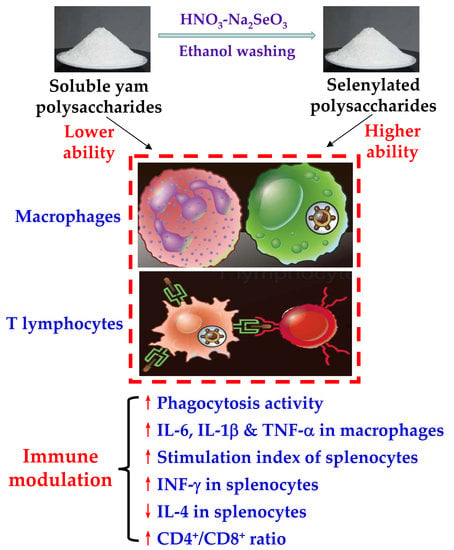
In Vitro Immunomodulation of the Polysaccharides from Yam (Dioscorea opposita Thunb.) in Response to a Selenylation of Lower Extent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Animals and Cells
2.3. Preparation and Assay of Yam Polysaccharides and Selenylated Polysaccharides
2.4. Assays of Macrophages Viability
2.5. Assays of Phagocytic Activity and Cytokine Secretion
2.6. Preparation of Splenocytes and Assays of Splenocytes Proliferation
2.7. Assays of T-Lymphocyte Subpopulations and Cytokine Secretion
2.8. Statistical Analysis
3. Results
3.1. Cytotoxicity of the Polysaccharide Samples and Na2SeO3 to Macrophages
3.2. Phagocytic Activity and Cytokine Production of the Macrophages in Response to the Polysaccharide Samples
3.3. Effects of the Polysaccharide Samples on Splenocyte Proliferation
3.4. Effect of the Polysaccharide Samples on Cytokine Production and T-Lymphocyte Subpopulations in Splenocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Shen, B.X.; Nie, S.L.; Duan, Z.H.; Chen, K.S. A combination of selenium and polysaccharides: Promising therapeutic potential. Carbohydr. Polym. 2019, 206, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.D.; Wang, X.; Wong, Y.S. Generation of selenium-enriched rice with enhanced grain yield, selenium content and bioavailability through fertilisation with selenite. Food Chem. 2013, 141, 2385–2393. [Google Scholar] [CrossRef]
- Gromadzinska, J.; Reszka, E.; Bruzelius, K.; Wasowicz, W.; Akesson, B. Selenium and cancer: Biomarkers of selenium status and molecular action of selenium supplements. Eur. J. Nutr. 2008, 47 (Suppl. 2), 29–50. [Google Scholar] [CrossRef]
- Chen, N.; Zhao, C.H.; Zhang, T.H. Selenium transformation and selenium-rich foods. Food Biosci. 2021, 40, 100875. [Google Scholar] [CrossRef]
- Li, S.J.; Xiong, Q.P.; Lai, X.P.; Li, X.; Wan, M.J.; Zhang, J.N.; Yan, Y.Y.; Cao, M.; Lu, L.; Guan, J.M.; et al. Molecular modification of polysaccharides and resulting bioactivities. Compr. Rev. Food Sci. Food Saf. 2016, 15, 237–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, W.Y.; Sun, L.J.; Miao, M.; Zhang, G.Y. Plant-sourced intrinsic dietary fiber: Physical structure and health function. Trends Food Sci. Tech. 2021, 118, 341–355. [Google Scholar] [CrossRef]
- Zhu, F. Dietary fiber polysaccharides of amaranth, buckwheat and quinoa grains: A review of chemical structure, biological functions and food uses. Carbohydr. Polym. 2020, 248, e116819. [Google Scholar] [CrossRef]
- Zhou, N.; Long, H.R.; Wang, C.H.; Zhu, Z.J.; Yu, L.; Yang, W.R.; Ren, X.Y.; Liu, X.L. Characterization of selenium-containing polysaccharide from Spirulina platensis and its protective role against Cd-induced toxicity. Int. J. Biol. Macromol. 2020, 164, 2465–2476. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Zhao, B.T.; Wang, X.F.; Yao, J.; Zhang, J. Synthesis of selenium-containing polysaccharides and evaluation of antioxidant activity in vitro. Int. J. Biol. Macromol. 2012, 51, 987–991. [Google Scholar] [CrossRef]
- Gao, Z.Z.; Chen, J.; Qiu, S.L.; Li, Y.Y.; Wang, D.Y.; Liu, C.; Li, X.P.; Hou, R.R.; Yue, C.J.; Liu, J.; et al. Optimization of selenylation modification for garlic polysaccharide based on immune-enhancing activity. Carbohydr. Polym. 2016, 136, 560–569. [Google Scholar] [CrossRef]
- Surhio, M.M.; Wang, Y.F.; Xu, P.; Shah, F.; Li, J.L.; Ye, M. Antihyperlipidemic and hepatoprotective properties of selenium modified polysaccharide from Lachnum sp. Int. J. Biol. Macromol. 2017, 99, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, Y.J.; Sun, P.L.; Zhang, F.M.; Linhardt, R.J.; Zhang, A.Q. Chemically modified polysaccharides: Synthesis, characterization, structure activity relationships of action. Int. J. Biol. Macromol. 2019, 132, 970–977. [Google Scholar] [CrossRef]
- Xie, L.; Shen, M.; Hong, Y.; Ye, H.; Huang, L.; Xie, J. Chemical modifications of polysaccharides and their anti-tumor activities. Carbohydr. Polym. 2020, 229, e115436. [Google Scholar] [CrossRef] [PubMed]
- Li, S.X.; Bao, F.Y.; Cui, Y. Immunoregulatory activities of the selenylated polysaccharides of Lilium davidii var. unicolor Salisb in vitro and in vivo. Int. Immunopharmacol. 2021, 94, e107445. [Google Scholar] [CrossRef]
- Yang, W.J.; Huang, G.L.; Chen, F.; Huang, H.L. Extraction/synthesis and biological activities of selenopolysaccharide. Trends Food Sci. Tech. 2021, 109, 211–218. [Google Scholar] [CrossRef]
- Loftus, R.M.; Finlay, D.K. Immunometabolism: Cellular metabolism turns immune regulator. J. Biol. Chem. 2016, 291, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Englinger, B.; Pirker, C.; Heffeter, P.; Terenzi, A.; Kowol, C.R.; Keppler, B.K.; Berger, W. Metal drugs and the anticancer immune response. Chem. Rev. 2019, 119, 1519–1624. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Jantan, I.; Ahmad, W.; Bukhari, S.N.A. Plant-derived immunomodulators: An insight on their preclinical evaluation and clinical trials. Front. Plant Sci. 2015, 6, 18. [Google Scholar] [CrossRef]
- Kim, Y.S.; Ahn, C.B.; Je, J.Y. Anti-inflammatory action of high molecular weight Mytilus edulis hydrolysates fraction in LPS-induced RAW264.7 macrophage via NF-kappaB and MAPK pathways. Food Chem. 2016, 202, 9–14. [Google Scholar] [CrossRef]
- Meng, X.; Liang, H.B.; Luo, L.X. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 2016, 424, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.Y.; Huang, G.L. Preparation and antioxidant activities of important traditional plant polysaccharides. Int. J. Biol. Macromol. 2018, 111, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Epping, J.; Laibach, N. An underutilized orphan tuber crop-Chinese yam: A review. Planta 2020, 252, e58. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.M.; Koo, S.J.; Hwang, J.K. Immune cell stimulating activity of mucopolysaccharide isolated from yam (Dioscorea batatas). J. Ethnopharmacol. 2004, 91, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Otegbayo, B.; Madu, T.; Oroniran, O.; Chijioke, U.; Fawehinmi, O.; Okoye, B.; Tanimola, A.; Adebola, P.; Obidiegwu, J. End-user preferences for pounded yam and implications for food product profile development. Int. J. Food Sci. Technol. 2021, 56, 1458–1472. [Google Scholar] [CrossRef]
- Zhao, J.L.; Ma, C.M.; Zhao, X.H.; Zhang, Q. Effects of yam (Dioscorea opposita Thunb.) juice on fermentation and textural attributes of set-style skimmed yoghurt. J. Food Meas. Charact. 2021, 15, 2220–2230. [Google Scholar] [CrossRef]
- Ma, F.; Wang, D.; Zhang, Y.; Li, M.; Qing, W.; Tikkanen-Kaukanen, C.; Liu, X.; Bell, A.E. Characterisation of the mucilage polysaccharides from Dioscorea opposita Thunb. with enzymatic hydrolysis. Food Chem. 2018, 245, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Ma, F.Y.; Zhang, Y.; Yao, Y.N.; Wen, Y.R.; Hu, W.P.; Zhang, J.; Liu, X.H.; Bell, A.E.; Tikkanen-Kaukanen, C. Chemical components and emulsification properties of mucilage from Dioscorea opposita Thunb. Food Chem. 2017, 228, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Xie, J.H.; Yu, Y.; Shen, M.Y. Recent progress in the research of yam mucilage polysaccharides: Isolation, structure and bioactivities. Int. J. Biol. Macromol. 2020, 155, 1262–1269. [Google Scholar] [CrossRef]
- Hao, L.X.; Zhao, X.H. Immunomodulatory potentials of the water-soluble yam (Dioscorea opposita Thunb) polysaccharides for the normal and cyclophosphamide-suppressed mice. Food Agric. Immunol. 2016, 27, 667–677. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.S.; Deng, J.S.; Chang, H.Y.; Chen, Y.C.; Lee, M.M.; Hou, W.C.; Lee, C.Y.; Huang, S.S.; Huang, G.J. Antioxidant and anti-inflammatory properties of Taiwanese yam (Dioscorea japonica Thunb. var. pseudojaponica (Hayata) Yamam.) and its reference compounds. Food Chem. 2013, 141, 1087–1096. [Google Scholar] [PubMed]
- Li, M.; Chen, L.X.; Chen, S.R.; Deng, Y.; Zhao, J.; Wang, Y.; Li, S.P. Non-starch polysaccharide from Chinese yam activated RAW 264.7 macrophages through the Toll-like receptor 4 (TLR4)-NF-κB signaling pathway. J. Funct. Foods 2017, 37, 491–500. [Google Scholar] [CrossRef]
- Cheng, L.Z.; Wang, Y.F.; He, X.X.; Wei, X.L. Preparation, structural characterization and bioactivities of Se-containing polysaccharide: A review. Int. J. Biol. Macromol. 2018, 120, 82–92. [Google Scholar] [CrossRef]
- Wróbel, K.; Wróbel, K.; Kannamkumarath, S.S.; Caruso, J.A.; Wysocka, I.A.; Bulska, E.; Świa̧tek, J.; Wierzbicka, M. HPLC–ICP-MS speciation of selenium in enriched onion leaves—A potential dietary source of Se-methylselenocysteine. Food Chem. 2004, 86, 617–623. [Google Scholar] [CrossRef]
- Li, S.Q.; Shah, N.P. Characterization, anti-Inflammatory and antiproliferative activities of natural and sulfonated exo-polysaccharides from Streptococcus thermophilus ASCC 1275. J. Food Sci. 2016, 81, M1167–M1176. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, X.H. Immune potentials of the Mucor-fermented Mao-tofu and especially its soluble extracts for the normal mice. Food Agric. Immunol. 2017, 28, 859–875. [Google Scholar] [CrossRef]
- Wang, M.C.; Jiang, C.X.; Ma, L.P.; Zhang, Z.J.; Cao, L.; Liu, J.; Zeng, X.X. Preparation, preliminary characterization and immunostimulatory activity of polysaccharide fractions from the peduncles of Hovenia dulcis. Food Chem. 2013, 138, 41–47. [Google Scholar] [CrossRef]
- Tanna, B.; Mishra, A. Nutraceutical potential of seaweed polysaccharides: Structure, bioactivity, safety, and toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 817–831. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, I.C.; Heleno, S.A.; Reis, F.S.; Stojkovic, D.; Queiroz, M.J.; Vasconcelos, M.H.; Sokovic, M. Chemical features of Ganoderma polysaccharides with antioxidant, antitumor and antimicrobial activities. Phytochemistry 2015, 114, 38–55. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jin, H.Y.; Yu, J.D.; Qu, C.H.; Wang, Q.; Yang, S.; Ma, S.C.; Ni, J. Quality control and immunological activity of lentinan samples produced in China. Int. J. Biol. Macromol. 2020, 159, 129–136. [Google Scholar] [CrossRef]
- Huang, L.X.; Shen, M.Y.; Morris, G.A.; Xie, J.H. Sulfated polysaccharides: Immunomodulation and signaling mechanisms. Trends Food Sci Tech. 2019, 92, 1–11. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Yue, C.J.; Hou, R.R.; Chen, J.; Lu, Y.; Li, X.P.; Li, R.J.; Liu, C.; Gao, Z.Z.; et al. Effect of selenylation modification on immune-enhancing activity of Atractylodes macrocephala polysaccharide. Int. J. Biol. Macromol. 2015, 72, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, Y.K.; Choi, Y.R.; Park, J.; Jung, S.K.; Chang, Y.H. The characterization, selenylation and anti-inflammatory activity of pectic polysaccharides extracted from Ulmus pumila L. Int. J. Biol. Macromol. 2018, 111, 311–318. [Google Scholar] [CrossRef]
- FAO/WHO. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Bangkok, Thailand, 2004; pp. 194–216. [Google Scholar]
- Vinceti, M.; Mandrioli, J.; Borella, P.; Michalke, B.; Tsatsakis, A.; Finkelstein, Y. Selenium neurotoxicity in humans: Bridging laboratory and epidemiologic studies. Toxicol. Lett. 2014, 230, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Zhu, Z.Y.; Sun, X.L.; Gao, H.; Zhang, Y.M. The preparation of three selenium-containing Cordyceps militaris polysaccharides: Characterization and anti-tumor activities. Int. J. Biol. Macromol. 2017, 99, 196–204. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, Y.K.; Chang, Y.H. Effects of selenylation modification on structural and antioxidant properties of pectic polysaccharides extracted from Ulmus pumila L. Int. J. Biol. Macromol. 2017, 104, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Yang, X.Q.; Kou, M.; Lu, C.Y.; Wang, Y.Y.; Peng, J.; Chen, P.; Jiang, J.H. Selenylation of polysaccharide from the sweet potato and evaluation of antioxidant, antitumor, and antidiabetic activities. J. Agric. Food. Chem. 2017, 65, 605–617. [Google Scholar] [CrossRef]
- Ren, G.M.; Yu, M.; Li, K.K.; Hu, Y.; Wang, Y.; Xu, X.H.; Qu, J.J. Seleno-lentinan prevents chronic pancreatitis development and modulates gut microbiota in mice. J. Funct. Foods 2016, 22, 177–188. [Google Scholar] [CrossRef]
- Qin, T.; Ren, Z.; Lin, D.D.; Song, Y.L.; Li, J.; Ma, Y.F.; Hou, X.H.; Huang, Y.F. Effects of selenizing Codonopsis pilosula polysaccharide on macrophage modulatory activities. J. Microbiol. Biotechnol. 2016, 26, 1358–1366. [Google Scholar] [CrossRef] [Green Version]
- Jie, D.; Gao, T.T.; Shan, Z.S.; Song, J.Y.; Zhang, M.; Kurskaya, O.; Sharshov, K.; Wei, L.X.; Bi, H.T. Immunostimulating effect of polysaccharides isolated from Ma-Nuo-Xi decoction in cyclophosphamide-immunosuppressed mice. Int. J. Biol. Macromol. 2020, 146, 45–52. [Google Scholar] [CrossRef]
- Sun, W.J.; Meng, K.; Qi, C.H.; Yang, X.Y.; Wang, Y.G.; Fan, W.T.; Yan, Z.G.; Zhao, X.N.; Liu, J.Z. Immune-enhancing activity of polysaccharides isolated from Atractylodis macrocephalae Koidz. Carbohydr. Polym. 2015, 126, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.B.; Fan, J.; Bo, H.Q.; Tian, X.; Bao, H.; Wang, X.H. Selenylation modification can enhance immune-enhancing activity of Chuanminshen violaceum polysaccharide. Carbohydr. Polym. 2016, 153, 302–311. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, Q.; Zhao, X.H.; Wang, L. The impact of caseinate oligochitosan-glycation by transglutaminase on amino acid compositions and immune-promoting activity in BALB/c mice of the tryptic caseinate hydrolysate. Food Chem. 2021, 350, e129302. [Google Scholar] [CrossRef]
- Hao, L.X.; Zhao, X.H. Immune activities of the water-soluble yam (Dioscorea opposite Thunb) polysaccharides as affected by thermal, acidic and enzymatic treatments. CyTA-J. Food. 2016, 14, 266–270. [Google Scholar] [CrossRef]
- Liu, W.N.; Shi, J.; Fu, Y.; Zhao, X.H. The stability and activity changes of apigenin and luteolin in human cervical cancer Hela cells in response to heat treatment and Fe2+/Cu2+ addition. Foods 2019, 8, 346. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wang, J.; Zhao, X.H. In vitro activities of the four structurally similar flavonols weakened by the prior thermal and oxidative treatments to a human colorectal cancer line. J. Food Biochem. 2017, 41, e12310. [Google Scholar] [CrossRef]
- Rong, Y.; Yang, R.L.; Yang, Y.Z.; Wen, Y.Z.; Liu, S.X.; Li, C.F.; Hu, Z.Y.; Cheng, X.R.; Li, W. Structural characterization of an active polysaccharide of longan and evaluation of immunological activity. Carbohydr. Polym. 2019, 213, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.Z.; Hao, R.; Zha, X.Q.; Pan, L.H.; Liu, J.; Luo, J.P. Polysaccharide of Dendrobium huoshanense activates macrophages via toll-like receptor 4-mediated signaling pathways. Carbohydr. Polym. 2016, 146, 292–300. [Google Scholar] [CrossRef]
- Xu, W.; Fang, S.J.; Cui, X.M.; Guan, R.; Wang, Y.; Shi, F.S.; Hu, S.H. Signaling pathway underlying splenocytes activation by polysaccharides from Atractylodis macrocephalae Koidz. Mol. Immunol. 2019, 111, 19–26. [Google Scholar] [CrossRef]
- Yu, Q.; Nie, S.P.; Wang, J.Q.; Huang, D.F.; Li, W.J.; Xie, M.Y. Signaling pathway involved in the immunomodulatory effect of Ganoderma atrum polysaccharide in spleen lymphocytes. J. Agric. Food Chem. 2015, 63, 2734–2740. [Google Scholar] [CrossRef]





| Index | Cell Group | Dose Level of Polysaccharide Sample (μg/mL) | ||
|---|---|---|---|---|
| 5 | 20 | 80 | ||
| CD4+ | Control | 29.4 ± 1.5 | ||
| YPS | 29.9 ± 2.5 | 30.7 ± 2.8 | 30.6 ± 4.4 | |
| SeYPS-1 | 29.3 ± 1.2 | 29.0 ± 2.4 | 29.7 ± 1.3 | |
| SeYPS-2 | 29.8 ± 0.6 | 29.6 ± 1.4 | 30.5 ± 3.3 | |
| CD8+ | Control | 16.4 ± 1.6 | ||
| YPS | 15.4 ± 3.5 | 15.4 ± 4.0 | 15.2 ± 2.4 | |
| SeYPS-1 | 14.9 ± 1.6 | 14.5 ± 2.2 | 13.9 ± 1.0 | |
| SeYPS-2 | 14.4 ± 0.8 | 13.9 ± 0.8 | 13.6 ± 1.2 | |
| CD4+/CD8+ | Control | 1.99 ± 0.02 | ||
| YPS | 1.94 ± 0.14 | 2.10 ± 0.44 | 2.12 ± 0.22 | |
| SeYPS-1 | 1.98 ± 0.15 | 2.17 ± 0.02 | 2.13 ± 0.04 | |
| SeYPS-2 | 2.07 ± 0.20 | 2.17 ± 0.09 | 2.24 ± 0.04 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Q.-Y.; Lin, Y.-R.; Li, L.-Y.; Tang, Z.-M.; Zhao, X.-H.; Shi, J. In Vitro Immunomodulation of the Polysaccharides from Yam (Dioscorea opposita Thunb.) in Response to a Selenylation of Lower Extent. Foods 2021, 10, 2788. https://doi.org/10.3390/foods10112788
Guan Q-Y, Lin Y-R, Li L-Y, Tang Z-M, Zhao X-H, Shi J. In Vitro Immunomodulation of the Polysaccharides from Yam (Dioscorea opposita Thunb.) in Response to a Selenylation of Lower Extent. Foods. 2021; 10(11):2788. https://doi.org/10.3390/foods10112788
Chicago/Turabian StyleGuan, Qing-Yun, Ya-Ru Lin, Ling-Yu Li, Zhi-Mei Tang, Xin-Huai Zhao, and Jia Shi. 2021. "In Vitro Immunomodulation of the Polysaccharides from Yam (Dioscorea opposita Thunb.) in Response to a Selenylation of Lower Extent" Foods 10, no. 11: 2788. https://doi.org/10.3390/foods10112788
APA StyleGuan, Q.-Y., Lin, Y.-R., Li, L.-Y., Tang, Z.-M., Zhao, X.-H., & Shi, J. (2021). In Vitro Immunomodulation of the Polysaccharides from Yam (Dioscorea opposita Thunb.) in Response to a Selenylation of Lower Extent. Foods, 10(11), 2788. https://doi.org/10.3390/foods10112788