Microalgae Xanthophylls: From Biosynthesis Pathway and Production Techniques to Encapsulation Development
Abstract
:1. Introduction
2. Main Xanthophylls Present in Microalgae
2.1. Fucoxanthin
2.2. Astaxanthin (ASX)
2.3. Lutein
2.4. Zeaxanthin
2.5. Violaxanthin
2.6. Canthaxanthin
2.7. β-Cryptoxanthin
2.8. Diatoxanthin
2.9. Diadinoxanthin
| Common Names | IUPAC Nomenclature | Molecular Formulas | Chemical Structures | References |
|---|---|---|---|---|
| Fucoxanthin | 3,5′-Dihydroxy-8-oxo-6′,7′-didehydro-5,6-epoxy-5,6,7,8,5′,6′-hexahydro-β,β-caroten-3′-yl acetate | C42H58O6 | 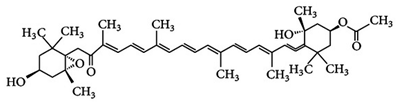 3S,5R,6S,3′S’,5′R,6′R)-3,5′-dihydroxy-8-oxo-6′,7′-didehydro-5,6-epoxy-5,6,7,8,5′,6′-hexahydro-β,β-caroten-3′-yl acetate | [91] |
| ASX | 3,3′-Dihydroxy-β,β-carotene-4,4′-dione | C40H52O4 |  (3S,30S)-3,30-dihydroxy-β,β-carotene-4,40-dione | [12] |
| Lutein | β- ε-Carotene-3,3′-diol | C40H56O2 |  (3R,3′R,6′R)-β,ε-carotene-3,3′-diol | [92] |
| Zeaxanthin | β,β-Carotene-3,3′-diol | C40H56O2 | 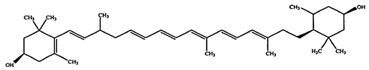 (3R,30R)-β,β-carotene-3,3′-diol | [92] |
| Violaxanthin | 5,5′,6,6′-Tetrahydro-5,6:5′,6′-diepoxy-β,β-carotene-3,3′-diol | C40H56O4 | 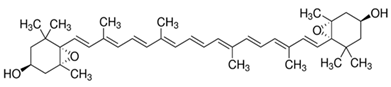 S,3′S,5R,5′R,6S,6′S)-5,5′,6,6′-tetrahydro-5,6:5′,6′-diepoxy-β,β-carotene-3 | [93] |
| Canthaxanthin | β,β-Carotene-4,40-dione | C40H52O2 |  trans-β-carotene-4,4′-dione | [94] |
| β-Cryptoxanthin | β,β-Caroten-3-ol | C40H56O | 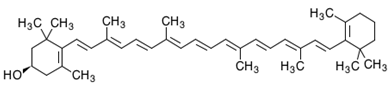 (3R)-β,β-Caroten-3-ol | [95] |
| Diadinoxanthin | 5,6-Epoxy-7’,8’-didehydro-5,6-dihydro-b,b-carotene-3,3-diol | C40H54O3 | 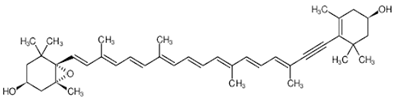 (3S,3’R,5R,6R)-7’,8’-Didehydro-3,6-epoxy-5,6-dihydro- β, β -carotene-3’,5-diol | [61] |
| Diatoxanthin | 3,3′-7,8-Didehydro-ß,ß-carotene-3,3’-diol | C40H54O2 |  (3R,3’R)-7,8-Didehydro- β, β -carotene-. 3,3’-diol | [61] |
3. Structures of Xanthophylls
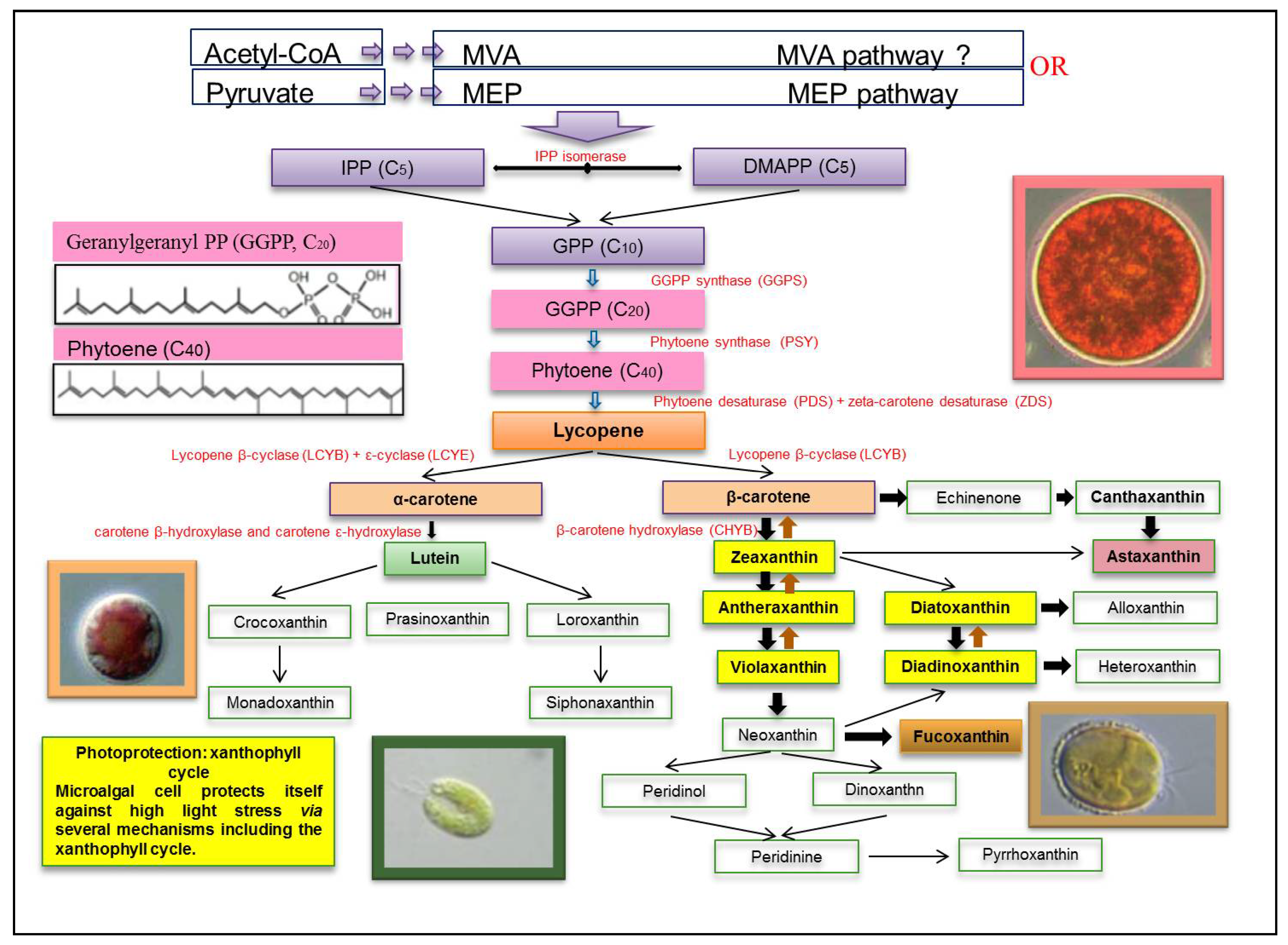
4. Biosynthesis of Xanthophylls
5. Cellular Location and Function of Xanthophylls
6. Recent Applications in Metabolic Engineering for Xanthophylls Production
7. Bioprocess for Xanthophylls Production by Microalgae
7.1. Cultivation Systems
7.1.1. Open Systems
7.1.2. Closed Systems
- -
- The tubular type is the most appropriate kind of PBR for producing satisfying high-quality cyanobacteria and microalgae biomasses in outdoor environments [123,128]. It is generally built with glass or plastic tubes, allowing a large illuminated surface area. In this system, the culture homogenization is generally assured by means of air pumps. It is characterized by some defects, such as pH variation, dissolved oxygen, fouling, and CO2 heterogeneity. There are many studies indicating the suitability of using this PBR kind for high-quality microalgae and cyanobacteria productions.
- -
- Flat PBRs have a large surface exposed to light and are characterized by high algal productivities, which is generally greater than those produced by tubular PBR. This culture system is constructed from a rigid transparent material to optimize light capture and to facilitate sterilization. It is suitable for outdoor cultivation, ideal for cell immobilization and is relatively inexpensive. The only drawback of this type of system is the difficulty in controlling the temperature of algal cultures [129]. Flat PBRs have been tested for culturing the marine diatom Phaeodactylum tricornutum for the production of fucoxanthin and chrysolaminarin [130]. The AlgaTechnologies industry (https://www.algatech.com/, accessed on 15 April 2021) also established a Haematococcus cultivation facility back in the late 1990s. Quite different to other American industries, the AlgaTechnologies Company used glass tubular PBRs for both green and red phases to phototrophically cultivate Haematococcus [131].
7.2. Factors Determining Xanthophylls Production
7.2.1. Light
7.2.2. Temperature
7.2.3. Salinity
7.2.4. Nutrient-Related Stresses
Nitrogen Starvation
Iron Supplementation
Sulfur Limitation
8. Encapsulation of Xanthophylls
9. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Afreen, R.; Tyagi, S.; Singh, G.P.; Singh, M. Challenges and Perspectives of Polyhydroxyalkanoate Production from Microalgae/Cyanobacteria and Bacteria as Microbial Factories: An Assessment of Hybrid Biological System. Front. Bioeng. Biotechnol. 2021, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.K.; Kausar, H.; Jaferi, S.S.; Drouet, S.; Hano, C.; Abbasi, B.H.; Anjum, S. An insight into the algal evolution and genomics. Biomolecules 2020, 10, 1524. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, K.K.; Bhattarai, S.; Bhandari, P. Handbook of Flowering Plants of Nepal (Vol. 1 Gymnosperms and Angiosperms: Cycadaceae-Betulaceae); Scientific Publishers: Norwood, NJ, USA, 2018. [Google Scholar]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef] [PubMed]
- Dammak, M.; Haase, S.M.; Miladi, R.; Ben Amor, F.; Barkallah, M.; Gosset, D.; Pichon, C.; Huchzermeyer, B.; Fendri, I.; Denis, M.; et al. Enhanced lipid and biomass production by a newly isolated and identified marine microalga. Lipids Health Dis. 2016, 15, 209. [Google Scholar] [CrossRef] [Green Version]
- Dammak, M.; Hadrich, B.; Barkallah, M.; Hentati, F.; Ben Hlima, H.; Pichon, C.; Denis, M.; Fendri, I.; Michaud, P.; Abdelkafi, S. Modelling Tetraselmis sp. growth-kinetics and optimizing bioactive-compound production through environmental conditions. Bioresour. Technol. 2018, 249, 510–518. [Google Scholar] [CrossRef]
- Ben Hlima, H.; Bohli, T.; Kraiem, M.; Ouederni, A.; Mellouli, L.; Michaud, P.; Abdelkafi, S.; Smaoui, S. Combined effect of Spirulina platensis and Punica granatum peel extacts: Phytochemical content and antiphytophatogenic activity. Appl. Sci. 2019, 9, 5475. [Google Scholar]
- Elleuch, F.; Ben Hlima, H.; Barkallah, M.; Baril, P.; Abdelkafi, S.; Pichon, C.; Fendri, I. Carotenoids overproduction in Dunaliella sp.: Transcriptional changes and new insights through lycopene cyclase regulation. Appl. Sci. 2019, 9, 5389. [Google Scholar] [CrossRef] [Green Version]
- Barkallah, M.; Ben Slima, A.; Fendri, I.; Pichon, C.; Abdelkafi, S.; Baril, P. Protective role of Spirulina platensis against bifenthrin-induced reprotoxicity in adult male mice by reversing expression of altered histological, biochemical, and molecular markers including microRNAs. Biomolecules 2020, 10, 7539. [Google Scholar] [CrossRef]
- Jacob-Lopes, E.; Maroneze, M.M.; Deprá, M.C.; Sartori, R.B.; Dias, R.R.; Zepka, L.Q. Bioactive food compounds from microalgae: An innovative framework on industrial biorefineries. Curr. Opin. Food Sci. 2019, 25, 1–7. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid metabolism in plants. Mol. Plant. 2015, 8, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from marine organisms: Biological functions and industrial applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef] [Green Version]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential allies of cardiovascular health? Food. Nutr. Res. 2015, 59, 26762. [Google Scholar] [PubMed] [Green Version]
- LaFountain, A.M.; Prum, R.O.; Frank, H.A. Diversity, physiology, and evolution of avian plumage carotenoids and the role of carotenoid–protein interactions in plumage color appearance. Arch. Biochem. Biophys. 2015, 572, 201–212. [Google Scholar] [CrossRef]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef] [PubMed]
- Torregrosa-Crespo, J.; Montero, Z.; Fuentes, J.L.; García-Galbis, M.R.; Garbayo, I.; Vílchez, C.; Martínez-Espinosa, R.M. Exploring the Valuable Carotenoids for the Large-Scale Production by Marine Microorganisms. Mar. Drugs 2018, 16, 203. [Google Scholar] [CrossRef] [Green Version]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frigola, A. Analytical methods for determining bio-availability andbio-accessibility of bioactive compounds from fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Ojulari, O.V.; Gi Lee, S.; Nam, J.O. Therapeutic Effect of Seaweed Derived Xanthophyl Carotenoid on Obesity Management; Overview of the Last Decade. Int. J. Mol. Sci. 2020, 21, 2502. [Google Scholar] [CrossRef] [Green Version]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Inno Gonçalves vative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.C.; Ferreira, I.C.F.R.; Dias, M.M.; Barreiro, M.F. Microalgae-Derived Pigments: A 10-Year Bibliometric Review andIndustry and Market Trend Analysis. Molecules 2020, 25, 3406. [Google Scholar] [CrossRef]
- Jain, A.; Sirisha, V.L. Algal Carotenoids: Understanding Their Structure, Distribution and Potential Applications in Human Health. Encycl. Mar. Biotechnol. 2020, 1, 33–64. [Google Scholar]
- da Silva Vaz, B.; Moreira, J.B.; de Morais, M.G.; Costa, J.A.V. Microalgae as a new source of bioactive compounds in food supplements. Curr. Opin. Food Sci. 2016, 7, 73–77. [Google Scholar]
- Mulders, K.J.M.; Lamers, P.P.; Martens, D.E.; Wijffels, R.H. Phototrophic Pigment Production with Microalgae: Biological Constraints and Opportunities. J. Phycol. 2014, 50, 229–242. [Google Scholar] [CrossRef]
- de Oliveira-Júnior, R.G.; Grougnet, R.; Bodet, P.-E.; Bonnet, A.; Nicolau, E.; Jebali, A.; Rumin, J.; Picot, L. Updated pigment composition of Tisochrysis lutea and purification of fucoxanthin using centrifugal partition chromatography coupled to flash chromatography for the chemosensitization of melanoma cells. Algal Res. 2020, 51, 102035. [Google Scholar] [CrossRef]
- Petrushkina, M.; Gusev, E.; Sorokin, B.; Zotko, N.; Mamaeva, A.; Filimonova, A.; Kulikovskiy, M.; Maltsev, Y.; Yampolsky, I.; Guglya, E.; et al. Fucoxanthin production by heterokont microalgae. Algal Res. 2017, 24, 387–393. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, S.W.; Kwon, O.N.; Chung, D.; Pan, C.H. Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: Characterization of extraction for commercial application. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 477–483. [Google Scholar] [CrossRef]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef]
- Marella, T.K.; Tiwari, A. Marine diatom Thalassiosira weissflogii based biorefinery for co-production of eicosapentaenoic acid and fucoxanthin. Bioresour. Technol. 2020, 307, 123245. [Google Scholar] [CrossRef]
- Panis, G.; Carreon, J.R. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016, 18, 175–190. [Google Scholar] [CrossRef] [Green Version]
- Aflalo, C.; Meshulam, Y.; Zarka, A.; Boussiba, S. On the relative efficiency of two-vs. one-stage production of astaxanthin by the green alga Haematococcus pluvialis. Biotechnol. Bioeng. 2007, 98, 300–305. [Google Scholar] [CrossRef]
- Torzillo, G.; Goksan, T.; Faraloni, C.; Kopecky, J.; Masojídek, J. Interplay between Photochemical Activities and Pigment Composition in an Outdoor Culture of Haematococcus pluvialis during the Shift from the Green to Red Stage. J. Appl. Phycol. 2003, 15, 127–136. [Google Scholar] [CrossRef]
- Ranga, R.; Sarada, A.R.; Baskaran, V.; Ravishankar, G.A. Identification of carotenoids from green alga Haematococcus pluvialis by HPLC and LC-MS (APCI) and their antioxidant properties. J. Microbiol. Biotechnol. 2009, 19, 1333–1341. [Google Scholar]
- Molino, A.; Rimauro, J.; Casella, P.; Cerbone, A.; Larocca, V.; Chianese, S.; Karatza, D.; Mehariya, S.; Ferraro, A.; Hristoforou, E. Extraction of Astaxanthin from Microalga Haematococcus pluvialis in Red Phase by Using Generally Recognized as Safe Solvents and Accelerated Extraction. J. Biotechnol. 2018, 283, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Yao, J.; Lee, C.; Park, J.; Choi, Y. A novel approach to enhance astaxanthin production in Haematococcus lacustris using a microstructure-based culture platform. Algal Res. 2019, 39, 101464. [Google Scholar] [CrossRef]
- Fábryová, T.; Cheel, J.; Kubáč, D.; Hrouzek, P.; Tůmová, L.; Kopecký, J. Purification of lutein from the green microalgae Chlorella vulgaris by integrated use of a new extraction protocol and a multi-injection high performance counter-current chromatography (HPCCC). Algal Res. 2019, 41, 101574. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Dhanarajan, G.; Dash, S.K.; Sen, R. An advanced hybrid medium optimization strategy for the enhanced productivity of lutein in Chlorella minutissima. Algal Res. 2015, 7, 24–32. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Liu, C.-C. Optimization of lutein production with a two-stage mixotrophic cultivation system with Chlorella sorokiniana MB-1. Bioresour. Technol. 2018, 262, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Minhas, A.K.; Hodgson, P.; Barrow, C.J.; Sashidhar, B.; Adholeya, A. The isolation and identification of new microalgal strains producing oil and carotenoid simultaneously with biofuel potential. Bioresour. Technol. 2016, 211, 556–565. [Google Scholar] [CrossRef]
- Serejo, M.L.; Posadas, E.; Boncz, M.A.; Blanco, S.; García-Encina, P.; Muñoz, R. Influence of Biogas Flow Rate on Biomass Composition during the Optimization of Biogas Upgrading in Microalgal-Bacterial Processes. Environ. Sci. Technol. 2015, 49, 3228–3236. [Google Scholar]
- Sun, Z.; Li, T.; Zhou, Z.G.; Jiang, Y. Microalgae as a source of lutein: Chemistry, biosynthesis, and carotenogenesis. Microalgae Biotechnol. 2015, 37–58. [Google Scholar]
- Shi, X.M.; Jiang, Y.; Chen, F. High-Yield Production of Lutein by the Green Microalga Chlorella Protothecoides in Heterotrophic Fed-Batch Culture. Biotechnol. Prog. 2002, 18, 723–727. [Google Scholar] [CrossRef]
- Schüler, L.M.; Gangadhar, K.N.; Duarte, P.; Placines, C.; Molina-Márquez, A.M.; Léon-Bañares, R.; Sousa, V.S.; Varela, J.; Barreira, L. Improvement of carotenoid extraction from a recently isolated, robust microalga, Tetraselmis sp. CTP4 (chlorophyta). Bioprocess. Biosyst. Eng. 2020, 43, 785–796. [Google Scholar] [CrossRef]
- Ma, R.; Zhao, X.; Xie, Y.; Ho, S.H.; Chen, J. Enhancing lutein productivity of Chlamydomonas sp. via high-intensity light exposure with corresponding carotenogenic genes expression profiles. Bioresour. Technol. 2019, 275, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.M.; Moreno, J.; Del Campo, J.A.; Rivas, J.; Guerrero, M.G. Outdoor cultivation of lutein-rich cells of Muriellopsis sp. in open ponds. Appl. Microbiol. Biotechnol. 2007, 73, 1259–1266. [Google Scholar] [CrossRef]
- Garbayo, I.; Cuaresma, M.; Vílchez, C.; Vega, J.M. Effect of abiotic stress on the production of lutein and β-carotene by Chlamydomonas acidophila. Process. Biochem. 2008, 43, 1158–1161. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Karatza, D.; Chianese, S.; Iovine, A.; Casella, P. Bench-Scale Cultivation of Microalgae Scenedesmus almeriensis for CO2 Capture and Lutein Production. Energies 2019, 12, 2806. [Google Scholar] [CrossRef] [Green Version]
- Ho, S.H.; Chan, M.C.; Liu, C.C.; Chen, C.Y.; Lee, W.L.; Lee, D.J.; Chang, J.S. Enhancing lutein productivity of an indigenous microalga Scenedesmus obliquus FSP-3 using light-related strategies. Bioresour. Technol. 2014, 152, 275–282. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, X.; Chen, J.; Yang, X.; Ho, S.H.; Wang, B.; Chang, J.S.; Shen, Y. Enhancing cell growth and lutein productivity of Desmodesmus sp. F51 by optimal utilization of inorganic carbon sources and ammonium salt. Bioresour. Technol. 2017, 244, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Liau, B.C.; Hong, S.E.; Chang, L.P.; Shen, C.T.; Li, Y.C.; Wu, Y.P.; Jong, T.T.; Shieh, C.J.; Hsu, S.L.; Chang, C.M.J. Separation of Sight-Protecting Zeaxanthin from Nannochloropsis Oculata by Using Supercritical Fluids Extraction Coupled with Elution Chromatography. Sep. Purif. Technol. 2011, 78, 1–8. [Google Scholar] [CrossRef]
- Koo, S.Y.; Cha, K.H.; Song, D.G.; Chung, D.; Pan, C.H. Optimization of Pressurized Liquid Extraction of Zeaxanthin from Chlorella Ellipsoidea. J. Appl. Phycol. 2012, 24, 725–730. [Google Scholar] [CrossRef]
- Plaza, M.; Santoyo, S.; Jaime, L.; García-Blairsy Reina, G.; Herrero, M.; Señoráns, F.J.; Ibáñez, E. Screening for Bioactive Compounds from Algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef]
- Soontornchaiboon, W.; Joo, S.S.; Kim, S.M. Anti-inflammatory effects of violaxanthin isolated from microalga Chlorella ellipsoidea in RAW 264.7 macrophages. Biol. Pharm. Bull. 2012, 35, 1137–1144. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lee, S.; Baek, K.; Jin, E. Site-specific gene knock-out and on-site heterologous gene overexpression in Chlamydomonas reinhardtii via a CRISPR-Cas9-mediated knock-in method. Front. Plant. Sci. 2020, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Hattori, H.; Hirano, M. Accumulation and antioxidant activity of secondary carotenoids in the aerial microalga Coelastrella striolata var. multistriata. Food Chem. 2007, 100, 656–661. [Google Scholar] [CrossRef]
- Cha, K.H.; Koo, S.Y.; Lee, D.U. Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J. Agric. Food Chem. 2008, 56, 10521–10526. [Google Scholar] [CrossRef] [PubMed]
- Careri, M.; Furlattini, L.; Mangia, A.; Musci, M.; Anklam, E.; Theobald, A.; Von Holst, C. Supercritical Fluid Extraction for Liquid Chromatographic Determination of Carotenoids in Spirulina pacifica Algae: A Chemometric Approach. J. Chromatogr. A 2001, 912, 61–71. [Google Scholar] [CrossRef]
- Othman, R.; Noh, N.H.; Hatta, F.A.M.; Jamaludin, M.A. Natural Carotenoid Pigments of 6 Chlorophyta Freshwater Green Algae Species. J. Pharm. Nutr. Sci. 2018, 8, 1–5. [Google Scholar] [CrossRef]
- Geisert, M.; Rose, T.; Bauer, W.; Zahn, R.K. Occurrence of carotenoids and sporopollenin in Nanochlorum eucaryotum, a novel marine alga with unusual characteristics. Biosystems 1987, 20, 133–142. [Google Scholar] [CrossRef]
- Kuczynska, P.; Jemiola-Rzeminska, M. Isolation and Purification of All-Trans Diadinoxanthin and All-Trans Diatoxanthin from Diatom Phaeodactylum tricornutum. J. Appl. Phycol. 2017, 29, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer chemoprevention by carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Eun, J.B. Marine carotenoids: Bioactivities and potential benefits to human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2600–2610. [Google Scholar] [CrossRef] [PubMed]
- Kotake-Nara, E.; Yonekura, L.; Nagao, A. Lysoglyceroglycolipids improve the intestinal absorption of micellar fucoxanthin by Caco-2 cells. J. Oleo Sci. 2015, 64, 1207–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.R.; Hosokawa, M.; Miyashita, K. Fucoxanthin: A Marine Carotenoid Exerting Anti-Cancer Effects by Affecting Multiple Mechanisms. Mar. Drugs 2013, 5130–5147. [Google Scholar] [CrossRef] [Green Version]
- Maoka, T.; Fujiwara, Y.; Hashimoto, K.; Akimoto, N. Characterization of Fucoxanthin and Fucoxanthinol Esters in the Chinese Surf Clam, Mactra Chinensis. J. Agric. Food Chem. 2007, 55, 1563–1567. [Google Scholar] [CrossRef]
- Mikami, K.; Hosokawa, M. Biosynthetic pathway and health benefits of fucoxanthin, an algae-specific xanthophyll in brown seaweeds. Int. J. Mol. Sci. 2013, 14, 13763–13781. [Google Scholar] [CrossRef] [Green Version]
- Heo, S.-J.; Ko, S.-C.; Kang, S.-M.; Kang, H.-S.; Kim, J.-P.; Kim, S.-H.; Lee, K.-W.; Cho, M.-G.; Jeon, Y.-J. Cytoprotective Effect of Fucoxanthin Isolated from Brown Algae Sargassum Siliquastrum against H2O2-Induced Cell Damage. Eur. Food Res. Technol. 2008, 228, 145–151. [Google Scholar]
- Market Reports World. Global Fucoxanthin Market Report 2017; Market Reports World: Pune, India, 2017. [Google Scholar]
- Fassett, R.G.; Coombes, J.S. Astaxanthin in cardiovascular health and disease. Molecules 2012, 17, 2030–2048. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Li, Y.; Hu, Q. Astaxanthin in Microalgae: Pathways, Functions and Biotechnological Implications. Algae 2013, 28, 131–147. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 2, 128–152. [Google Scholar] [CrossRef]
- Butler, T.; Golan, Y. Astaxanthin production from microalgae. In Microalgae Biotechnology for Food, Health and High Value Products; Springer: Singapore, 2020; pp. 175–242. [Google Scholar]
- Naziri, D.; Hamidi, M.; Hassanzadeh, S.; Tarhriz, V.; Maleki Zanjani, B.; Nazemyieh, H.; Hejazi, M.A.; Hejazi, M.S. Analysis of carotenoid production by Halorubrum sp. TBZ126; an extremely halophilic archeon from Urmia Lake. Adv. Pharm. Bull. 2014, 4, 61–67. [Google Scholar]
- Cuaresma, M.; Casal, C.; Forján, E.; Vílchez, C. Productivity and selective accumulation of carotenoids of the novel extremophile microalga Chlamydomonas acidophila grown with different carbon sources in batch systems. J. Ind. Microbiol. Biotechnol. 2011, 38, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.-M.; Zhang, X.-W.; Chen, F. Heterotrophic production of biomass and lutein by Chlorella protothecoides on various nitrogen sources. Enzyme Microb. Technol. 2000, 27, 312–318. [Google Scholar] [CrossRef]
- Sánchez, J.F.; Fernández-Sevilla, J.M.; Acién, F.G.; Cerón, M.C.; Pérez-Parra, J.; Molina-Grima, E. Biomass and lutein productivity of Scenedesmus almeriensis: Influence of irradiance, dilution rate and temperature. Appl. Microbiol. Biotechnol. 2008, 79, 719–729. [Google Scholar] [CrossRef]
- Sun, H.; Kong, Q.; Geng, Z.; Duan, L.; Yang, M.; Guan, B. Enhancement of cell biomass and cell activity of astaxanthin-rich Haematococcus pluvialis. Bioresour. Technol. 2015, 186, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Firdous, A.P.; Kuttan, G.; Kuttan, R. Anti-inflammatory potential of carotenoid meso-zeaxanthin and its mode of action. Pharm. Biol. 2015, 53, 961–967. [Google Scholar] [CrossRef] [Green Version]
- Li, X.R.; Tian, G.Q.; Shen, H.J.; Liu, J.Z. Metabolic Engineering of Escherichia Coli to Produce Zeaxanthin. J. Ind. Microbiol. Biotechnol. 2015, 42, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, V.; Morisset, P.; Ihammouine, S.; Chepied, A.; Aumailley, L.; Berard, J.B.; Serive, B.; Kaas, R.; Lanneluc, I.; Thiery, V.; et al. Antiproliferative activity of violaxanthin isolated from bioguided fractionation of Dunaliella tertiolecta extracts. Mar. Drugs 2011, 9, 819–831. [Google Scholar] [CrossRef] [Green Version]
- Kathiresan, S.; Chandrashekar, A.; Ravishankar, G.A.; Sarada, R. Regulation of astaxanthin and its intermediates through cloning and genetic transformation of β-carotene ketolase in Haematococcus pluvialis. J. Biotechnol. 2015, 196–197, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Reuss, L.; Wang, Y. β-Cryptoxanthin: Chemistry, occurrence, and potential health benefits. Curr. Pharmacol. Rep. 2019, 5, 20–34. [Google Scholar] [CrossRef]
- Burri, B.J.; La Frano, M.R.; Zhu, C. Absorption, metabolism, and functions of β-cryptoxanthin. Nutr. Rev. 2016, 74, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Gastineau, R.; Davidovich, N.; Hansen, G.; Rines, J.; Wulff, A.; Kaczmarska, I.; Ehrman, J.; Hermann, D.; Maumus, F.; Hardivillier, Y.; et al. Haslea ostrearia-like diatoms: Biodiversity out of the blue. Adv. Bot. Res. 2014, 71, 441–465. [Google Scholar]
- Kooistra, W.H.; Gersonde, R.; Medlin, L.K.; Mann, D.G. The origin and evolution of the diatoms: Their adaptation to a planktonic existence. Evol. Prim. Prod. Sea 2007, 207–249. [Google Scholar] [CrossRef]
- Tanno, Y.; Kato, S.; Takahashi, S.; Tamaki, S.; Takaichi, S.; Kodama, Y.; Sonoike, K.; Shinomura, T. Light Dependent Accumulation of β-Carotene Enhances Photo-Acclimation of Euglena Gracilis. J. Photochem. Photobiol. B Biol. 2020, 209, 111950. [Google Scholar] [CrossRef]
- Dambek, M.; Eilers, U.; Breitenbach, J.; Steiger, S.; Büchel, C.; Sandmann, G. Biosynthesis of Fucoxanthin and Diadinoxanthin and Function of Initial Pathway Genes in Phaeodactylum tricornutum. J. Exp. Bot. 2012, 63, 5607–5612. [Google Scholar] [CrossRef]
- Faraloni, C.; Torzillo, G. Synthesis of antioxidant carotenoids in microalgae in response to physiological stress. In Carotenoids; InTechOpen: London, UK, 2017; pp. 143–157. [Google Scholar]
- Goodwin, T.W.; Britton, G. Distribution and analysis of carotenoids. Plant Pigment. 1988, 61–132. [Google Scholar]
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Fact. 2014, 13, 1–11. [Google Scholar]
- Rodrigo-Baños, M.; Garbayo, I.; Vílchez, C.; Bonete, M.J.; Martínez-Espinosa, R.M. Carotenoids from Haloarchaea and their potential in biotechnology. Mar. Drugs 2015, 13, 5508–5532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–468. [Google Scholar] [PubMed] [Green Version]
- Yatsunami, R.; Ando, A.; Yang, Y.; Takaichi, S.; Kohno, M.; Matsumura, Y.; Ikeda, H.; Fukui, T.; Nakasone, K.; Fujita, N. Identification of carotenoids from the extremely halophilic archaeon Haloarcula japonica. Front. Microbiol. 2014, 5, 100–105. [Google Scholar] [CrossRef]
- Varela, J.C.; Pereira, H.; Vila, M.; León, R. Production of carotenoids by microalgae: Achievements and challenges. Photosynth. Res. 2015, 125, 423–436. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Maoka, T. Allenic and cumulenic lipids. Prog. Lipid Res. 2007, 46, 328–375. [Google Scholar] [CrossRef] [PubMed]
- Raposo, M.F.D.J.; De Morais, A.M.M.B.; De Morais, R.M.S.C. Carotenoids from marine microalgae: A valuable natural source for the prevention of chronic diseases. Mar. Drugs 2015, 13, 5128–5155. [Google Scholar] [CrossRef]
- Barredo, J.-L. Microbial Carotenoids from Bacteria and Microalgae. Methods and Protocols. Methods Mol. Biol. 2012, 892, 133–141. [Google Scholar]
- Gwak, Y.; Hwang, Y.S.; Wang, B.; Kim, M.; Jeong, J.; Lee, C.G.; Hu, Q.; Han, D.; Jin, E. Comparative analyses of lipidomes and transcriptomes reveal a concerted action of multiple defensive systems against photooxidative stress in Haematococcus pluvialis. J. Exp. Bot. 2014, 65, 4317–4334. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.N. The non-mevalonate pathway of isoprenoid precursor biosynthesis. J. Biol. Chem. 2007, 282, 21573–21577. [Google Scholar] [CrossRef] [Green Version]
- Jahns, P.; Latowski, D.; Strzalka, K. Mechanism and regulation of the violaxanthin cycle: The role of antenna proteins and membrane lipids. Biochim. Biophys. Acta. 2009, 1787, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.J.; Lin, S.; Xu, W.; Cheung, P.C.K. Occurrence and biosynthesis of carotenoids in phytoplankton. Biotechnol. Adv. 2017, 35, 597–618. [Google Scholar] [CrossRef]
- Rabbani, S.; Beyer, P.; Lintig, J.V.; Hugueney, P.; Kleinig, H. Induced β-carotene synthesis driven by triacylglycerol deposition in the unicellular alga Dunaliella bardawil. Plant Phys. 1998, 116, 1239–1248. [Google Scholar] [CrossRef] [Green Version]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.; Feth, B.; Melis, A. A mutant of the green alga Dunaliella salina constitutively accumulates zeaxanthin under all growth conditions. Biotechnol. Bioeng. 2003, 81, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Abu-Ghosh, S.; Fixler, D.; Dubinsky, Z.; Iluz, D. Flashing light in microalgae biotechnology. Bioresour. Technol. 2016, 203, 357–363. [Google Scholar] [CrossRef]
- Polle, J.E.; Qin, S. Development of Genetics and Molecular Tool Kits for Species of the Unicellular Green Alga Dunaliella (Chlorophyta). Alga Dunaliella 2009, 17, 403–422. [Google Scholar]
- Huang, W.; Lin, Y.; He, M.; Gong, Y.; Huang, J. Induced high-yield production of zeaxanthin, lutein, and β-carotene by a mutant of Chlorella zofingiensis. J. Agric. Food Chem. 2018, 66, 891–897. [Google Scholar] [CrossRef]
- Sarnaik, V.; Nambissan, R.; Pandit, A.; Lali, A. Recombinant Synechococcus elongatus PCC 7942 for improved zeaxanthin production under natural light conditions. Algal Res. 2018, 36, 139–151. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Astaxanthin as feed supplement in aquatic animals. Rev. Aquac. 2018, 10, 738–773. [Google Scholar] [CrossRef]
- Sproles, A.E.; Fields, F.J.; Smalley, T.N.; Le, C.H.; Badary, A.; Mayfield, S.P. Recent advancements in the genetic engineering of microalgae. Algal Res. 2021, 53, 102158. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, Y.; Chen, J.; Qin, S.; Chen, G. Metabolic engineering of Synechocystis sp. PCC6803 to produce astaxanthin. Algal Res. 2019, 44, 101679. [Google Scholar] [CrossRef]
- Perozeni, F.; Cazzaniga, S.; Baier, T.; Zanoni, F.; Zoccatelli, G.; Lauersen, K.J.; Wobbe, L.; Ballottari, M. Turning a green alga red: Engineering astaxanthin biosynthesis by intragenic pseudogene revival in Chlamydomonas reinhardtii. Plant Biotechnol. J. 2020, 18, 2053–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galarza, J.I.; Gimpel, J.A.; Rojas, V.; Arredondo-Vega, B.O.; Henríquez, V. Over-accumulation of astaxanthin in Haematococcus pluvialis through chloroplast genetic engineering. Algal Res. 2018, 31, 291–297. [Google Scholar] [CrossRef]
- Manfellotto, F.; Stella, G.R.; Falciatore, A.; Brunet, C.; Ferrante, M.I. Engineering the Unicellular Alga Phaeodactylum tricornutum for Enhancing Carotenoid Production. Antioxidants 2020, 9, 757. [Google Scholar] [CrossRef]
- Kadono, T.; Kira, N.; Suzuki, K.; Iwata, O.; Ohama, T.; Okada, S.; Nishimura, T.; Akakabe, M.; Tsuda, M.; Adachi, M. Effect of an introduced phytoene synthase gene expression on carotenoid biosynthesis in the marine diatom Phaeodactylum tricornutum. Mar. Drugs 2015, 13, 5334–5357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eilers, U.; Bikoulis, A.; Breitenbach, J.; Büchel, C.; Sandmann, G. Limitations in the biosynthesis of fucoxanthin as targets for genetic engineering in Phaeodactylum tricornutum. J. Appl. Phycol. 2016, 28, 123–129. [Google Scholar] [CrossRef]
- Cordero, B.F.; Obraztsova, I.; Couso, I.; Leon, R.; Vargas, M.A.; Rodriguez, H. Enhancement of lutein production in Chlorella sorokiniana (Chorophyta) by improvement of culture conditions and random mutagenesis. Mar. Drugs 2011, 9, 1607–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Mao, X.; Zhou, W.; Guarnieri, M.T. Simultaneous production of triacylglycerol and high-value carotenoids by the astaxanthin-producing oleaginous green microalga Chlorella zofingiensis. Bioresour. Technol. 2016, 214, 319–327. [Google Scholar] [CrossRef]
- Rathod, J.P.; Vira, C.; Lali, A.M.; Prakash, G. Metabolic Engineering of Chlamydomonas reinhardtii for Enhanced β-Carotene and Lutein Production. Appl. Biochem. Biotechnol. 2020, 190, 1457–1469. [Google Scholar] [CrossRef]
- Cezare-Gomes, E.A.; Mejia-da-Silva, L.D.C.; Pérez-Mora, L.S.; Matsudo, M.C.; Ferreira-Camargo, L.S.; Singh, A.K.; de Carvalho, J.C.M. Potential of microalgae carotenoids for industrial application. Appl. Biochem. Biotechnol. 2019, 1188, 602–634. [Google Scholar] [CrossRef]
- Carvalho, J.C.; Bezerra, R.P.; Matsudo, M.C.; Sato, S. Cultivation of Arthrospira (Spirulina) platensis by fed-batch process. In Advanced Biofuels and Bioproducts; Springer: New York, NY, USA, 2013; pp. 781–805. [Google Scholar]
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Commercial production of microalgae: Ponds, tanks, tubes and fermenters. J. Biotechnol. 1999, 70, 313–321. [Google Scholar] [CrossRef]
- Singh, R.N.; Sharma, S. Development of suitable photobioreactor for algae production—A review. Renew. Sust. Energ. Rev. 2012, 16, 2347–2353. [Google Scholar] [CrossRef]
- Chia, S.R.; Chew, K.W.; Leong, H.Y.; Ho, S.H.; Munawaroh, H.S.H.; Show, P.L. CO2 mitigation and phycoremediation of industrial flue gas and wastewater via microalgae-bacteria consortium: Possibilities and challenges. Chem. Eng. Sci. 2021, 425, 131436. [Google Scholar] [CrossRef]
- Ferreira, L.S.; Rodrigues, M.S.; Converti, A.; Sato, S.; Carvalho, J.C. Kinetic and growth parameters of Arthrospira (Spirulina) platensis cultivated in tubular photobioreactor under different cell circulation systems. Biotechnol. Bioeng. 2012, 109, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Eze, C.N.; Ogbonna, J.C.; Ogbonna, I.O.; Aoyagi, H. A novel flat plate air-lift photobioreactor with inclined reflective broth circulation guide for improved biomass and lipid productivity by Desmodesmus subspicatus LC172266. J. Appl. Phycol. 2017, 29, 2745–2754. [Google Scholar] [CrossRef]
- Gao, B.; Xia, S.; Lei, X.; Zhang, C. Combined effects of different nitrogen sources and levels and light intensities on growth and fatty acid and lipid production of oleaginous Eustigmatophycean microalga Eustigmatos cf. polyphem. J. Appl. Phycol. 2017, 30, 215–229. [Google Scholar] [CrossRef]
- Boussiba, S. Carotenogenesis in the green alga Haematococcus pluvialis: Cellular physiology and stress response. Physiol. Plantarum. 2000, 108, 111–117. [Google Scholar] [CrossRef]
- Huang, G.; Chen, F.; Wei, D.; Zhang, X.; Chen, G. Biodiesel production by microalgal biotechnology. Appl. Energy 2010, 87, 38–46. [Google Scholar] [CrossRef]
- Doucha, J.; Lívanský, K. Production of high-density Chlorella culture grown in fermenters. J. Appl. Phycol. 2011, 24, 35–43. [Google Scholar] [CrossRef]
- De Swaaf, M.E.; Sijtsma, L.; Pronk, J.T. High-cell-density fed-batch cultivation of the docosahexaenoic acid producing marine alga Crypthecodinium cohnii. Biotechnol. Bioeng. 2003, 81, 666–672. [Google Scholar] [CrossRef]
- Shi, X.; Wu, Z.; Chen, F. Kinetic modeling of lutein production by heterotrophic Chlorella at various pH and temperatures. Mol. Nutr. Food Res. 2006, 50, 763–768. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, X. Optimization for high-density cultivation of heterotrophic Chlorella based on a hybrid neural network model. Lett. Appl. Microbiol. 2007, 44, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Huntley, M.E.; Johnson, Z.I.; Brown, S.L.; Sills, D.L.; Gerber, L.; Archibald, I.; Machesky, S.C.; Granados, J.; Beal, C.; Greene, C.H. Demonstrated large-scale production of marine microalgae for fuels and feed. Algal Res. 2015, 10, 249–265. [Google Scholar] [CrossRef] [Green Version]
- Olaizola, M.; Huntley, M.E. Recent advances in commercial production of astaxanthin from microalgae. In Recent Advances in Marine Biotechnology; Fingerman, M., Nagabbushaman, R., Eds.; Science Publishers: Enfield, NH, USA, 2003; pp. 143–164. [Google Scholar]
- Jeon, Y.C.; Cho, C.W.; Yun, Y.S. Combined effects of light intensity and acetate concentration on the growth of unicellular microalga Haematococcus pluvialis. Enzym. Microb. Technol. 2006, 39, 490–495. [Google Scholar] [CrossRef]
- Jeon, Y.C.; Cho, C.W.; Yun, Y.S. Oxygen evolution rate of photosynthetic microalga Haematococcus pluvialis depending on light intensity and quality. In Studies in Surface Science and Catalysis; Rhee, H.K., Nam, I.-S., Park, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 157–160. [Google Scholar]
- Pang, N.; Fu, X.; Fernandez, J.S.M.; Chen, S. Multilevel heuristic LED regime for stimulating lipid and bioproducts biosynthesis in Haematococcus pluvialis under mixotrophic conditions. Bioresour. Technol. 2019, 288, 121525. [Google Scholar] [CrossRef]
- Azizi, M.; Hejazi, M.A.; Hashemi, M. Supplementation with polyalcohols and se-quential mixotrophy dilution photoinduction strategy boost the accumulation of astaxanthin by Haematococcus pluvialis. Aquaculture 2019, 511, 734225. [Google Scholar] [CrossRef]
- Lemoine, Y.; Schoefs, B. Secondary ketocarotenoid astaxanthin biosynthesis in algae: A multifunctional response to stress. Photosynth. Res. 2010, 106, 155–177. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Kakizono, T.; Nagai, S. Enhanced carotenoid biosynthesis by oxidative stress in acetate-induced cyst cells of a green unicellular alga, Haematococcus pluvialis. Appl. Environ. Microbiol. 1993, 59, 867–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Solovchenko, A.E.; Khozin-Goldberg, I.; Recht, L.; Boussiba, S. Stressinduced changes in optical properties, pigment and fatty acid content of Nannochloropsis sp.: Implications for non-destructive assay of total fatty acids. Mar. Biotechnol. 2011, 13, 527–535. [Google Scholar] [CrossRef]
- Ben-Amotz, A. Industrial production of microalgal cell-mass and secondary products-major industrial species. Handb. Microalgal Cult. Biotechnol. Appl. Phycol. 2004, 255, 273. [Google Scholar]
- Del Campo, J.A.; Rodríguez, H.; Moreno, J.; Vargas, M.A.; Rivas, J.; Guerrero, M.G. Accumulation of astaxanthin and lutein in Chlorella zofingiensis (Chlorophyta). Appl. Microbiol. Biotechnol. 2004, 64, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ho, S.H.; Chen, C.N.N.; Chen, C.Y.; Ng, I.S.; Jing, K.J.; Chang, J.S.; Lu, Y. Phototrophic cultivation of a thermo-tolerant Desmodesmus sp. for lutein production: Effects of nitrate concentration, light intensity and fed-batch operation. Bioresour. Technol. 2013, 144, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Remmers, I.M.; Martens, D.E.; Wijffels, R.H.; Lamers, P.P. Dynamics of triacylglycerol and EPA production in Phaeodactylum tricornutum under nitrogen starvation at different light intensities. PLoS ONE 2017, 12, e0175630. [Google Scholar] [CrossRef]
- Pal, D.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl. Microbiol. Biotechnol. 2011, 90, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Kurpan Nogueira, D.P.; Silva, A.F.; Araujo, O.Q.F.; Chaloub, R.M. Impact of temperature and light intensity on triacylglycerol accumulation in marine microalgae. Biomass Bioenergy. 2015, 72, 280–287. [Google Scholar] [CrossRef]
- Zhao, Y.; Yue, C.; Ding, W.; Li, T.; Xu, J.-W.; Zhao, P. Butylated hydroxytoluene induces astaxanthin and lipid production in Haematococcus pluvialis under high-light and nitrogen-deficiency conditions. Bioresour. Technol. 2018, 266, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Coesel, S.; Marques, A.; Rodrigues, M.; Baumgartner, A.; Noronha, J.; Rauter, A.; Brenig, B.; Varela, J. Isolation and characterization of a stress-inducible Dunaliella salina Lcy-b gene encoding a functional lycopene b-cyclase. Appl. Microbiol. Biot. 2008, 79, 819–828. [Google Scholar] [CrossRef]
- Coesel, S.N.; Baumgartner, A.C.; Teles, L.M.; Ramos, A.A.; Henriques, N.M.; Cancela, L.; Varela, J.C.S. Nutrient limitation is the main regulatory factor for carotenoid accumulation and for Psy and Pds steady state transcript levels in Dunaliella salina (Chlorophyta) exposed to high light and salt stress. Mar. Biotechnol. 2008, 10, 602–611. [Google Scholar] [CrossRef]
- Chekanov, K.; Lobakova, E.; Selyakh, I.; Semenova, L.; Sidorov, R.; Solovchenko, A. Accumulation of astaxanthin by a new Haematococcus pluvialis strain BM1 from the White Sea coastal rocks (Russia). Mar. Drugs 2014, 12, 4504–4520. [Google Scholar] [CrossRef] [Green Version]
- Kovacic, P. Review of free radicals in biology and medicine Barry Halliwell and John M. C. Gutteridge. The Clarendon Press, Oxford University Press, New York, NY 10016. J. Pharm. Sci. 1986, 75, 105–106. [Google Scholar] [CrossRef]
- García-González, M.; Moreno, J.; Manzano, C.; Florencio, F.J.; Guerrero, M.G. Production of Dunaliella salina biomass rich in 9-cis β-carotene and lutein in a closed tubular photobioreactor. J. Biotechnol. 2005, 115, 81–90. [Google Scholar] [CrossRef]
- Bhosale, P. Environmental and cultural stimulants in the production of carotenoids from microorganisms. Appl. Microbiol. Biotechnol. 2004, 63, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Pick, U. Adaptation of the halotolerant alga Dunaliella to high salinity. In Salinity: Environment–Plants–Molecules; Läuchli, A., Lüttge, U., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 97–112. [Google Scholar]
- Ding, W.; Cui, J.; Zhao, Y.T.; Han, B.Y.; Li, T.; Zhao, P.; Xu, J.W.; Yu, X. Enhancing Haematococcus pluvialis biomass and g-aminobutyric acid accumulation by two-step cultivation and salt supplementation. Bioresour. Technol. 2019, 285, 121334. [Google Scholar] [CrossRef] [PubMed]
- Lamers, P.P.; Janssen, M.; De Vos, R.C.; Bino, R.J.; Wijffels, R.H. Exploring and exploiting carotenoid accumulation in Dunaliella salina for cell-factory applications. Trends Biotechnol. 2008, 26, 631–638. [Google Scholar] [CrossRef]
- Fu, L.; Cui, X.; Li, Y.; Xu, L.; Zhang, C.; Xiong, R.; Zhou, D.; Crittenden, J.C. Excessive phosphorus enhances Chlorella regularis lipid production under nitrogen starvation stress during glucose heterotrophic cultivation. Chem. Eng. J. 2017, 330, 566–572. [Google Scholar] [CrossRef]
- Menegol, T.; Diprat, A.B.; Rodrigues, E.; Rech, R. Effect of temperature and nitrogen concentration on biomass composition of Heterochlorella luteoviridis. Food Sci. Technol. 2017, 37, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Del Campo, J.A.; Moreno, J.; Rodriguez, H.; Vargas, M.A.; Rivas, J.; Guerrero, M.G. Carotenoid content of chlorophycean microalgae: Factors determining lutein accumulation in Muriellopsis sp. (Chlorophyta). J. Biotechnol. 2000, 76, 51–59. [Google Scholar] [CrossRef]
- Urreta, I.; Ikaran, Z.; Janices, I.; Ibanez, E.; Castro-Puyana, M.; Castanon, S.; Suárez-Alvarez, S. Revalorization of Neochloris oleoabundans biomass as source of biodiesel by concurrent production of lipids and carotenoids. Algal Res. 2014, 5, 16–22. [Google Scholar] [CrossRef]
- Lamers, P.P.; Janssen, M.; De Vos, R.C.H.; Bino, R.J.; Wijffels, R.H. Carotenoid and fatty acid metabolism in nitrogen-starved Dunaliella salina, a unicellular green microalga. J. Biotechnol. 2012, 162, 21–27. [Google Scholar] [CrossRef]
- Chen, G.; Wang, B.; Han, D.; Sommerfeld, M.; Lu, Y.; Chen, F.; Hu, Q. Molecular mechanisms of the coordination between astaxanthin and fatty acid biosynthesis in Haematococcus pluvialis (Chlorophyceae). Plant J. 2015, 81, 95–107. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Huisman, J.M.; Osborn, A. Culture of the astaxanthin-producing green alga Haematococcus pluvialis 1. Effects of nutrients on growth and cell type. J. Appl. Phycol. 1991, 3, 295–304. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, P.; Duncan, J.; Barber, J. Astaxanthin accumulation in the green alga Haematococcus pluvialis: Effects of cultivation parameters. J. Integr. Plant Biol. 2007, 49, 447–451. [Google Scholar] [CrossRef]
- Cai, M.; Li, Z.; Qi, A. Effects of iron electrovalence and species on growth and astaxanthin production of Haematococcus pluvialis. Chin. J. Oceanol. Limnol. 2009, 27, 370–375. [Google Scholar] [CrossRef]
- Boussiba, S.; Vonshak, A. Astaxanthin accumulation in the green alga Haematococcus pluvialis 1. Plant Cell Physiol. 1991, 32, 1077–1082. [Google Scholar] [CrossRef] [Green Version]
- Carfagna, S.; Bottone, C.; Cataletto, P.R.; Petriccione, M.; Pinto, G.; Salbitani, G.; Vona, V.; Ciniglia, C. Impact of sulfur starvation in autotrophic and heterotrophic cultures of the extremophilic microalga Galdieria phlegrea (Cyanidiophyceae). Plant. Cell. Physiol. 2016, 57, 1890–1898. [Google Scholar] [CrossRef] [Green Version]
- Carmen Ruiz-Dominguez, M.; Vaquero, I.; Obregon, V.; de la Morena, B.; Vilchez, C.; Vega, J.M. Lipid accumulation and antioxidant activity in the eukaryotic acidophilic microalga Coccomyxa sp. (strain onubensis) under nutrient starvation. J. Appl. Phycol. 2015, 27, 1099–1108. [Google Scholar] [CrossRef]
- Machado, F.R., Jr.; Trevisol, T.C.; Boschetto, D.L.; Burkert, J.F.; Ferreira, S.R.; Oliveira, J.V.; Burkert, C.A.V. Technological process for cell disruption, extraction and encapsulation of astaxanthin from Haematococcus pluvialis. J. Biotechnol. 2016, 218, 108–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, F.R., Jr.; Reis, D.F.; Boschetto, D.L.; Burkert, J.F.; Ferreira, S.R.; Oliveira, J.V.; Burkert, C.A.V. Encapsulation of astaxanthin from Haematococcus pluvialis in PHBV by means of SEDS technique using supercritical CO2. Ind. Crops Prod. 2014, 54, 17–21. [Google Scholar] [CrossRef]
- Park, S.A.; Ahn, J.B.; Choi, S.H.; Lee, J.S.; Lee, H.G. The effects of particle size on the physicochemical properties of optimized astaxanthin-rich Xanthophyllomyces dendrorhous-loaded microparticles. LWT-Food Sci. Technol. 2014, 55, 638–644. [Google Scholar] [CrossRef]
- Bustos-Garza, C.; Yáñez-Fernández, J.; Barragán-Huerta, B.E. Thermal and pH stability of spray-dried encapsulated astaxanthin oleoresin from Haematococcus pluvialis using several encapsulation wall materials. Food Res. Int. 2013, 54, 641–649. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M.; Argüelles-Monal, W. Microencapsulation of astaxanthin in a chitosan matrix. Carbohydr. Polym. 2004, 56, 41–45. [Google Scholar] [CrossRef]
- Kittikaiwan, P.; Powthongsook, S.; Pavasant, P.; Shotipruk, A. Encapsulation of Haematococcus pluvialis using chitosan for astaxanthin stability enhancement. Carbohydr. Polym. 2007, 70, 378–385. [Google Scholar] [CrossRef]
- Hong, H.L.; Suo, Q.L.; Han, L.M.; Li, C.P. Study on precipitation of astaxanthin in supercritical fluid. Powder Technol. 2009, 191, 294–298. [Google Scholar] [CrossRef]
- Tachaprutinun, A.; Udomsup, T.; Luadthong, C.; Wanichwecharungruang, S. Preventing the thermal degradation of astaxanthin through nanoencapsulation. Int. J. Pharm. 2009, 374, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Niizawa, I.; Espinaco, B.Y.; Zorrilla, S.E.; Sihufe, G.A. Natural astaxanthin encapsulation: Use of response surface methodology for the design of alginate beads. Int. J. Biol. Macromol. 2019, 121, 601–608. [Google Scholar] [CrossRef]
- Lin, S.F.; Chen, Y.C.; Chen, R.N.; Chen, L.C.; Ho, H.O.; Tsung, Y.H.; Sheu, M.T.; Liu, D.Z. Improving the stability of astaxanthin by microencapsulation in calcium alginate beads. PLoS ONE 2016, 11, e0153685. [Google Scholar] [CrossRef]
- Boonlao, N.; Shrestha, S.; Sadiq, M.B.; Anal, A.K. Influence of whey protein-xanthan gum stabilized emulsion on stability and in vitro digestibility of encapsulated astaxanthin. J. Food Eng. 2020, 272, 109859. [Google Scholar] [CrossRef]
- Zanoni, F.; Vakarelova, M.; Zoccatelli, G. Development and characterization of astaxanthin-containing whey protein-based nanoparticles. Mar. Drugs 2019, 17, 627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Yang, L.; Xu, J.; Qiao, X.; Li, Z.; Wang, Y.; Xue, C. Evaluation of the physicochemical stability and digestibility of microencapsulated esterified astaxanthins using in vitro and in vivo models. Food Chem. 2018, 260, 73–81. [Google Scholar] [CrossRef]
- Vakarelova, M.; Zanoni, F.; Lardo, P.; Rossin, G.; Mainente, F.; Chignola, R.; Menin, A.; Rizzi, C.; Zoccatelli, G. Production of stable food-grade microencapsulated astaxanthin by vibrating nozzle technology. Food Chem. 2017, 221, 289–295. [Google Scholar] [CrossRef]
- Pu, J.; Bankston, J.D.; Sathivel, S. Production of microencapsulated crawfish (Procambarus clarkii) astaxanthin in oil by spray drying technology. Dry. Technol. 2011, 29, 1150–1160. [Google Scholar] [CrossRef]
- Niamnuy, C.; Devahastin, S.; Soponronnarit, S.; Raghavan, G.V. Kinetics of astaxanthin degradation and color changes of dried shrimp during storage. J. Food. Eng. 2008, 87, 591–600. [Google Scholar] [CrossRef]
- Takeungwongtrakul, S.; Benjakul, S. Astaxanthin degradation and lipid oxidation of Pacific white shrimp oil: Kinetics study and stability as affected by storage conditions. Int. Aquat. Res. 2016, 8, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Bustamante, A.; Masson, L.; Velasco, J.; Del Valle, J.M.; Robert, P. Microencapsulation of H. pluvialis oleoresins with different fatty acid composition: Kinetic stability of astaxanthin and alpha-tocopherol. Food Chem. 2016, 190, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Foo, S.C.; Khong, N.M.; Yusoff, F.M. Physicochemical, microstructure and antioxidant properties of microalgae-derived fucoxanthin rich microcapsules. Algal Res. 2020, 51, 102061. [Google Scholar] [CrossRef]
- Koo, S.Y.; Mok, I.K.; Pan, C.H.; Kim, S.M. Preparation of fucoxanthin-loaded nanoparticles composed of casein and chitosan with improved fucoxanthin bioavailability. J. Agric. Food. Chem. 2016, 64, 9428–9435. [Google Scholar] [CrossRef]
| Xanthophylls | Microalgae | Extraction Processes | Concentrations | Applications | References |
|---|---|---|---|---|---|
| Fucoxanthin | Tisochrysis lutea | Ultrasonic-assisted extraction | 0.25 mg/g dw | Nutraceutical, cosmetic and pharmaceutical applications | [26] |
| Cyclotella meneghiniana | 2.3 mg/g | [27] | |||
| Mallomonas sp. | 26.6 mg/g | ||||
| Nitzschia cf. carinospeciosa | 5.5 mg/g | ||||
| Phaeodactylum tricornutum | 10 mg/g | ||||
| Paralia longispina | 1.4 mg/g | ||||
| Isochrysis aff. galbana | 1.8% dw | [28] | |||
| Odontella aurita | up to 2.2% dw | [29] | |||
| Thalassiosira weissflogii | Solvent extraction | 5.1 mg/L/d | [30] | ||
| ASX | Haematococcus pluvialis | Conventional extraction | 900 kg/2 ha/year | Antioxidant, anti-cancer, anti-inflammatory, ocular protective effect, antidiabetic, coloring agent | [31] |
| Two-stage system | 3.8% dw | [32] | |||
| Enzyme extraction | 3.8% dw | [33] | |||
| Conventional extraction | 2–3% dw | [34] | |||
| Pressurized extraction | 99% of total AS | [35] | |||
| Haematococcus lacustris | Mechanical extraction | 18.8 mg/L | [36] | ||
| Lutein | Chlorella vulgaris | Heptane–ethanol– water extraction | 30 mg/g | Antioxidant, light-filtering, eye protection, colorant, potential therapeutic use against several chronic diseases, lower risk of cancer, anti-inflammatory benefits | [37] |
| Chlorella minutissima | Solvent extraction | 5.58 mg/g | [38] | ||
| Chlorella sorokiniana | Solvent extraction | 7.62 mg/L/d | [39] | ||
| Scenedesmus bijugus | 2.9 mg/g | [40] | |||
| Dunaliella salina | Conventional extraction | 15.4 mg/m2/d | [41] | ||
| Chlorella protothecoides | Maceration | 83.8 mg/L | [42] | ||
| Chlorella protothecoides | Mechanical | 4.92 mg/g | [43] | ||
| Tetraselmis sp. CTP4 | Mechanical | 3.17 mg/g dw | [44] | ||
| Chlamydomonas sp. | Solvent extraction | 5.08 mg/L/d | [45] | ||
| Muriellopsis sp. | Solvent extraction | 100 mg/m2/d | [46] | ||
| Chlamydomonas acidophila | Solvent extraction | 20 mg/L | [47] | ||
| Scenedesmus almeriensis | Accelerated solvent extraction | 8.54 mg/g | [48] | ||
| Scenedesmus obliquus | Solvent extraction | 3.63 mg/g | [49] | ||
| Desmodesmus sp. | Solvent extraction | 5.22 mg/L/d | [50] | ||
| Coelastrella sp. | Accelerated solvent extraction | 6.49 mg/g | [40] | ||
| Zeaxanthin | Heterochlorella luteoviridis | Moderate electric field | 244 µg/g | Antioxidant, anti-inflammatory, eyes and UV light protection, prevention of coronary syndromes, anti-tumoral, anti-cardiovascular diseases, and structural actions in neural tissue | [21] |
| Nannochloropsis oculata | Supercritical fluids extraction | 13.17 mg/g | [51] | ||
| Chlorella ellipsoidea | Pressurized liquid extraction | 4.26 mg/g | [52] | ||
| Synechocystis sp. | Pulse electric field | 1.64 mg/g | [53] | ||
| Violaxanthin | Chlorella ellipsodea | Solvent extraction | not determined | Anti-inflammatory activity | [54] |
| Chlorella vulgaris | Solvent extraction Mechanical extraction | 3.7 mg/g | [55] | ||
| Canthaxanthin | Coelastrella striolata var. multistriata | 4.75% dw | Anti-oxidant property Create a tan color | [56] | |
| Chlorella vulgaris | 45% Total carotenoids | [57] | |||
| Cryptoxanthin | Spirulina platensis | Supercritical fluid extraction | 7.5 mg/100 g | Antioxidant, anti-inflammatory, anticancer, improves respiratory functions, stimulates bone formation and protection, decreases risk of degenerative diseases | [58] |
| Pandorina morum | Maceration | 2.38 µg/g DW | [59] | ||
| Nanochlorum eucaryotum | Enzyme extraction | not determined | [60] | ||
| Diadinoxanthin | Odontella aurita | Ethanol extraction | 10% total carotenoids | Antioxidant | [29] |
| Phaeodactylum tricornutum | 19% of total pigments | [61] | |||
| Diatoxanthin | Phaeodactylum tricornutum | Methanol extraction | 17% of total pigments | Antioxidant | [61] |
| Bioactive Compounds | Wall Materials | Encapsulation Techniques | Encapsulation Preparations | Main Findings | References |
|---|---|---|---|---|---|
| ASX from Haematococcus pluvialis | PHBV (Poly(hydroxybutyrate-co-hydroxyvalerate)) | Co-precipitation |
|
| [176] |
| ASX from Haematococcus pluvialis | PHBV (Poly(hydroxybutyrate-co-hydroxyvalerate)) | Co-precipitation |
|
| [177] |
| ASX from Haematococcus pluvialis | Precirol ATO 5 or Stearic acid | Hot Homogenization method: SUPRAS/NLCs mixture |
|
| [87] |
| ASX from Haematococcus pluvialis | GA and WP single or mixed with MD or IN | Spray drying |
|
| [179] |
| ASX from Haematococcus pluvialis | WPC | Emulsification–solvent Evaporation |
|
| [187] |
| Esterified ASX from Haematococcus pluvialis | WP and GA | Complex coacervation |
|
| [188] |
| ASX-enriched oil from Haematococcus pluvialis | C6H7NaO6; and low-methoxyl pectin | Vibrating nozzle technology |
|
| [189] |
| Fucoxanthin from Chaetoceros calcitrans | Maltodextrin and GA | Spray and freeze drying |
|
| [194] |
| Fucoxanthin from Phaeodactylum tricornutum (FX) | Chitosan (CN) | Electrospraying |
|
| [195] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smaoui, S.; Barkallah, M.; Ben Hlima, H.; Fendri, I.; Mousavi Khaneghah, A.; Michaud, P.; Abdelkafi, S. Microalgae Xanthophylls: From Biosynthesis Pathway and Production Techniques to Encapsulation Development. Foods 2021, 10, 2835. https://doi.org/10.3390/foods10112835
Smaoui S, Barkallah M, Ben Hlima H, Fendri I, Mousavi Khaneghah A, Michaud P, Abdelkafi S. Microalgae Xanthophylls: From Biosynthesis Pathway and Production Techniques to Encapsulation Development. Foods. 2021; 10(11):2835. https://doi.org/10.3390/foods10112835
Chicago/Turabian StyleSmaoui, Slim, Mohamed Barkallah, Hajer Ben Hlima, Imen Fendri, Amin Mousavi Khaneghah, Philippe Michaud, and Slim Abdelkafi. 2021. "Microalgae Xanthophylls: From Biosynthesis Pathway and Production Techniques to Encapsulation Development" Foods 10, no. 11: 2835. https://doi.org/10.3390/foods10112835
APA StyleSmaoui, S., Barkallah, M., Ben Hlima, H., Fendri, I., Mousavi Khaneghah, A., Michaud, P., & Abdelkafi, S. (2021). Microalgae Xanthophylls: From Biosynthesis Pathway and Production Techniques to Encapsulation Development. Foods, 10(11), 2835. https://doi.org/10.3390/foods10112835









