Structural and Emulsifying Properties of Soybean Protein Isolate–Sodium Alginate Conjugates under High Hydrostatic Pressure
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SPI-SA Conjugates and Mixtures
2.3. Measurement of Intermediate Content and Browning Intensity
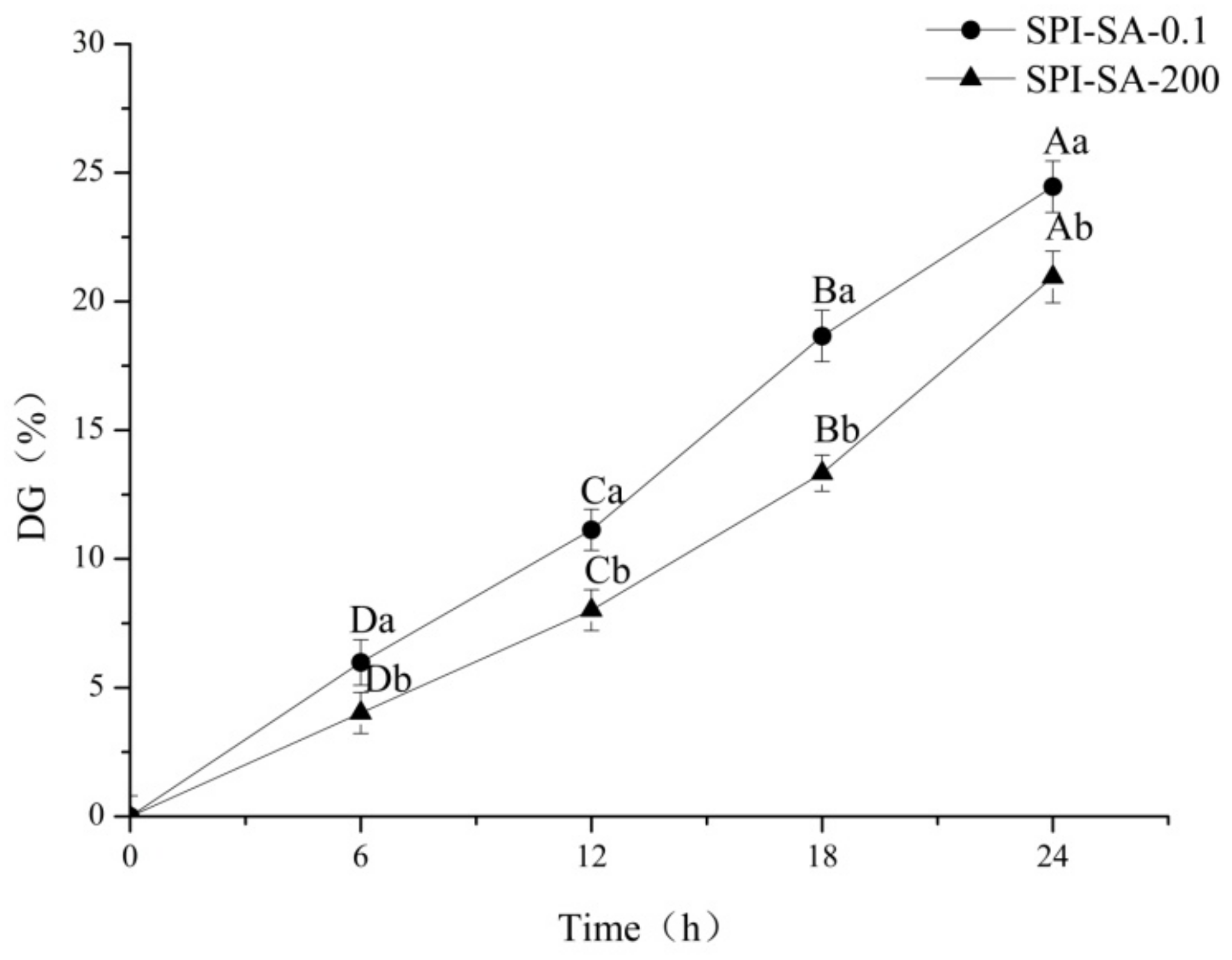
2.4. Determination of the Degree of Grafting (DG)
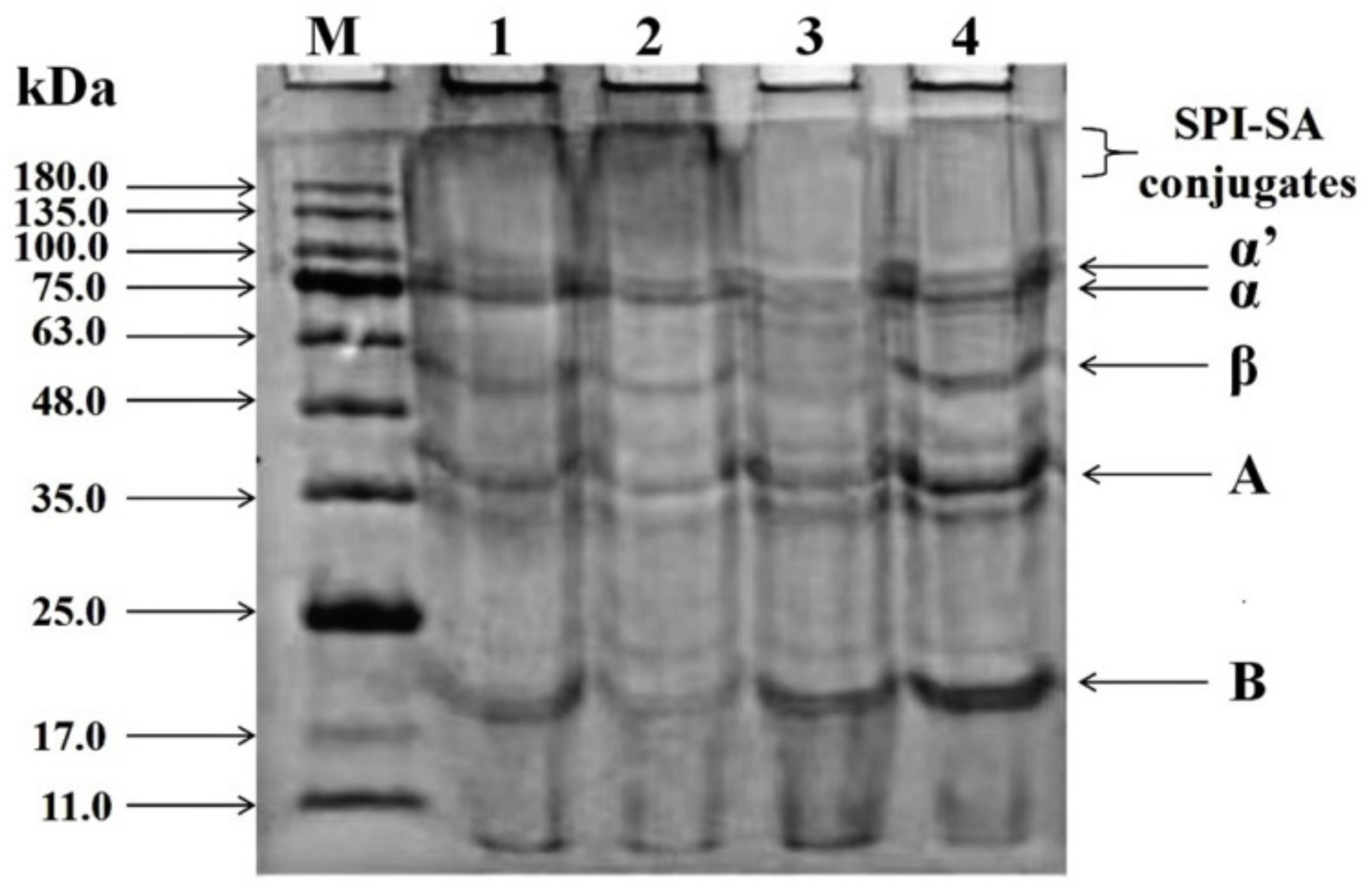
2.5. Sodium Dodecyl Sulphate–Polyacrylamide gel Electrophoresis (SDS-PAGE)
2.6. Circular Dichroism (CD) Spectroscopy
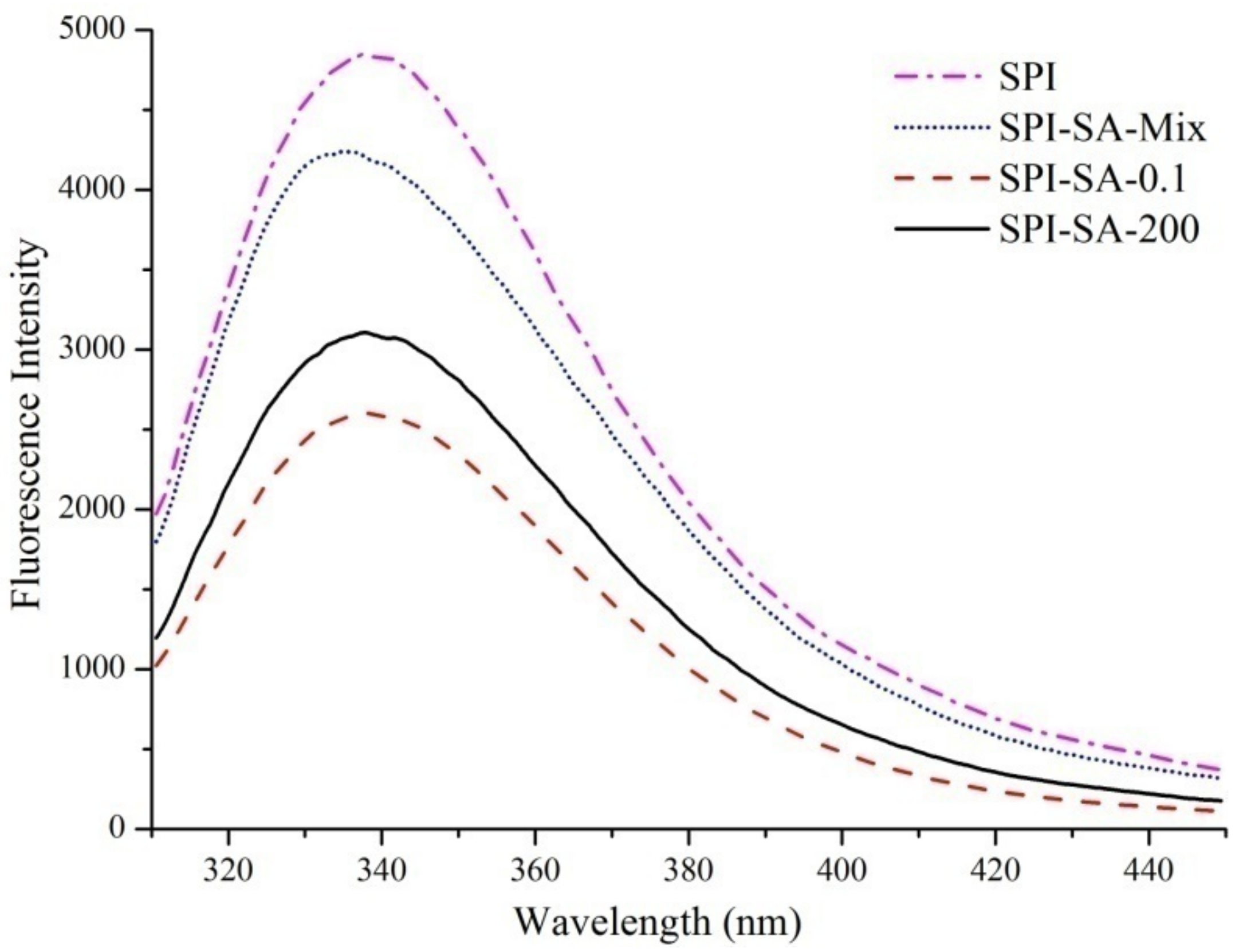
2.7. Intrinsic Fluorescence Spectroscopy
2.8. Emulsifying Property Analysis
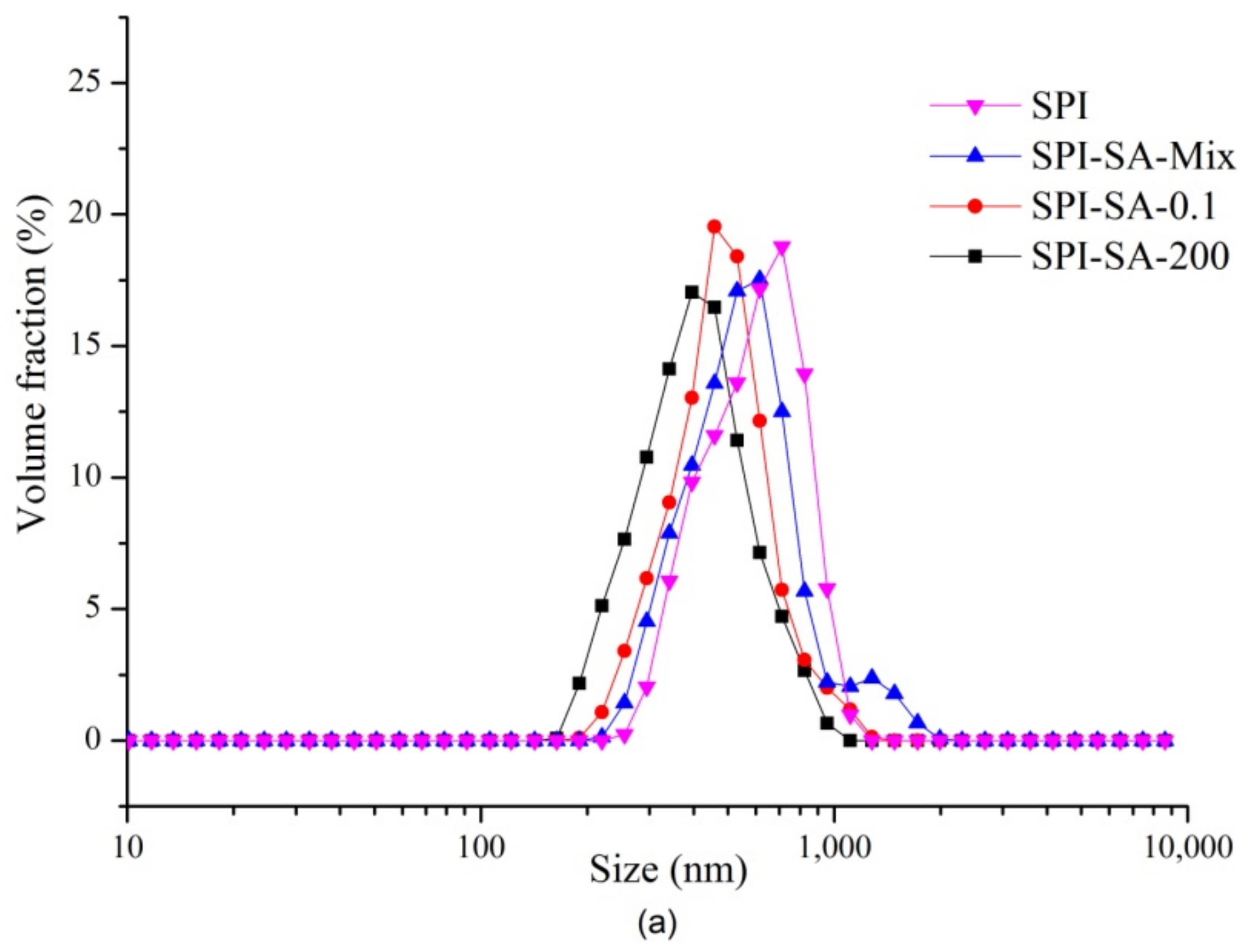
2.9. Emulsion Particle Size
2.10. Emulsion Zeta Potential
2.11. CLSM
2.12. Statistical Analysis
3. Results and Discussion
3.1. Effect of HHP on the Maillard Reaction between SPI and SA
3.2. SDS-PAGE Analysis
3.3. Circular Dichroism (CD) Spectral Analysis
3.4. Intrinsic Fluorescence Spectroscopy Analysis
3.5. Emulsifying Properties
3.5.1. Emulsifying Activity and Stability
3.5.2. Particle Sizes of Emulsions
3.5.3. Zeta Potential of Emulsions
3.5.4. Emulsion Microstructure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, L.; Ding, X.; Li, Y.; Ma, H. The aggregation, structures and emulsifying properties of soybean protein isolate induced by ultrasound and acid. Food Chem. 2019, 279, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ru, Q.; Ding, Y. Glycation a promising method for food protein modification: Physicochemical properties and structure, a review. Food Res. Int. 2012, 49, 170–183. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G.O. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- McClements, D.J. Critical review of techniques and methodologies for characterization of emulsion stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef]
- Damodaran, S. Protein stabilization of emulsions and foams. J. Food Sci. 2005, 70, R54–R66. [Google Scholar] [CrossRef]
- Lam, R.S.H.; Nickerson, M.T. Food proteins: A review on their emulsifying properties using a structure-function approach. Food Chem. 2013, 141, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Interfacial structure and stability of food emulsions as affected by protein-polysaccharide interactions. Soft Matter 2008, 4, 932–942. [Google Scholar] [CrossRef]
- Wang, X.S.; Tang, C.H.; Li, B.S.; Yang, X.Q.; Li, L.; Ma, C.Y. Effects of high-pressure treatment on some physicochemical and functional properties of soy protein isolates. Food Hydrocoll. 2008, 22, 560–567. [Google Scholar] [CrossRef]
- Xu, K.; Guo, M.M.; Du, J.H.; Zhang, Z.H. Okra polysaccharide: Effect on the texture and microstructure of set yoghurt as a new natural stabilizer. Int. J. Biol. Macromol. 2019, 133, 117–126. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Gomaa, M.; Hifney, A.F.; Abdel-Gawad, K.M. Optimization of alginate alkaline extraction technology from Sargassum latifolium and its potential antioxidant and emulsifying properties. Carbohydr. Polym. 2017, 157, 1903–1912. [Google Scholar] [CrossRef]
- Zhao, N.; Zou, H.; Sun, S.; Yu, C. The interaction between sodium alginate and myofibrillar proteins: The rheological and emulsifying properties of their mixture. Int. J. Biol. Macromol. 2020, 161. [Google Scholar] [CrossRef]
- De Oliveira, F.C.; dos Reis Coimbra, J.S.; de Oliveira, E.B.; Giraldo Zuniga, A.D.; Garcia Rojas, E.E. Food Protein-polysaccharide Conjugates Obtained via the Maillard Reaction: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1108–1125. [Google Scholar] [CrossRef]
- Kasran, M.; Cui, S.W.; Goff, H.D. Emulsifying properties of soy whey protein isolate-fenugreek gum conjugates in oil-in-water emulsion model system. Food Hydrocoll. 2013, 30, 691–697. [Google Scholar] [CrossRef]
- Troise, A.D. Analytical strategies to depict the fate of the Maillard reaction in foods. Curr. Opin. Food Sci. 2018, 19, 15–22. [Google Scholar] [CrossRef]
- Zhu, D.; Damodaran, S.; Lucey, J.A. Formation of whey protein isolate (WPI)-dextran conjugates in aqueous solutions. J. Agric. Food Chem. 2008, 56, 7113–7118. [Google Scholar] [CrossRef]
- Kasran, M.; Cui, S.W.; Goff, H.D. Covalent attachment of fenugreek gum to soy whey protein isolate through natural Maillard reaction for improved emulsion stability. Food Hydrocoll. 2013, 30, 552–558. [Google Scholar] [CrossRef]
- Akhtar, M.; Dickinson, E. Emulsifying properties of whey protein–dextran conjugates at low pH and different salt concentrations. Colloids Surf. B Biointerfaces 2003, 31, 125–132. [Google Scholar] [CrossRef]
- Jimenez-Castano, L.; Villamiel, M.; Lopez-Fandino, R. Glycosylation of individual whey proteins by Maillard reaction using dextran of different molecular mass. Food Hydrocoll. 2007, 21, 433–443. [Google Scholar] [CrossRef]
- Dhakal, S.; Giusti, M.M.; Balasubramaniam, V.M. Effect of high pressure processing on dispersive and aggregative properties of almond milk. J. Sci. Food Agric. 2016, 96, 3821–3830. [Google Scholar] [CrossRef]
- Torres, J.A.; Velazquez, G. Commercial opportunities and research challenges in the high pressure processing of foods. J. Food Eng. 2005, 67, 95–112. [Google Scholar] [CrossRef]
- Zhao, R.J.; Huo, C.Y.; Qian, Y.; Ren, D.F.; Lu, J. Ultra-high-pressure processing improves proteolysis and release of bioactive peptides with activation activities on alcohol metabolic enzymes in vitro from mushroom foot protein. Food Chem. 2017, 231, 25–32. [Google Scholar] [CrossRef]
- Tauscher, B. Pasteurization of food by hydrostatic high pressure: Chemical aspects. Z. Lebensm. Unters. Forsch. 1995, 200, 3–13. [Google Scholar] [CrossRef]
- Alt, N.; Schieberle, P. Model studies on the influence of high hydrostatic pressure on the formation of glycated arginine modifications at elevated temperatures. J. Agric. Food Chem. 2005, 53, 5789–5797. [Google Scholar] [CrossRef]
- Tamaoka, T.; Itoh, N.; Hayashi, R. High Pressure Effect on Maillard Reaction. Agric. Biol. Chem. 1991, 55, 2071–2074. [Google Scholar] [CrossRef]
- Moreno, F.J.; Molina, E.; Olano, A.; López-Fandiño, R. High-pressure effects on Maillard reaction between glucose and lysine. J. Agric. Food Chem. 2003, 51, 394–400. [Google Scholar] [CrossRef]
- Kobayashi, A.; Gomikawa, S.; Oguro, A.; Maeda, S.; Yamazaki, A.; Sato, S.; Maekawa, H. The Effect of High Hydrostatic Pressure on Acrylamide Generation in Aqueous Reaction Systems Using Asparagine and Glucose. Food Sci. Technol. Res. 2019, 25, 587–596. [Google Scholar] [CrossRef]
- Gu, F.l.; Abbas, S.; Zhang, X.m. Optimization of Maillard reaction products from casein-glucose using response surface methodology. LWT-Food Sci. Technol. 2009, 42, 1374–1379. [Google Scholar] [CrossRef]
- Chen, W.; Ma, X.; Wang, W.; Lv, R.; Guo, M.; Ding, T.; Ye, X.; Miao, S.; Liu, D. Preparation of modified whey protein isolate with gum acacia by ultrasound maillard reaction. Food Hydrocoll. 2019, 95, 298–307. [Google Scholar] [CrossRef]
- Guan, J.J.; Zhang, T.B.; Hui, M.; Yin, H.C.; Qiu, A.Y.; Liu, X.Y. Mechanism of microwave-accelerated soy protein isolate-saccharide graft reactions. Food Res. Int. 2011, 44, 2647–2654. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, C.; Wang, J.; Ni, S.; Wang, Y. Maillard reaction with ribose, galacto-oligosaccharide or chitosan-oligosaccharide reduced the allergenicity of shrimp tropomyosin by inducing conformational changes. Food Chem. 2019, 274, 789–795. [Google Scholar] [CrossRef]
- Miles, A.J.; Ramalli, S.G.; Wallace, B.A. DichroWeb, a website for calculating protein secondary structure from circular dichroism spectroscopic data. Protein Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhu, W.; Yi, J.; Liu, N.; Cao, Y.; Lu, J.; Decker, E.; Mcclements, D.J. Effects of sonication on the physicochemical and functional properties of walnut protein isolate. Food Res. Int. 2018, 106, 853–861. [Google Scholar] [CrossRef]
- Matmaroh, K.; Benjakul, S.; Tanaka, M. Effect of reactant concentrations on the Maillard reaction in a fructose-glycine model system and the inhibition of black tiger shrimp polyphenoloxidase. Food Chem. 2006, 98, 1–8. [Google Scholar] [CrossRef]
- de Oliveira, F.C.; dos Reis Coimbra, J.S.; de Oliveira, E.B.; Raposo Branco Rodrigues, M.Q.; Sabioni, R.C.; Silva de Souza, B.W.; Boggione Santos, I.J. Acacia gum as modifier of thermal stability, solubility and emulsifying properties of alpha-lactalbumin. Carbohydr. Polym. 2015, 119, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, N.S.; Coulson, M. Effect of pressure on processes modelling the Maillard reaction. J. Phys. Org. Chem. 1996, 9, 639–644. [Google Scholar] [CrossRef]
- Martínez, M.; Velazquez, G.; Cando, D.; Nunez-Flores, R.; Borderías, A.J.; Moreno, H.M. Effects of high pressure processing on protein fractions of blue crab (Callinectes sapidus) meat. Innov. Food Sci. Emerg. Technol. 2017, 41, 323–329. [Google Scholar] [CrossRef]
- Considine, T.; Patel, H.A.; Anema, S.G.; Singh, H.; Creamer, L.K. Interactions of milk proteins during heat and high hydrostatic pressure treatments—A review. Innov. Food Sci. Emerg. Technol. 2007, 8, 1–23. [Google Scholar] [CrossRef]
- Bristow, M.; Isaacs, N.S. The effect of high pressure on the formation of volatile products in a model Maillard reaction. J. Chem. Soc. Perkin Trans. 1999, 2, 2213–2218. [Google Scholar] [CrossRef]
- Buckow, R.; Wendorff, J.; Hemar, Y. Conjugation of Bovine Serum Albumin and Glucose under Combined High Pressure and Heat. J. Agric. Food Chem. 2011, 59, 3915–3923. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, L.; Wang, Y.; Li, Z.; Han, J. Effect of high hydrostatic pressure on solubility and conformation changes of soybean protein isolate glycated with flaxseed gum. Food Chem. 2020, 333, 127530. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, N.; Lan, T.; Yang, X. Improvement in emulsifying properties of soy protein isolate by conjugation with maltodextrin using high-temperature, short-time dry-heating Maillard reaction. Int. J. Food Sci. Technol. 2014, 49, 460–467. [Google Scholar] [CrossRef]
- Ma, X.; Chen, W.; Yan, T.; Wang, D.; Liu, D. Comparison of citrus pectin and apple pectin in conjugation with soy protein isolate (SPI) under controlled dry-heating conditions. Food Chem. 2019, 309, 125501. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, G.; Zhao, M.; Ren, J.; Bao, Y. Improvement of functional properties of peanut protein isolate by conjugation with dextran through Maillard reaction. Food Chem. 2012, 131, 901–906. [Google Scholar] [CrossRef]
- Li, C.; Huang, X.; Peng, Q.; Shan, Y.; Xue, F. Physicochemical properties of peanut protein isolate-glucomannan conjugates prepared by ultrasonic treatment. Ultrason. Sonochem. 2014, 21, 1722–1727. [Google Scholar] [CrossRef]
- Zheng, J.X.; Yin, H.; Shen, C.C.; Zhang, L.; Lu, J. Functional and structural properties of Spirulina phycocyanin modified by ultra-high-pressure composite glycation. Food Chem. 2019, 306, 125615. [Google Scholar] [CrossRef]
- Tang, C.H.; Sun, X.; Foegeding, E.A. Modulation of physicochemical and conformational properties of kidney bean vicilin (phaseolin) by glycation with glucose: Implications for structure-function relationships of legume vicilins. J. Agric. Food Chem. 2011, 59, 10114–10123. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Chen, L.; Zhu, X.; Wanga, L.; Pan, S. Comparative studies on the physicochemical properties of soy protein isolate-maltodextrin and soy protein isolate-gum acacia conjugate prepared through Maillard reaction. Food Res. Int. 2013, 51, 490–495. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Huang, G.Q.; Xu, T.C.; Liu, L.-N.; Xiao, J.X. Comparative study on the Maillard reaction of chitosan oligosaccharide and glucose with soybean protein isolate. Int. J. Biol. Macromol. 2019, 131, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E.; Galazka, V.B. Emulsion stabilization by ionic and covalent complexes of β-lactoglobulin with polysaccharides. Food Hydrocoll. 1991, 5, 281–296. [Google Scholar] [CrossRef]
- Telis, V. O/W Emulsions Stabilized by Interactions Between Proteins and Polysaccharides. Encycl. Food Chem. 2019, 494–498. [Google Scholar] [CrossRef]
- Yin, S.W.; Tang, C.H.; Wen, Q.B.; Yang, X.Q.; Li, L. Functional properties and in vitro trypsin digestibility of red kidney bean (Phaseolus vulgaris L.) protein isolate: Effect of high-pressure treatment. Food Chem. 2008, 110, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Diftis, N.; Kiosseoglou, V. Improvement of emulsifying properties of soybean protein isolate by conjugation with carboxymethyl cellulose. Food Chem. 2003, 81, 1–6. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Agyare, K.K.; Addo, K. Hydrolyzed wheat gluten suppresses transglutaminase-mediated gelation but improves emulsification of pork myofibrillar protein. Meat Sci. 2008, 80, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Zha, F.C.; Dong, S.Y.; Rao, J.J.; Chen, B.C. Pea protein isolate-gum Arabic Maillard conjugates improves physical and oxidative stability of oil-in-water emulsions. Food Chem. 2019, 285, 130–138. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Y.; Song, T.; Bao, M.; Li, Y.; Li, X. Improvement in emulsifying properties of whey protein–Rhamnolipid conjugates through short-time heat treatment. Colloids Surf. B Biointerfaces 2019, 181, 688–695. [Google Scholar] [CrossRef]

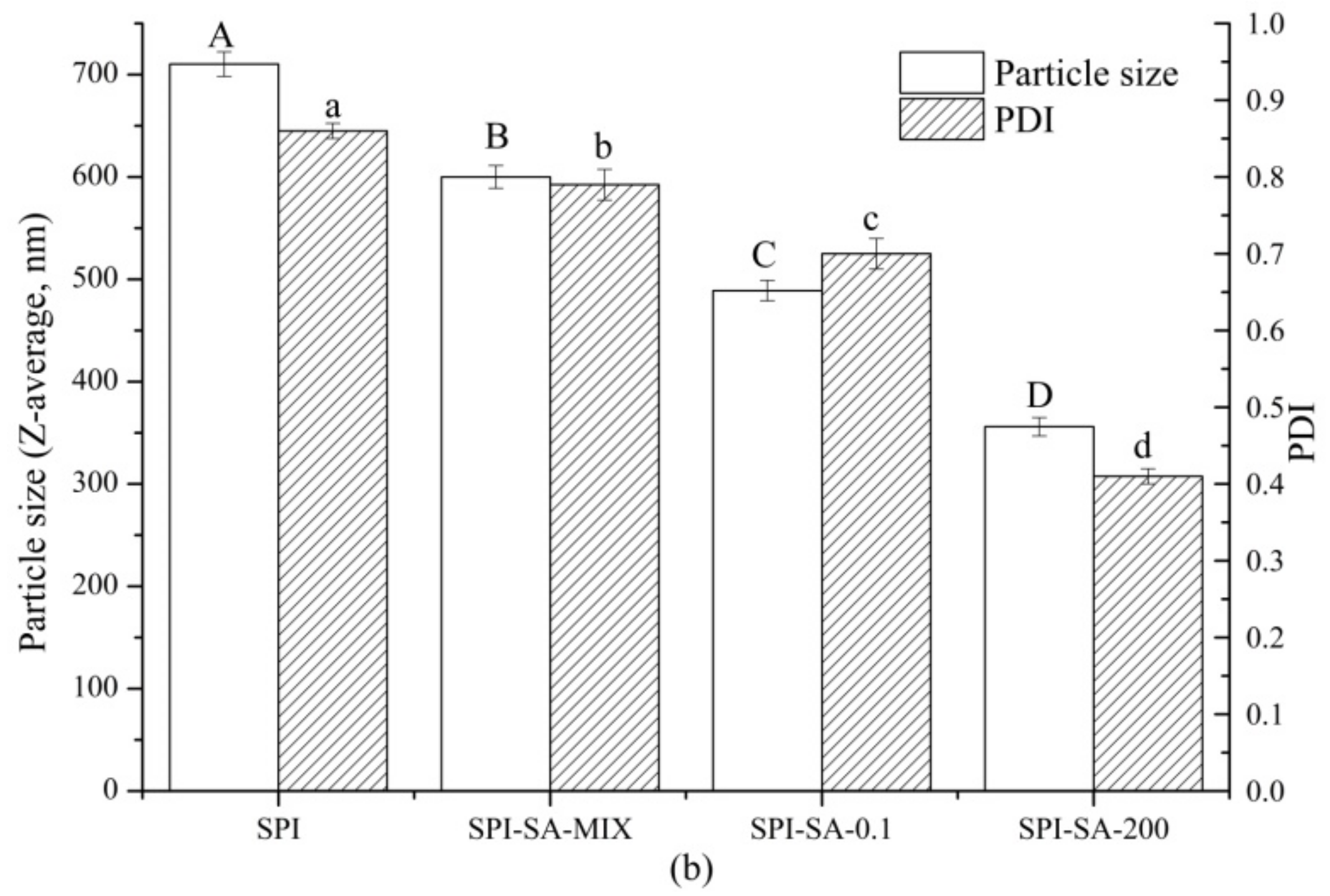
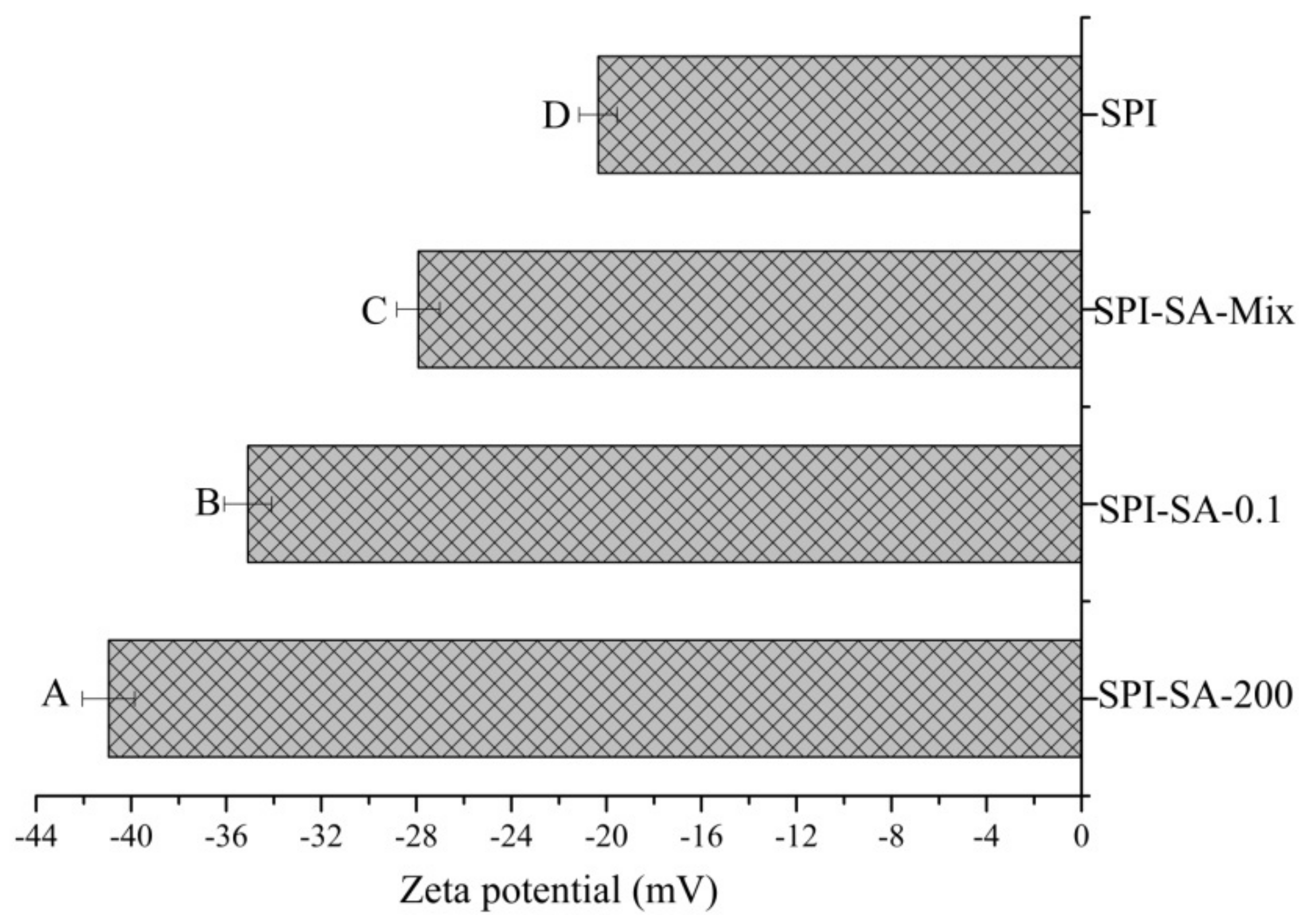
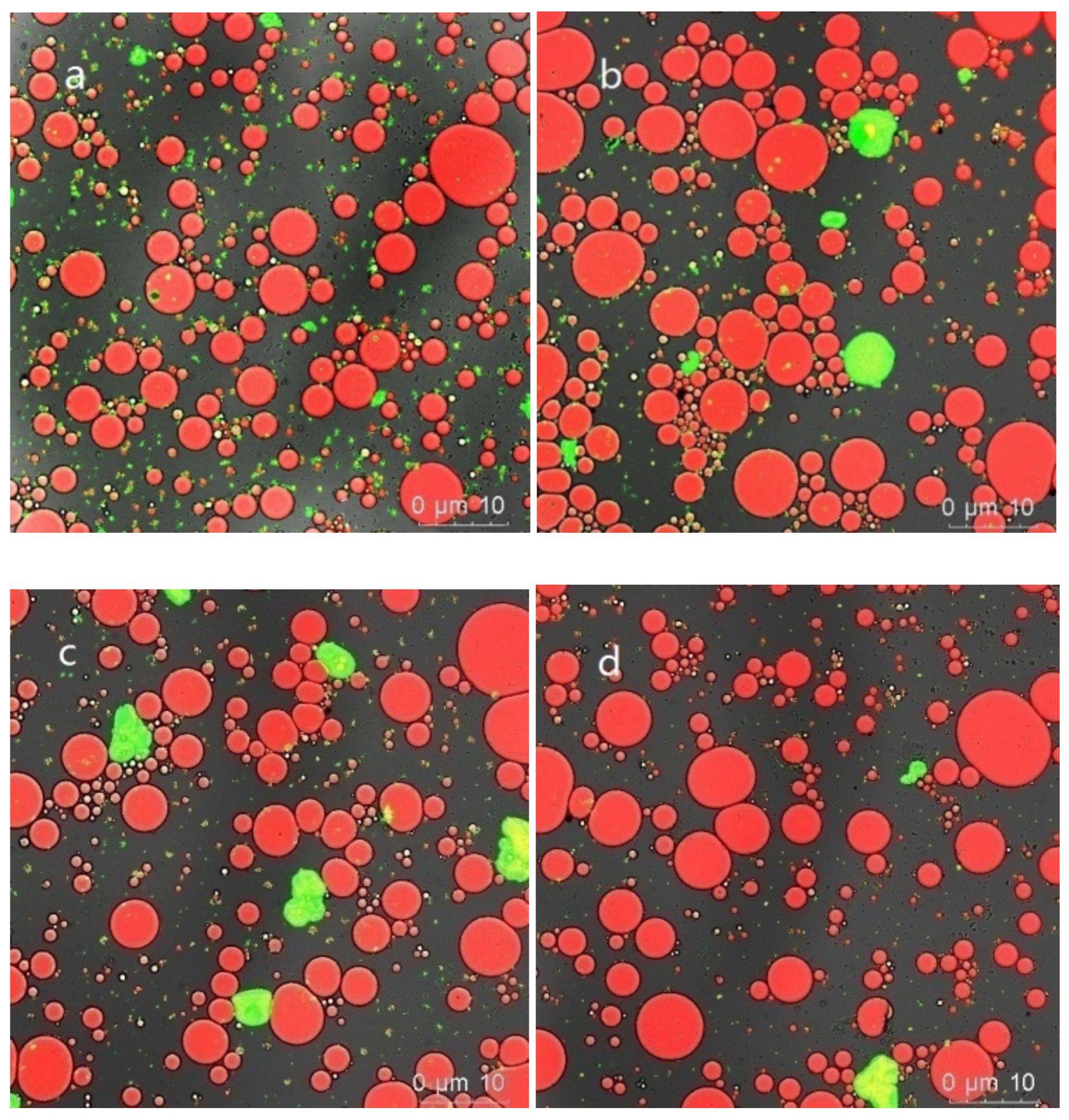
| Samples | SPI | SPI-SA-Mix | SPI-SA-0.1 | SPI-SA-200 |
|---|---|---|---|---|
| A294 | 0.09 ± 0.013 d | 0.12 ± 0.007 c | 0.82 ± 0.03 a | 0.54 ± 0.01 b |
| A420 | 0.02 ± 0.003 d | 0.03 ± 0.005 c | 0.17 ± 0.008 a | 0.10 ± 0.003 b |
| Sample | α-Helix (%) | β-Sheet (%) | β-Turns (%) | Random Coil (%) |
|---|---|---|---|---|
| SPI | 14.60 a | 27.03 b | 10.22 c | 48.15 d |
| SPI-SA-Mix | 13.10 b | 27.44 a | 9.58 d | 49.88 c |
| SPI-SA-0.1 | 11.51 c | 26.09 c | 11.96 a | 50.44 b |
| SPI-SA-200 | 9.08 d | 25.32 d | 10.98 b | 54.62 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Gong, S.; Wang, Y.; Liu, D.; Han, J. Structural and Emulsifying Properties of Soybean Protein Isolate–Sodium Alginate Conjugates under High Hydrostatic Pressure. Foods 2021, 10, 2829. https://doi.org/10.3390/foods10112829
Wang Z, Gong S, Wang Y, Liu D, Han J. Structural and Emulsifying Properties of Soybean Protein Isolate–Sodium Alginate Conjugates under High Hydrostatic Pressure. Foods. 2021; 10(11):2829. https://doi.org/10.3390/foods10112829
Chicago/Turabian StyleWang, Zihuan, Shaoying Gong, Yucong Wang, Danyi Liu, and Jianchun Han. 2021. "Structural and Emulsifying Properties of Soybean Protein Isolate–Sodium Alginate Conjugates under High Hydrostatic Pressure" Foods 10, no. 11: 2829. https://doi.org/10.3390/foods10112829
APA StyleWang, Z., Gong, S., Wang, Y., Liu, D., & Han, J. (2021). Structural and Emulsifying Properties of Soybean Protein Isolate–Sodium Alginate Conjugates under High Hydrostatic Pressure. Foods, 10(11), 2829. https://doi.org/10.3390/foods10112829







