1056. [Google Scholar] [CrossRef]
- Guo, L.; Sun, Y.; Zhu, Y.; Wang, B.; Xu, L.; Huang, M.; Li, Y.; Sun, J. The antibacterial mechanism of ultrasound in combination with sodium hypochlorite in the control of Escherichia coli. Food Res. Int. 2020, 129, 108887. [Google Scholar] [CrossRef] [PubMed]
- Byun, M.W.; Kim, J.H.; Kim, D.H.; Kim, H.J.; Jo, C. Effects of irradiation and sodium hypochlorite on the micro-organisms attached to a commercial food container. Food Microbiol. 2007, 24, 544–548. [Google Scholar] [CrossRef]
- Meireles, A.; Machado, I.; Fulgêncio, R.; Mergulhão, F.; Melo, L.; Simões, M. Efficacy of antimicrobial combinations to reduce the use of sodium hypochlorite in the control of planktonic and sessile Escherichia coli. Biochem. Eng. J. 2015, 104, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Park, K.M.; Yoon, S.-G.; Choi, T.-H.; Kim, H.J.; Park, K.J.; Koo, M. The bactericidal effect of a combination of food-grade compounds and their application as alternative antibacterial agents for food contact surfaces. Foods 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.-N.; Huang, C.-H. Formation of disinfection byproducts in wash water and lettuce by washing with sodium hypochlorite and peracetic acid sanitizers. Food Chem. X 2019, 1, 100003. [Google Scholar] [CrossRef]
- Luongo, G.; Previtera, L.; Ladhari, A.; Di Fabio, G.; Zarrelli, A. Peracetic acid vs. Sodium hypochlorite: Degradation and transformation of drugs in wastewater. Molecules 2020, 25, 2294. [Google Scholar] [CrossRef]
- Cao, P.; Li, W.W.; Morris, A.R.; Horrocks, P.D.; Yuan, C.Q.; Yang, Y. Investigation of the antibiofilm capacity of peptide-modified stainless steel. R. Soc. Open Sci. 2018, 5, 172165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedlander, A.; Nir, S.; Reches, M.; Shemesh, M. Preventing biofilm formation by dairy-associated bacteria using peptide-coated surfaces. Front. Microbiol. 2019, 10, 1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faure, E.; Vreuls, C.; Falentin-Daudré, C.; Zocchi, G.; van de Weerdt, C.; Martial, J.; Jérôme, C.; Duwez, A.S.; Detrembleur, C. A green and bio-inspired process to afford durable anti-biofilm properties to stainless steel. Biofouling 2012, 28, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gómez, P.; Muro-Fraguas, I.; Múgica-Vidal, R.; Sainz-García, A.; Sainz-García, E.; González-Raurich, M.; Álvarez-Ordóñez, A.; Prieto, M.; López, M.; López, M.; et al. Development and characterization of anti-biofilm coatings applied by Non-Equilibrium Atmospheric Plasma on stainless steel. Food Res. Int. 2020, 109891. [Google Scholar] [CrossRef]
- Zhong, L.J.; Pang, L.Q.; Che, L.M.; Wu, X.E.; Chen, X.D. Nafion coated stainless steel for anti-biofilm application. Colloids Surf. B Biointerfaces 2013, 111, 252–256. [Google Scholar] [CrossRef]
- Alvarez-Molina, A.; Cobo-Díaz, J.F.; López, M.; Prieto, M.; de Toro, M.; Alvarez-Ordóñez, A. Unraveling the emergence and population diversity of Listeria monocytogenes in a newly built meat facility through whole genome sequencing. Int. J. Food Microbiol. 2021, 340, 109043. [Google Scholar] [CrossRef] [PubMed]
- The European Parliament and the Council of the European Union. Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 Laying Down Specific Hygiene Rules for Food of Animal Origin; L226; The European Parliament and the Council of the European Union: Brussels, Belgium, 2004; pp. 22–82. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Stalder, A.F.; Melchior, T.; Müller, M.; Sage, D.; Blu, T.; Unser, M. Low-bond axisymmetric drop shape analysis for surface tension and contact angle measurements of sessile drops. Colloids Surf. A Physicochem. Eng. Asp. 2010, 364, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Short, R.D.; Dorn, S.O.; Kuttler, S. The crystallization of sodium hypochlorite on gutta-percha cones after the rapid-sterilization technique: An SEM study. J. Endod. 2003, 29, 670–673. [Google Scholar] [CrossRef]
- Kim, K.-W.; Lee, E.-H.; Chung, D.-Y.; Moon, J.-K.; Shin, H.-S.; Kim, J.-S.; Shin, D.-W. Manufacture characteristics of metal oxide—hydroxides for the catalytic decomposition of a sodium hypochlorite solution. Chem. Eng. J. 2012, 200–202, 52–58. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, X.; Jing, K.; Chang, L.; Bao, W. Study on the pore structure and oxygen-containing functional groups devoting to the hydrophilic force of dewatered lignite. Appl. Surf. Sci. 2015, 324, 90–98. [Google Scholar] [CrossRef]
- Muzammil, I.; Li, Y.; Lei, M. Tunable wettability and pH-responsiveness of plasma copolymers of acrylic acid and octafluorocyclobutane. Plasma Process. Polym. 2017, 14, e1700053. [Google Scholar] [CrossRef]
- Wang, T.; Canetta, E.; Weerakkody, T.G.; Keddie, J.L.; Rivas, U. pH dependence of the properties of waterborne pressure-sensitive adhesives containing acrylic acid. ACS Appl. Mater. Interfaces 2009, 1, 631–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, T.; Jańczewski, D.; Guo, S.; Man, S.M.; Jiang, S.; Tan, W.S. Stable pH responsive layer-by-layer assemblies of partially hydrolysed poly(2-ethyl-2-oxazoline) and poly(acrylic acid) for effective prevention of protein, cell and bacteria surface attachment. Colloids Surf. B Biointerfaces 2018, 161, 269–278. [Google Scholar] [CrossRef]
- Wei, T.; Yu, Q.; Zhan, W.; Chen, H. A smart antibacterial surface for the on-demand killing and releasing of bacteria. Adv. Healthc. Mater. 2016, 5, 449–456. [Google Scholar] [CrossRef]
- Dong, R.; Lindau, M.; Ober, C.K. Dissociation behavior of weak polyelectrolyte brushes on a planar surface. Langmuir 2009, 25, 4774–4779. [Google Scholar] [CrossRef]
- Lei, H.; Wang, M.; Tang, Z.; Luan, Y.; Liu, W.; Song, B.; Chen, H. Control of lysozyme adsorption by pH on surfaces modified with polyampholyte brushes. Langmuir 2014, 30, 501–508. [Google Scholar] [CrossRef]
- Sainz-García, E.; Alba-Elías, F.; Múgica-Vidal, R.; Pantoja-Ruiz, M. Promotion of tribological and hydrophobic properties of a coating on TPE substrates by atmospheric plasma-polymerization. Appl. Surf. Sci. 2016, 371, 50–60. [Google Scholar] [CrossRef]
- Tang, Y.; Xia, X. Improvement of the hydrophilicity of Cu/LDPE composite and its influence on the release of cupric ions. Mater. Sci. Forum 2013, 745–746, 46–52. [Google Scholar] [CrossRef]

| Sample | Sanitizing Solution | Elemental Composition (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C1s | N1s | O1s | Si2p | Fe2p | Cr2p | Na1s | Cl2p | ||
| SS AISI 316 | None | 53.99 | 1.33 | 33.56 | - | 9.30 | 1.83 | - | - |
| C2H4O3 (0.5%) | 47.37 | 2.13 | 30.32 | - | 15.68 | 3.59 | - | - | |
| NaOCl (0.05%) | 59.13 | 0.73 | 26.69 | 0.71 | 6.76 | 0.97 | 4.19 | 0.82 | |
| AP10 + AA6 | None | 66.70 | 2.52 | 30.63 | 0.15 | - | - | - | - |
| C2H4O3 (0.5%) | 66.75 | 2.97 | 25.77 | 4.51 | - | - | - | - | |
| NaOCl (0.05%) | 58.33 | 3.49 | 28.91 | 5.86 | - | - | 2.07 | 1.34 | |
| AP10 | None | 48.55 | 5.10 | 35.21 | 11.13 | - | - | - | - |
| Sample | Sanitizing Solution | Contribution in the C1s Region (at %) | ||||
|---|---|---|---|---|---|---|
| C-C, C-H | C-O | C=O | O-C=O | Total Polar Groups (Sum of C-O, C=O, and O-C=O) | ||
| SS AISI 316 | None | 70.19 | 16.58 | 7.46 | 5.76 | 29.81 |
| C2H4O3 (0.5%) | 72.30 | 17.99 | 4.69 | 5.02 | 27.70 | |
| NaOCl (0.05%) | 75.86 | 14.07 | 4.49 | 5.57 | 24.14 | |
| AP10 + AA6 | None | 45.36 | 28.33 | 0.36 | 25.95 | 54.64 |
| C2H4O3 (0.5%) | 56.82 | 28.26 | 2.75 | 12.71 | 43.18 | |
| NaOCl (0.05%) | 52.81 | 25.21 | 15.36 | 6.62 | 47.19 | |
| AP10 | None | 53.59 | 36.36 | 10.05 | - | 46.41 |
| Sample | Sanitizing Solution | O/C Ratio | Oxygenated/Non-Oxygenated Carbon Ratio |
|---|---|---|---|
| SS AISI 316 | None | 0.62 | 0.42 |
| C2H4O3 (0.5%) | 0.64 | 0.38 | |
| NaOCl (0.05%) | 0.45 | 0.32 | |
| AP10 + AA6 | None | 0.46 | 1.20 |
| C2H4O3 (0.5%) | 0.39 | 0.76 | |
| NaOCl (0.05%) | 0.50 | 0.89 | |
| AP10 | None | 0.73 | 0.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muro-Fraguas, I.; Fernández-Gómez, P.; Múgica-Vidal, R.; Sainz-García, A.; Sainz-García, E.; Oliveira, M.; González-Raurich, M.; López, M.; Rojo-Bezares, B.; López, M.; et al. Durability Assessment of a Plasma-Polymerized Coating with Anti-Biofilm Activity against L. monocytogenes Subjected to Repeated Sanitization. Foods 2021, 10, 2849. https://doi.org/10.3390/foods10112849
Muro-Fraguas I, Fernández-Gómez P, Múgica-Vidal R, Sainz-García A, Sainz-García E, Oliveira M, González-Raurich M, López M, Rojo-Bezares B, López M, et al. Durability Assessment of a Plasma-Polymerized Coating with Anti-Biofilm Activity against L. monocytogenes Subjected to Repeated Sanitization. Foods. 2021; 10(11):2849. https://doi.org/10.3390/foods10112849
Chicago/Turabian StyleMuro-Fraguas, Ignacio, Paula Fernández-Gómez, Rodolfo Múgica-Vidal, Ana Sainz-García, Elisa Sainz-García, Márcia Oliveira, Montserrat González-Raurich, María López, Beatriz Rojo-Bezares, Mercedes López, and et al. 2021. "Durability Assessment of a Plasma-Polymerized Coating with Anti-Biofilm Activity against L. monocytogenes Subjected to Repeated Sanitization" Foods 10, no. 11: 2849. https://doi.org/10.3390/foods10112849
APA StyleMuro-Fraguas, I., Fernández-Gómez, P., Múgica-Vidal, R., Sainz-García, A., Sainz-García, E., Oliveira, M., González-Raurich, M., López, M., Rojo-Bezares, B., López, M., & Alba-Elías, F. (2021). Durability Assessment of a Plasma-Polymerized Coating with Anti-Biofilm Activity against L. monocytogenes Subjected to Repeated Sanitization. Foods, 10(11), 2849. https://doi.org/10.3390/foods10112849






