Glyphosate Use, Toxicity and Occurrence in Food
Abstract
:1. Introduction
2. Methodology
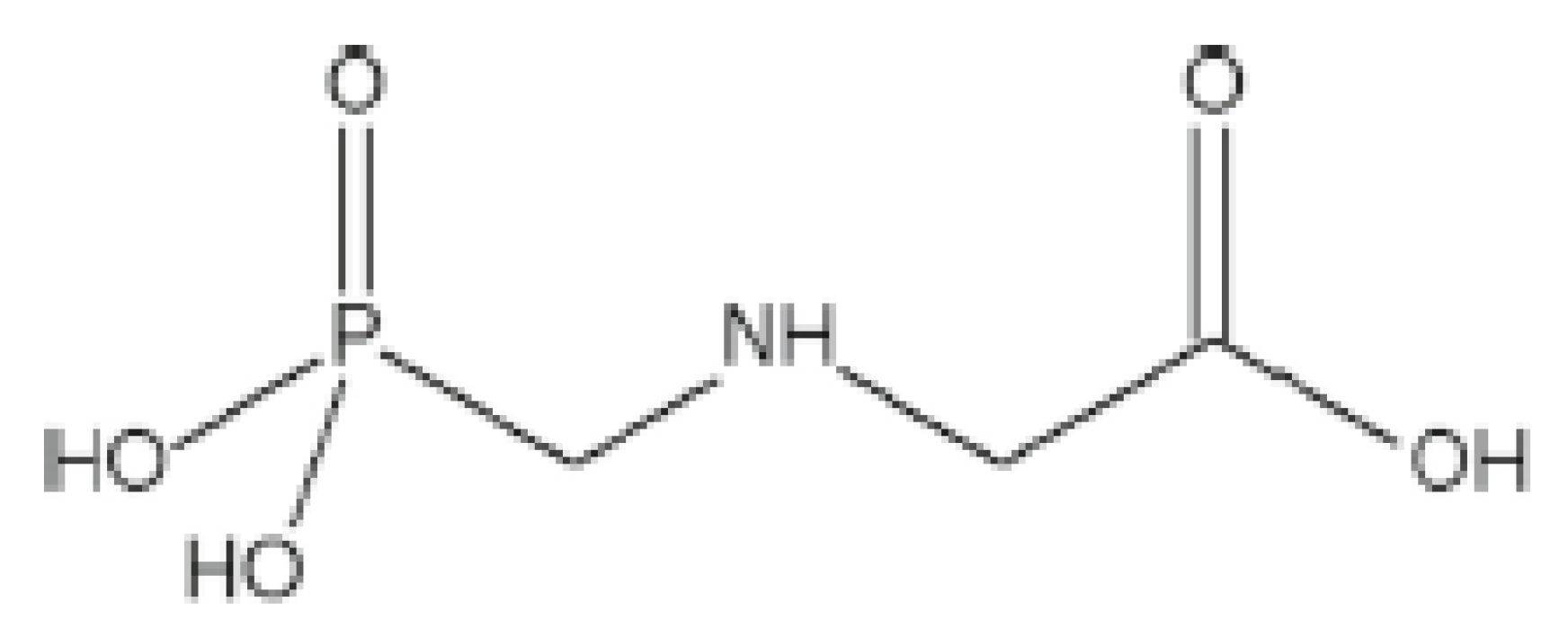
3. Physical and Chemical Properties
4. Glyphosate-Based Herbicides
4.1. Sales and Use
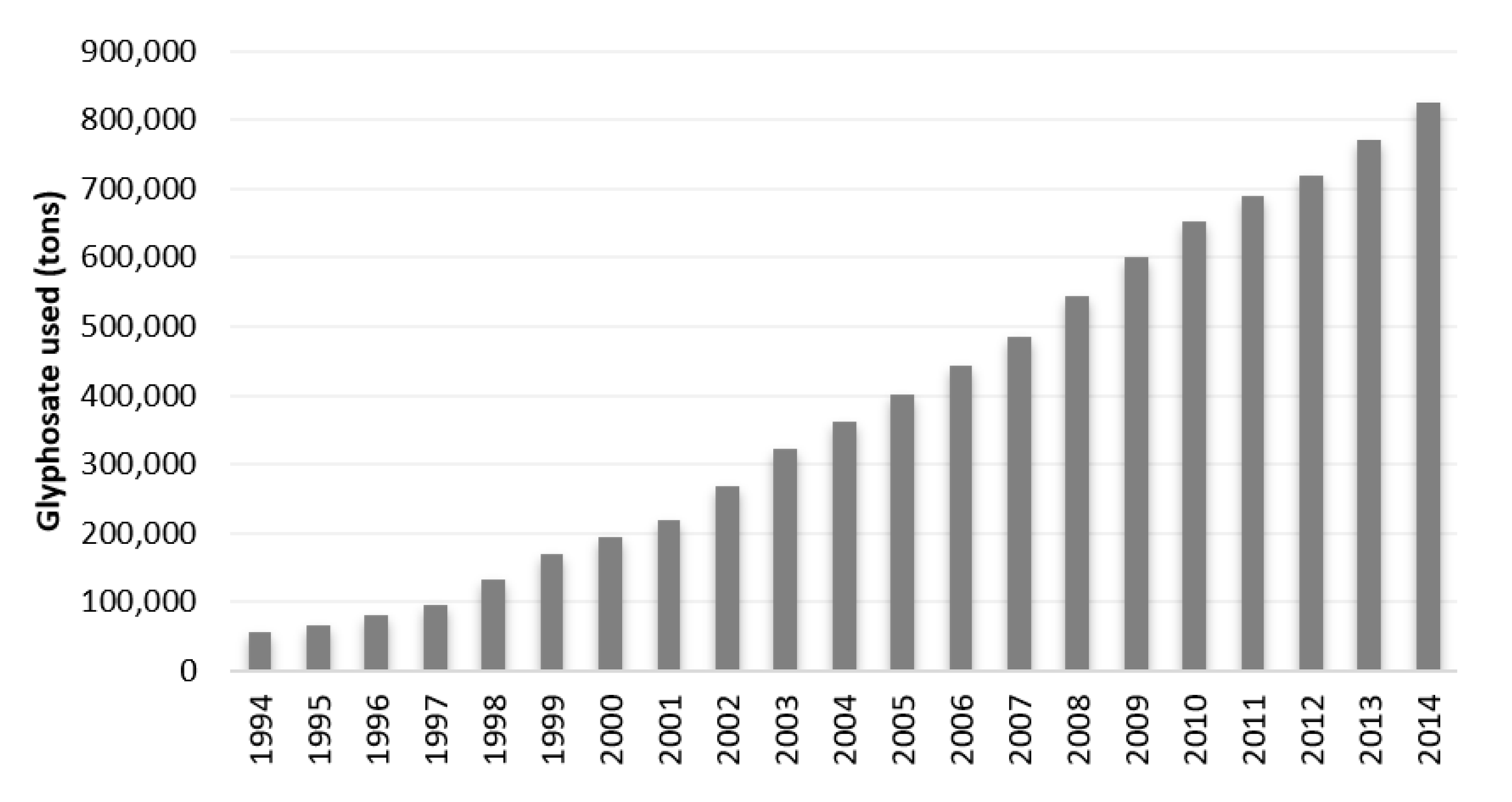
4.1.1. Worldwide
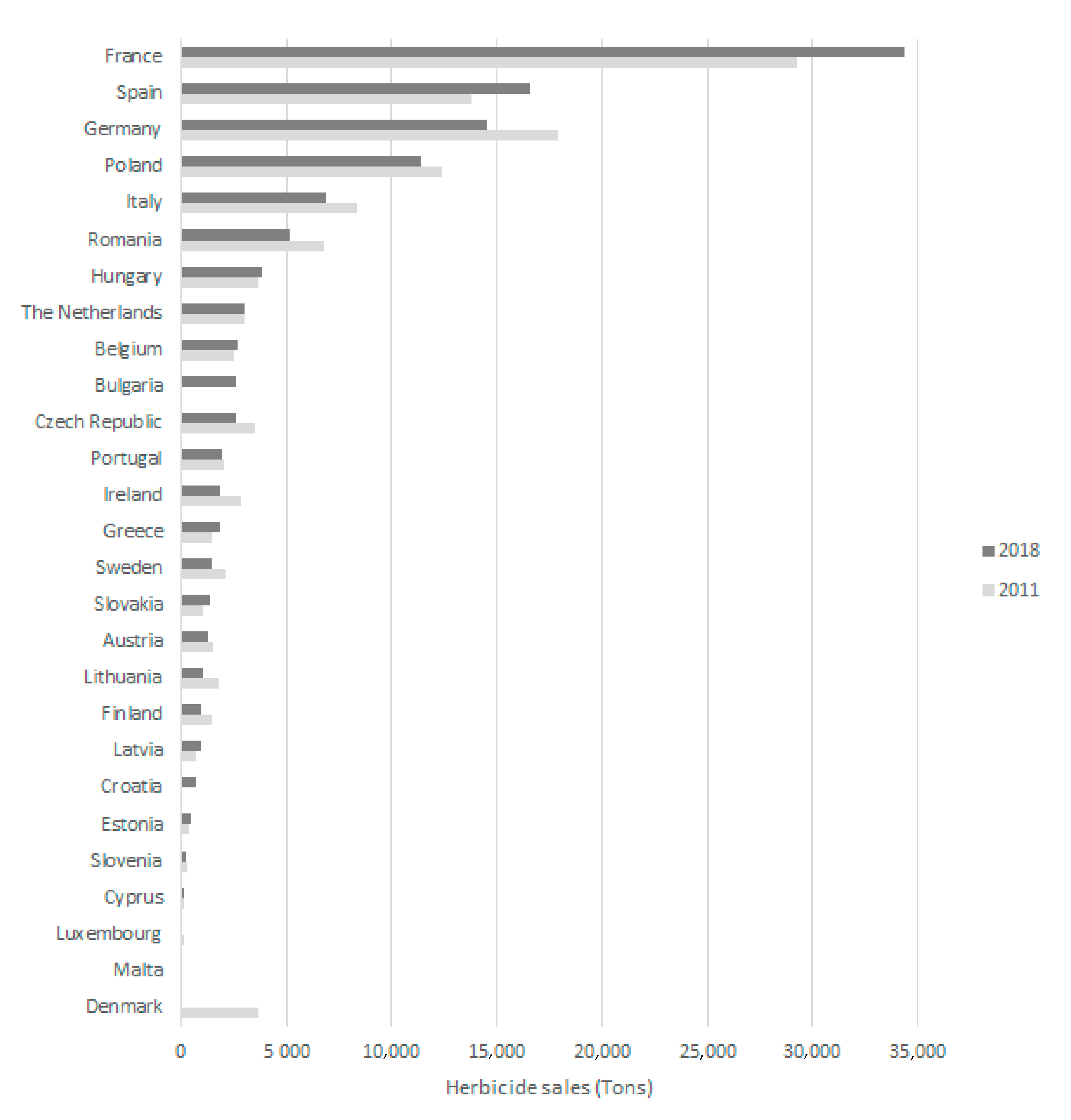
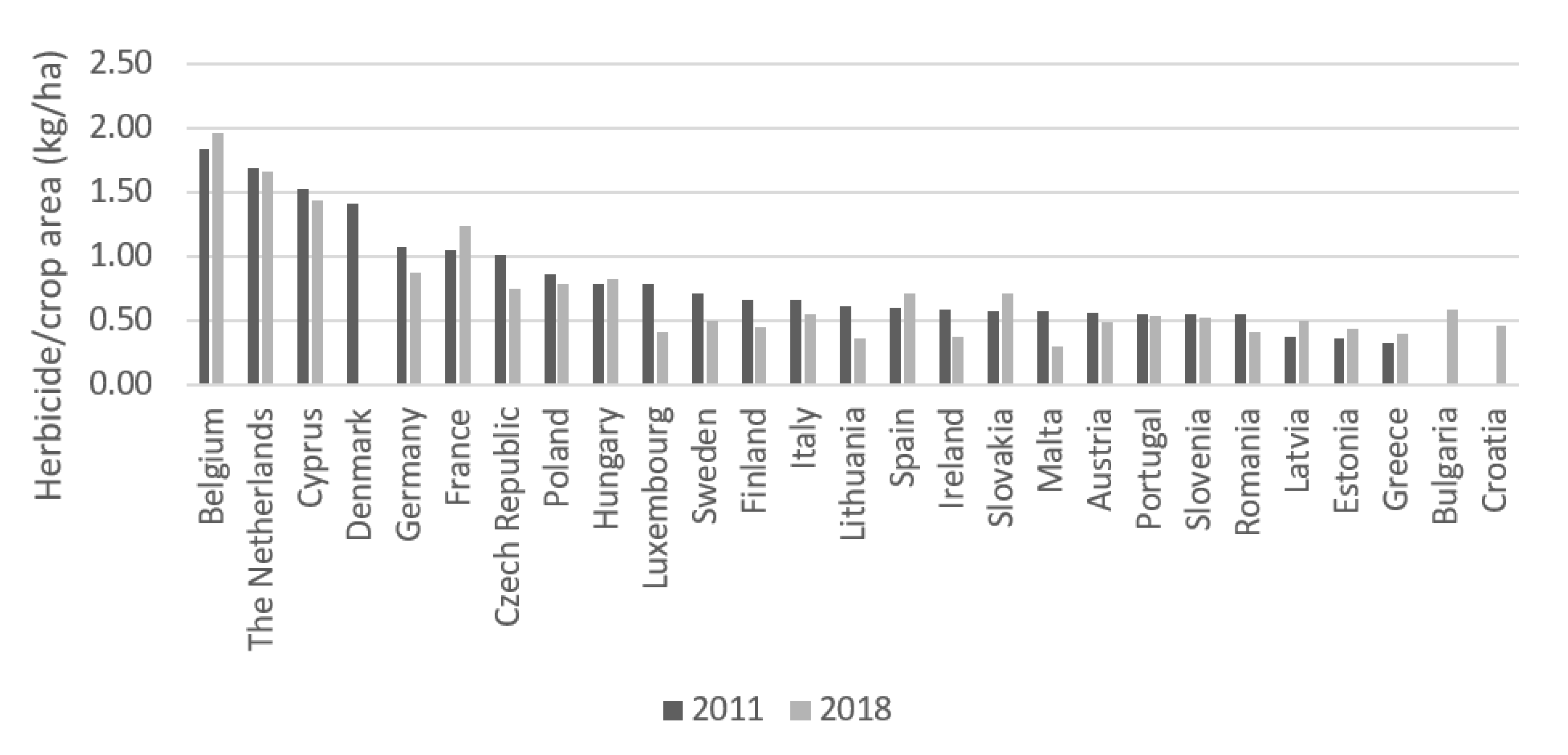
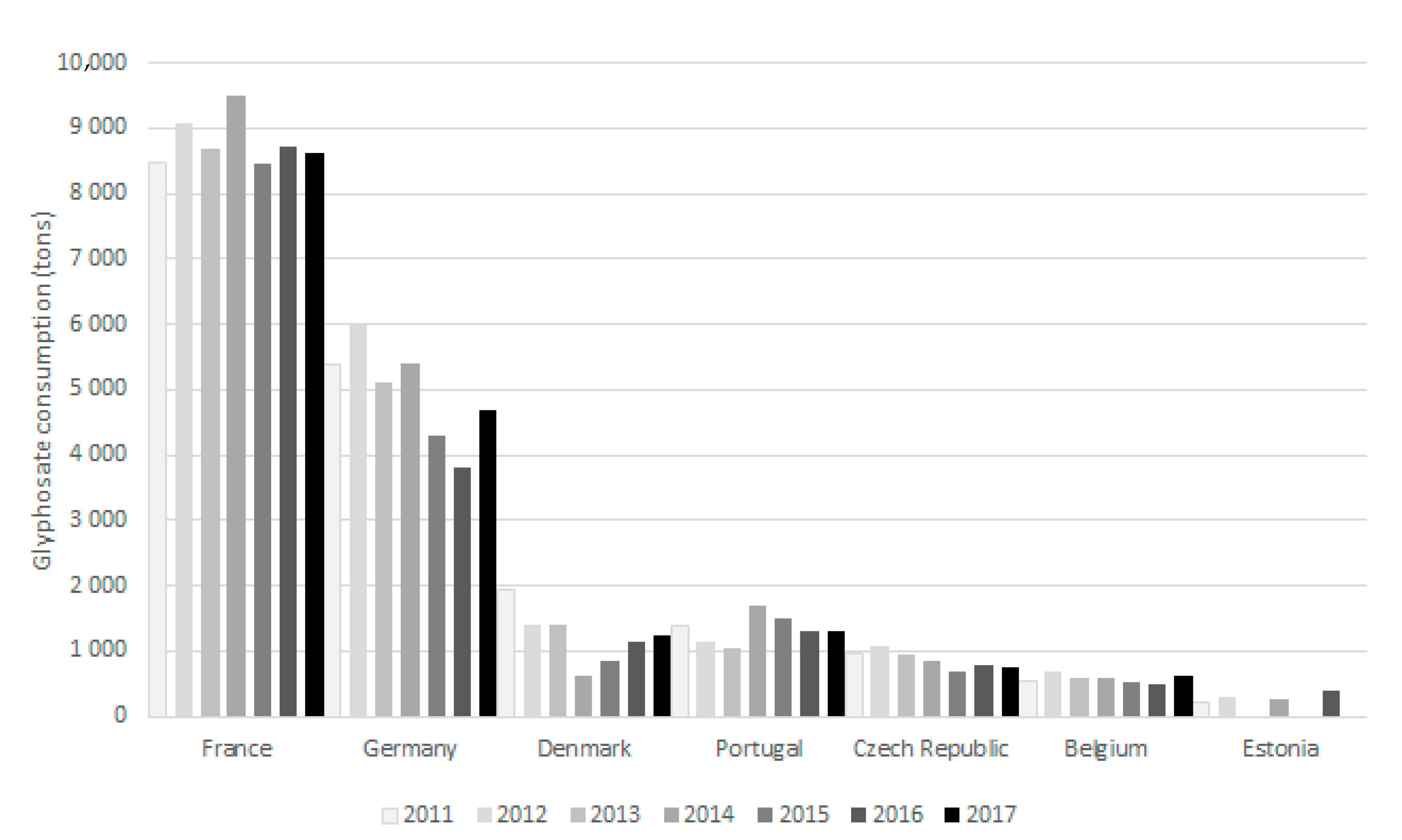
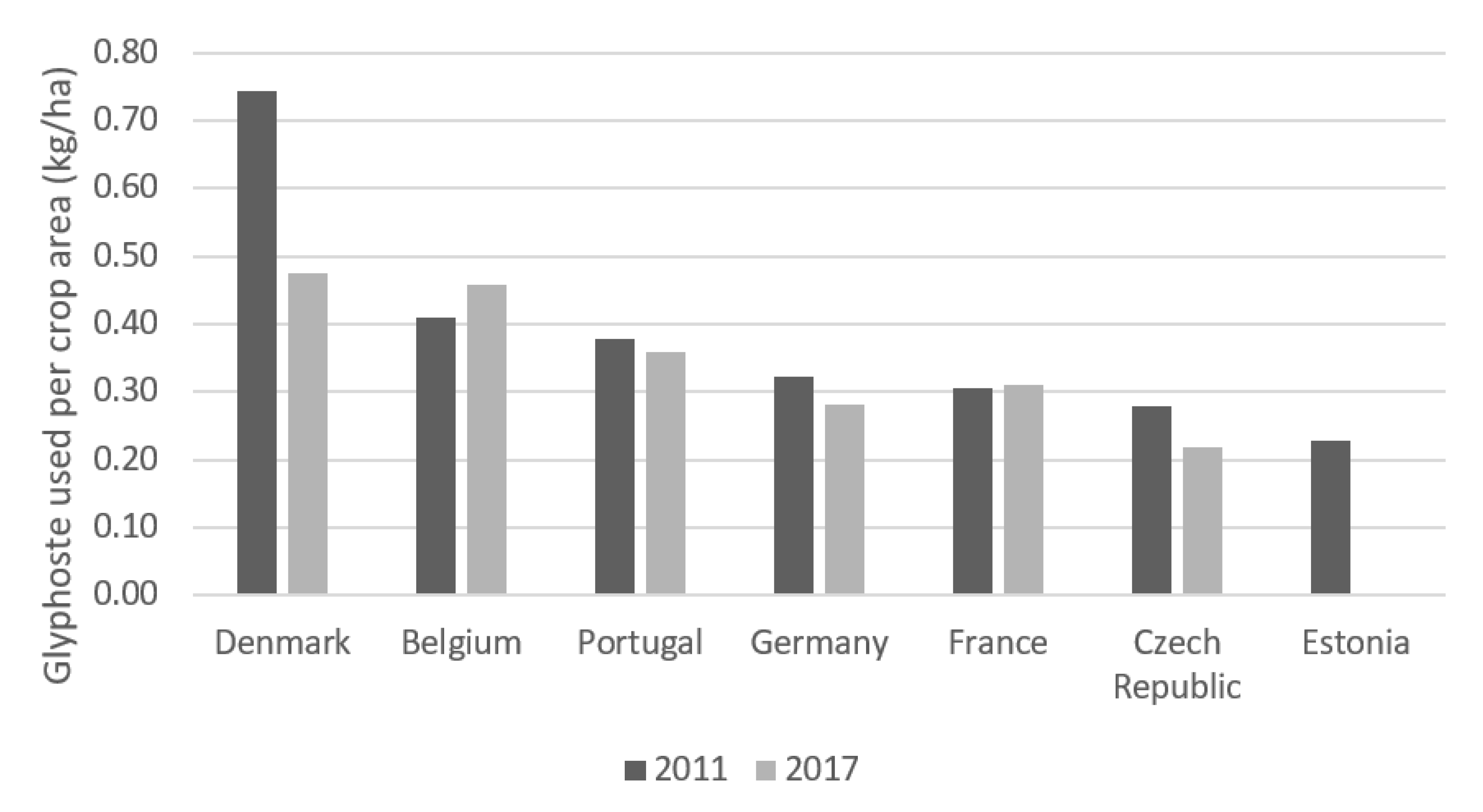
4.1.2. European Union
4.2. Action Mechanism in Plants
5. Toxicokinetics in Humans
5.1. Absorption
5.2. Distribution and Metabolism
5.3. Excretion
6. Human Health Impact
6.1. Toxicological Parameters
- No Observable Adverse Effect Level (NOAEL) of 100 mg/kg body weight per day.
- Acceptable Daily Intake (ADI) of 0.5 mg/kg of body weight per day.
- Acute Reference Dose (ARfD) of 0.5 mg/kg of body weight per day.
- Acceptable Operator Exposure Level (AOEL) of 0.1 mg/kg body weight per day.
6.2. Acute Toxicity
6.3. Chronic Toxicity
6.3.1. Target Organ Toxicity
6.3.2. Cytotoxicity
6.3.3. Carcinogenicity
6.3.4. Neurotoxicity
6.3.5. Genotoxicity
6.3.6. Teratogenic Effects
6.3.7. Endocrine Disruption
7. Environmental Impact
8. Legislation and Maximum Residue Levels in Food
9. Analytical Methodologies
10. Occurrence in Food
10.1. Olive Oil
10.2. Honey
10.3. Fruits and Nuts
10.4. Cereals and Cereal Products
10.5. Vegetables
10.6. Animal-Derived Products
10.7. Baby Food
10.8. Water
10.9. Alcoholic Beverages
11. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Comission Regulation (EU) 2017/269 of 16 February 2017; Official Journal of the European Union: Brussels, Belgium, 2017; L. 40/4.
- Davoren, M.J.; Schiestl, R.H. Glyphosate-based herbicides and cancer risk: A post-IARC decision review of potential mechanisms, policy and avenues of research. Carcinogenesis 2018, 39, 1207–1215. [Google Scholar] [CrossRef] [Green Version]
- Williams, G.M.; Kroes, R.; Munro, I.C. Safety Evaluation and Risk Assessment of the Herbicide Roundup and Its Active Ingredient, Glyphosate, for Humans. Regul. Toxicol. Pharmacol. 2000, 31, 117–165. [Google Scholar] [CrossRef] [Green Version]
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Tarazona, J.V.; Court-Marques, D.; Tiramani, M.; Reich, H.; Pfeil, R.; Istace, F.; Crivellente, F. Glyphosate toxicity and carcinogenicity: A review of the scientific basis of the European Union assessment and its differences with IARC. Arch. Toxicol. 2017, 91, 2723–2743. [Google Scholar] [CrossRef] [Green Version]
- Chiesa, L.M.; Nobile, M.; Panseri, S.; Arioli, F. Detection of glyphosate and its metabolites in food of animal origin based on ion-chromatography-high resolution mass spectrometry (IC-HRMS). Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 592–600. [Google Scholar] [CrossRef]
- Bai, S.H.; Ogbourne, S.M. Glyphosate: Environmental contamination, toxicity and potential risks to human health via food contamination. Environ. Sci. Pollut. Res. 2016, 23, 18988–19001. [Google Scholar] [CrossRef]
- Compound Summary-Glyphosate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Glyphosate (accessed on 29 October 2021).
- Villarreal-Chiu, J.F.; Acosta-Cortés, A.G.; Kumar, S.; Kaushik, G.; Singh, R. Green Technologies and Environmental Sustainability; Singh, R., Kumar, S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-50653-1. [Google Scholar]
- Oliveira, P.C.; Maximiano, E.M.; Oliveira, P.A.; Camargo, J.S.; Fiorucci, A.R.; Arruda, G.J. Direct electrochemical detection of glyphosate at carbon paste electrode and its determination in samples of milk, orange juice, and agricultural formulation. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2018, 53, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Feng, Y.; Fan, X.; Chen, S. Recent advances in glyphosate biodegradation. Appl. Microbiol. Biotechnol. 2018, 102, 5033–5043. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, E.; Cartaud, G.; Quinn, R.M.; Marotti, I.; Dinelli, G. An interlaboratory comparative study on the quantitative determination of glyphosate at low levels in wheat flour. J. AOAC Int. 2015, 98, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- Wumbei, A.; Goeteyn, L.; Lopez, E.; Houbraken, M.; Spanoghe, P. Glyphosate in yam from Ghana. Food Addit. Contam. Part B Surveill. 2019, 12, 231–235. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. EFSA J. 2016, 13. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G. Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 2018, 616–617, 255–268. [Google Scholar] [CrossRef]
- Agostini, L.P.; Dettogni, R.S.; dos Reis, R.S.; Stur, E.; dos Santos, E.V.W.; Ventorim, D.P.; Garcia, F.M.; Cardoso, R.C.; Graceli, J.B.; Louro, I.D. Effects of glyphosate exposure on human health: Insights from epidemiological and in vitro studies. Sci. Total Environ. 2020, 705, 135808. [Google Scholar] [CrossRef] [PubMed]
- IARC Glyphosate. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans-Volume 112: Some Organophosphate Insecticides and Herbicides; Agência Internacional para a Investigação do Cancro: Lyon, France, 2017; Volume 112, pp. 321–412. ISBN 9789283201786. [Google Scholar]
- Conrad, A.; Schröter-Kermani, C.; Hoppe, H.W.; Rüther, M.; Pieper, S.; Kolossa-Gehring, M. Glyphosate in German adults–Time trend (2001 to 2015) of human exposure to a widely used herbicide. Int. J. Hyg. Environ. Health 2017, 220, 8–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, T.S.; van den Heever, J.P.; Limanowka, R.E. Determination of glyphosate, AMPA, and glufosinate in honey by online solid-phase extraction-liquid chromatography-tandem mass spectrometry. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 434–446. [Google Scholar] [CrossRef]
- Muthman, R. The Use of Plant Protection Products in the European Union Data 1992–2003; Nadin, P., Ed.; Eurostat Statistical Books: Luxembourg, 2007; ISBN 9279038907. [Google Scholar]
- Eurostat Sales of Pesticides by Type of Pesticides. Available online: https://appsso.eurostat.ec.europa.eu/nui/submitViewTableAction.do (accessed on 10 August 2021).
- Eurostat. Agriculture, Forestry and Fishery Statistics; Publications Office of the European Union: Luxembourg, 2018; ISBN 9789279947582. [Google Scholar]
- Janson, M. So viel Glyphosat kommt in Deutschland zum Einsatz. Available online: https://de.statista.com/infografik/17416/glyphosatz-absatz-in-deutschland/ (accessed on 10 October 2021).
- Deutscher Bundestag. Folgen aus der Gefährdung von Bestäubern und der Umwelt durch Neonikotinoide und Andere Pestizidwirkstoffe; Bundesanzeiger Verlag GmbH: Köln, Germany, 2015. [Google Scholar]
- SPF. Santé publique Sécurité de la Chaîne Alimentaire et Environnement Données de vente des Produits Phytopharmaceutiques en Belgique. Available online: https://fytoweb.be/fr/plan-de-reduction/vigilance/donnes-de-vente (accessed on 14 July 2021).
- Rebane, R.; Vooro, K.; Nurk, G.; Leisk, Ü.; Laht, M.; Metsur, M. Uuring Pestitsiidide Koormuse Allikate ja Päritolu Selgitamiseks Nitraaditundlikul Alal; Eesti Keskkonnauuringute Keskus OÜ: Tallinn, Estonia, 2017. [Google Scholar]
- Ørum, J.E.; Martensen, K.Ø. Bekæmpelsesmiddelstatistik 2017: Behandlingshyppighed og Pesticidbelastning Baseret på salg og Forbrug; Miljøstyrelsen: København, Denmark, 2019; ISBN 9788770380539. [Google Scholar]
- Musil, B. Czech Republic-Usage of Active Substances in 2011. Available online: http://eagri.cz/public/web/file/439602/celek_2011_EN.pdf (accessed on 10 August 2021).
- Musil, B. Czech Republic-Usage of Active Substances in 2012. Available online: http://eagri.cz/public/web/file/439598/celek_2012_EN.pdf (accessed on 10 August 2021).
- Musil, B. Czech Republic-Usage of Active Substances in 2013. Available online: http://eagri.cz/public/web/file/439536/celek_2013_EN.pdf (accessed on 10 August 2021).
- Musil, B. Czech Republic-Usage of Active Substances in 2014. Available online: http://eagri.cz/public/web/file/439456/celek_2014_EN.pdf (accessed on 10 August 2021).
- Musil, B. Czech Republic-Usage of Active Substances in 2015. Available online: http://eagri.cz/public/web/file/477476/Spotreba_UL_2015_EN_CELEK.pdf (accessed on 10 August 2021).
- Musil, B. Czech Republic-Usage of Active Substances in 2016. Available online: http://eagri.cz/public/web/file/537723/celek_2016_EN.pdf (accessed on 10 August 2021).
- Musil, B. Czech Republic-Usage of Active Substances in 2017. Available online: http://eagri.cz/public/web/file/587988/celek_2017_EN.pdf (accessed on 10 August 2021).
- Commissariat général au développement durable. Plan de réduction des produits phytopharmaceutiques et sortie du glyphosate: État des lieux des ventes et des achats en France; DATALAB Essentiel: Paris, France, 2019. [Google Scholar]
- Direção Geral de Alimentação e Veterinára. Vendas de Produtos Fitofarmacêuticos em Portugal em 2011. Lisboa. 2012. Available online: http://dgav.pt (accessed on 8 September 2021).
- Direção Geral de Alimentação e Veterinára. Vendas de Produtos Fitofarmacêuticos em Portugal em 2012. Lisboa. 2013. Available online: http://dgav.pt (accessed on 8 September 2021).
- Direção Geral de Alimentação e Veterinára. Vendas de Produtos Fitofarmacêuticos em Portugal em 2013. Lisboa. 2015. Available online: http://dgav.pt (accessed on 8 September 2021).
- Direção Geral de Alimentação e Veterinára. Vendas de Produtos Fitofarmacêuticos em Portugal em 2014. Lisboa. 2016. Available online: http://dgav.pt (accessed on 8 September 2021).
- Direção Geral de Alimentação e Veterinára. Vendas de Produtos Fitofarmacêuticos em Portugal em 2015. Available online: https://www.dgav.pt/wp-content/uploads/2021/05/Vendas-de-Produtos-Fitofarmaceuticos-Portugal-2015.pdf (accessed on 8 September 2021).
- Direção Geral de Alimentação e Veterinára. Vendas de Produtos Fitofarmacêuticos em Portugal em 2016. Available online: https://www.dgav.pt/wp-content/uploads/2021/05/Relatorio-Vendas-2016_PF.pdf (accessed on 8 September 2021).
- Direção Geral de Alimentação e Veterinára. Vendas de Produtos Fitofarmacêuticos em Portugal em 2017. Available online: https://www.agroportal.pt/wp-content/uploads/vendas-2017.pdf (accessed on 8 September 2021).
- Chiarello, M.; Jiménez-Medina, M.L.; Marín Saéz, J.; Moura, S.; Garrido Frenich, A.; Romero-González, R. Fast analysis of glufosinate, glyphosate and its main metabolite, aminomethylphosphonic acid, in edible oils, by liquid chromatographycoupled with electrospray tandem mass spectrometry. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 1376–1384. [Google Scholar] [CrossRef]
- Autoridade de Segurança Alimentar. e Económica Sabe o que é o Glifosato. Available online: https://asae.gov.pt/ficheiros-externos-2016/sabe-o-que-e-o-glifosato-maio-aspx. (accessed on 30 July 2021).
- Forage Information System. Distinguish between Selective and Non-Selective Herbicides and Give an Example of Each. Available online: https://forages.oregonstate.edu/nfgc/eo/onlineforagecurriculum/instructormaterials/availabletopcis/weeds/herbicides. (accessed on 15 September 2021).
- FAO/WHO. Pesticide Residues in Food 2016; FAO/WHO: Rome, Italy, 2016. [Google Scholar]
- Zoller, O.; Rhyn, P.; Rupp, H.; Zarn, J.A.; Geiser, C. Glyphosate residues in Swiss market foods: Monitoring and risk evaluation. Food Addit. Contam. Part B Surveill. 2018, 11, 83–91. [Google Scholar] [CrossRef]
- Van Eenennaam, A.L.; Young, A.E. Detection of dietary DNA, protein, and glyphosate in meat, milk, and eggs. J. Anim. Sci. 2017, 95, 3247–3269. [Google Scholar] [CrossRef]
- European Chemicals Agency. Proposing Harmonised Classification and Labelling at EU Level of Glyphosate. Available online: https://echa.europa.eu/documents/10162/2f8b5c7f-030f-5d3a-e87e-0262fb392f38 (accessed on 10 September 2021).
- Gaur, H.; Bhargava, A. Glyphosate induces toxicity and modulates calcium and NO signaling in zebrafish embryos. Biochem. Biophys. Res. Commun. 2019, 513, 1070–1075. [Google Scholar] [CrossRef]
- Lanzarin, G.A.B.; Félix, L.M.; Santos, D.; Venâncio, C.A.S.; Monteiro, S.M. Dose-dependent effects of a glyphosate commercial formulation–Roundup® UltraMax-on the early zebrafish embryogenesis. Chemosphere 2019, 223, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, J.; Song, J.; Feng, Y.; Zhang, S.; Wang, N.; Liu, S.; Song, Z.; Lian, K.; Kang, W. Effects of low-concentration glyphosate and aminomethyl phosphonic acid on zebrafish embryo development. Ecotoxicol. Environ. Saf. 2021, 226, 112854. [Google Scholar] [CrossRef]
- Food Safety Comission of Japan Glyphosate. Glyphosate: Summary. Food Saf. 2016, 4, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Rueda-Ruzafa, L.; Cruz, F.; Roman, P.; Cardona, D. Gut microbiota and neurological effects of glyphosate. Neurotoxicology 2019, 75, 1–8. [Google Scholar] [CrossRef]
- Gress, S.; Lemoine, S.; Séralini, G.-E.; Puddu, P.E. Glyphosate-Based Herbicides Potently Affect Cardiovascular System in Mammals: Review of the Literature. Cardiovasc. Toxicol. 2015, 15, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, R.; Maradey, J.A.; Dearmin, R.S.; Belford, P.M.; Bhave, P.D. Electrocardiographic abnormalities associated with acute glyphosate toxicity. Hear. Case Rep. 2020, 6, 63–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesnage, R.; Defarge, N.; Spiroux de Vendômois, J.; Séralini, G.E. Potential toxic effects of glyphosate and its commercial formulations below regulatory limits. Food Chem. Toxicol. 2015, 84, 133–153. [Google Scholar] [CrossRef] [Green Version]
- Mesnage, R.; Renney, G.; Séralini, G.E.; Ward, M.; Antoniou, M.N. Multiomics reveal non-alcoholic fatty liver disease in rats following chronic exposure to an ultra-low dose of Roundup herbicide. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Chen, J.; Ding, F.; Chou, X.; Zhang, X.; Wan, Y.; Hu, J.; Wu, Q. Activation of the N-methyl-d-aspartate receptor is involved in glyphosate-induced renal proximal tubule cell apoptosis. J. Appl. Toxicol. 2019, 39, 1096–1107. [Google Scholar] [CrossRef]
- Gunarathna, S.; Gunawardana, B.; Jayaweera, M.; Manatunge, J.; Zoysa, K. Glyphosate and AMPA of agricultural soil, surface water, groundwater and sediments in areas prevalent with chronic kidney disease of unknown etiology, Sri Lanka. J. Environ. Sci. Health Part B 2018, 53, 729–737. [Google Scholar] [CrossRef]
- Meftaul, I.M.; Venkateswarlu, K.; Dharmarajan, R.; Annamalai, P.; Asaduzzaman, M.; Parven, A.; Megharaj, M. Controversies over human health and ecological impacts of glyphosate: Is it to be banned in modern agriculture? Environ. Pollut. 2020, 263, 114372. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, Y.; Ni, H.; Gao, J.; Yang, Y.; Xu, W.; Tao, L. Evaluation of the cytotoxic effects of glyphosate herbicides in human liver, lung, and nerve. J. Environ. Sci. Health Part B 2019, 54, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Guyton, K.Z.; Loomis, D.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Scoccianti, C.; Mattock, H.; Straif, K. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol. 2015, 16, 490–491. [Google Scholar] [CrossRef]
- APVMA. Regulatory Position: Consideration of the Evidence for a Formal Reconsideration of Glyphosate; APVMA: Kingston Act, Australia, 2017; ISBN 978-1-925390-67-4. [Google Scholar]
- Gillezeau, C.; van Gerwen, M.; Shaffer, R.M.; Rana, I.; Zhang, L.; Sheppard, L.; Taioli, E. The evidence of human exposure to glyphosate: A review. Environ. Health 2019, 18, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agency for Toxic Substances and Disease Registry Toxicological Profile for Lead. ATSDR’s Toxicological Profiles; CRC Press: Atlanta, Georgia, 2002. [Google Scholar]
- Vainio, H. Public health and evidence-informed policy-making: The case of a commonly used herbicide. Scand. J. Work. Environ. Health 2020, 46, 105–109. [Google Scholar] [CrossRef]
- Zhang, L.; Rana, I.; Shaffer, R.M.; Taioli, E.; Sheppard, L. Exposure to glyphosate-based herbicides and risk for non-Hodgkin lymphoma: A meta-analysis and supporting evidence. Mutat. Res. Rev. Mutat. Res. 2019, 781, 186–206. [Google Scholar] [CrossRef] [PubMed]
- Dragani, T. Difficulties in establishing a causal link between chemical exposures and cancer cannot be overcome by court assessments. Hum. Exp. Toxicol. 2020, 39, 1095–1107. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Reszka, E.; Woźniak, K.; Jabłońska, E.; Michałowicz, J.; Bukowska, B. DNA damage and methylation induced by glyphosate in human peripheral blood mononuclear cells (in vitro study). Food Chem. Toxicol. 2017, 105, 93–98. [Google Scholar] [CrossRef]
- Antoniou, M.; Habib, M.E.M., Howard; Fagan, J. Teratogenic Effects of Glyphosate-Based Herbicides: Divergence of Regulatory Decisions from Scientific Evidence. J. Environ. Anal. Toxicol. 2012, 4, 1–13. [Google Scholar] [CrossRef] [Green Version]
- National Pesticide Information Center Glyphosate General Fact Sheet. Available online: http://npic.orst.edu/factsheets/glyphogen.html (accessed on 22 August 2021).
- The European Parliament. Renewal of the Approval of the Active Substance Glyphosate; Official Journal of the European Union: Brussels, Belgium, 2016. [Google Scholar]
- The European Commission. Commission Implementing Regulation (Eu) 2017/2324 of 12 December 2017 Renewing the Approval of the Active Substance Glyphosate in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant pr; Official Journal of the European Union: Brussels, Belgium, 2017. [Google Scholar]
- The European Commission. Commission Regulation (Eu) No 293/2013 of 20 March 2013 Amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Emamectin Benzoate, Etofenprox, Etoxazole, Flutriafol, g; Official Journal of the European Union: Brussels, Belgium, 2013. [Google Scholar]
- EFSA. Review of the Existing Maximum Residue Levels for Glyphosate According to Article 12 of Regulation (EC) No 396/2005–Revised Version to Take into Account Omitted Data. EFSA J. 2019, 17, 5862. [Google Scholar]
- European Comission EU. Pesticides Database-Pesticides Residues and Maximum Residue Levels. Available online: https://eurlex.europa.eu/legal.content/EN/ALL/?uri=CELEZ%3A32013R0293 (accessed on 25 August 2021).
- Melo, K.G.; Nucci, D.G.; Trape, A.Z. Brief review analytical methods for the determination of glyphosate. MOJ Toxicol. 2018, 4, 86–89. [Google Scholar] [CrossRef]
- Karise, R.; Raimets, R.; Bartkevics, V.; Pugajeva, I.; Pihlik, P.; Keres, I.; Williams, I.H.; Viinalass, H.; Mänd, M. Are pesticide residues in honey related to oilseed rape treatments? Chemosphere 2017, 188, 389–396. [Google Scholar] [CrossRef]
- Ciasca, B.; Pecorelli, I.; Lepore, L.; Paoloni, A.; Catucci, L.; Pascale, M.; Lattanzio, V.M.T. Rapid and reliable detection of glyphosate in pome fruits, berries, pulses and cereals by flow injection–Mass spectrometry. Food Chem. 2020, 310, 125813. [Google Scholar] [CrossRef] [PubMed]
- Melton, L.M.; Taylor, M.J.; Flynn, E.E. The utilisation of ion chromatography and tandem mass spectrometry (IC-MS/MS) for the multi-residue simultaneous determination of highly polar anionic pesticides in fruit and vegetables. Food Chem. 2019, 298, 125028. [Google Scholar] [CrossRef]
- Savini, S.; Bandini, M.; Sannino, A. An Improved, Rapid, and Sensitive Ultra-High-Performance Liquid Chromatography-High-Resolution Orbitrap Mass Spectrometry Analysis for the Determination of Highly Polar Pesticides and Contaminants in Processed Fruits and Vegetables. J. Agric. Food Chem. 2019, 67, 2716–2722. [Google Scholar] [CrossRef] [PubMed]
- Chamkasem, N. Determination of Glyphosate, Maleic Hydrazide, Fosetyl Aluminum, and Ethephon in Grapes by Liquid Chromatography/Tandem Mass Spectrometry. J. Agric. Food Chem. 2017, 65, 7535–7541. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-X.; Cao, Z.-Y.; Jiang, Y.; Zhu, Z.-W. Direct determination of glyphosate and its major metabolite, aminomethylphosphonic acid, in fruits and vegetables by mixed-mode hydrophilic interaction/weak anion-exchange liquid chromatography coupled with electrospray tandem mass spectrometry. J. Chromatogr. A 2013, 1272, 90–99. [Google Scholar] [CrossRef]
- Hsu, C.C.; Whang, C.W. Microscale solid phase extraction of glyphosate and aminomethylphosphonic acid in water and guava fruit extract using alumina-coated iron oxide nanoparticles followed by capillary electrophoresis and electrochemiluminescence detection. J. Chromatogr. A 2009, 1216, 8575–8580. [Google Scholar] [CrossRef]
- Gotti, R.; Fiori, J.; Bosi, S.; Dinelli, G. Field-amplified sample injection and sweeping micellar electrokinetic chromatography in analysis of glyphosate and aminomethylphosphonic acid in wheat. J. Chromatogr. A 2019, 1601, 357–364. [Google Scholar] [CrossRef]
- Santilio, A.; Pompili, C.; Giambenedetti, A. Determination of glyphosate residue in maize and rice using a fast and easy method involving liquid chromatography–mass spectrometry (LC/MS/MS). J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2019, 54, 205–210. [Google Scholar] [CrossRef]
- Granby, K.; Johannesen, S.; Vahl, M. Analysis of glyphosate residues in cereals using liquid chromatography-mass spectrometry (LC-MS/MS). Food Addit. Contam. 2003, 20, 692–698. [Google Scholar] [CrossRef]
- Chamkasem, N.; Harmon, T. Direct determination of glyphosate, glufosinate, and AMPA in soybean and corn by liquid chromatography/tandem mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 4995–5004. [Google Scholar] [CrossRef]
- Jansons, M.; Pugajeva, I.; Bartkevičs, V. Occurrence of glyphosate in beer from the Latvian market. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 1767–1775. [Google Scholar] [CrossRef]
- Liao, Y.; Berthion, J.M.; Colet, I.; Merlo, M.; Nougadère, A.; Hu, R. Validation and application of analytical method for glyphosate and glufosinate in foods by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2018, 1549, 31–38. [Google Scholar] [CrossRef]
- Berg, C.J.; Peter King, H.; Delenstarr, G.; Kumar, R.; Rubio, F.; Glaze, T. Glyphosate residue concentrations in honey attributed through geospatial analysis to proximity of large-scale agriculture and transfer off-site by bees. PLoS ONE 2018, 13, e0198876. [Google Scholar] [CrossRef] [PubMed]
- EFSA. The 2017 European Union report on pesticide residues in food. EFSA J. 2019, 17, e05743. [Google Scholar] [CrossRef] [Green Version]
- Direção Geral de Alimentação e Veterinára. Controlo Nacional de Resíduos de Pesticidas em Produtos de Origem Vegetal no ano de 2017. Available online: https://www.dgav.pt/wp-content/uploads/2021/03/Controlo-residuos-2017.pdf (accessed on 24 June 2021).
- Gauch, R.; Leuenberger, U.; Müller, U. Bestimmung des Herbicids Glyphosat und dessen Hauptmetabolit Aminomethylphosphonsäure (AMPA) in Trinkwasser mit Hilfe der HPLC. Z. Lebensm. Unters. Forsch. 1989, 188, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Rendón-Von Osten, J.; Dzul-Caamal, R. Glyphosate residues in groundwater, drinking water and urine of subsistence farmers from intensive agriculture localities: A survey in Hopelchén, Campeche, Mexico. Int. J. Environ. Res. Public Health 2017, 14, 595. [Google Scholar] [CrossRef]
- Skark, C.; Zullei-Seibert, N.; Schöttler, U.; Schlett, C. The occurrence of glyphosate in surface water. Int. J. Environ. Anal. Chem. 1998, 70, 93–104. [Google Scholar] [CrossRef]
- Battaglin, W.A.; Meyer, M.T.; Kuivila, K.M.; Dietze, J.E. Glyphosate and its degradation product AMPA occur frequently and widely in U.S. soils, surface water, groundwater, and precipitation. J. Am. Water Resour. Assoc. 2014, 50, 275–290. [Google Scholar] [CrossRef]
- Horth, H.; Blackmore, K. Survey of Glyphosate and AMPA in Groundwaters and Surface Waters in Europe. Available online: http://www.egeis.org/cd-info/WRC-report-UC8073-02-December-2009-Glyphosate-monitoring-in-water.pdf (accessed on 7 August 2021).
- The Council of the European Union. Council Directive 98/83/EC of November 1998 on the Quality of Water Intended for Human Consumption. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31998L0083&from=EN (accessed on 6 August 2021).
| Active Substance | Glyphosate |
|---|---|
| Family | Organophosphorus compounds |
| Function | Herbicide |
| IUPAC name | N-(phosphonomethyl) glycine |
| CAS number | 1071-83-6 |
| Molecular formula | C3H8NO5P |
| Molecular weight | 169.1 g/mol |
| Solubility | In water: 10.5 g/L a 20 °C Insoluble in organic solvents |
| Melting point (°C) | 189 °C |
| Boiling point (°C) | Not defined (Glyphosate is decomposed during melting process) |
| Temperature of decomposition (°C) | 200 °C |
| Dissociation constant | pKa1 = 2.0; pKa2 = 2.6; pKa3 = 5.6; pKa4 = 10.6 |
| Log Kow | −0.40 |
| Herbicide | Quantity (in Tons) | Market Share (%) | |
|---|---|---|---|
| 1 | Glyphosate | 1 | 1 |
| 2 | Isoproturon | 12,073 | 14.3 |
| 3 | MCPA | 5293 | 6.3 |
| 4 | Pendimethaline | 3141 | 3.7 |
| 5 | 2,4-D | 1 | 1 |
| 6 | Trifluraline | 2899 | 3.4 |
| 7 | Acetochlor | 2332 | 2.8 |
| 8 | S-Metalachlor | 1 | 1 |
| 9 | Atrazine | 1885 | 2.2 |
| 10 | Metazachlor | 1740 | 2.1 |
| Matrix | Extraction Method | Analytical Method | LOD (μg/kg) | LOQ (μg/kg) | References |
|---|---|---|---|---|---|
|
Honey Fish Beef | Sonication with a mixture of acidified water at 1% and methanol (7:3) | IC-HRMS | nd | 43 51 65 | [6] |
| Honey | SPE followed by derivatization | HPLC-MS/MS | nd | 1 | [19] |
| Honey | Centrifugation with methanol | UHPLC-MS/MS | nd | 50 | [79] |
| Yam | Centrifugation followed by derivatization | HPLC-MS/MS | 40 | 120 | [13] |
|
Yam Grape Chickpea | SPE | FI-MS/MS | nd | 500 500 2000 | [80] |
| Fruits and Vegetables | Centrifugation with a mixture of water and methanol (1:1) | IC-MS/MS | 25 | nd | [81] |
|
Fruits juice Vegetables Fruit puree | Centrifugation with acidified methanol | UHPLC-MS/MS | nd | 3 | [82] |
| Grape | SPE | HPLC-MS/MS | 60 | 190 | [83] |
| Fruits and Vegetables | SPE | HPLC-MS/MS | 1.2 | 5 | [84] |
| Guava | SPE | CE-ECL | 10 | nd | [85] |
| Wheat | SPE followed by derivatization | FASI-MEKC | 30 | 100 | [86] |
|
Rice Corn | Centrifugation with a mixture of water and acidified methanol at 1% (1:1) | HPLC-MS/MS | 2 4 | 10 | [87] |
| Cereals | Ultrasonication with water | HPLC-MS/MS | 20 | nd | [88] |
|
Soy Corn | SPE | HPLC-MS/MS | 140 150 | 420 450 | [89] |
| Oil | Centrifugation with acidified water at 1% | HPLC-MS/MS | 3.3 | 10 | [43] |
| Beer | SPE | HPLC-MS/MS | 0.2 | 0.5 | [90] |
|
Several aliments Several beverages | SPE | HPLC-MS/MS | 0.3 0.2 | 1 0.5 | [47] |
| Several aliments | SPE followed by derivatization | HPLC-MS/MS | 1.7 | 5 | [91] |
| Country | Number of Samples | Detection Frequency (%) | Minimum (μg/kg) | Mean (μg/kg) | Maximum (μg/kg) | References |
|---|---|---|---|---|---|---|
| Canada | 200 | 98.5 | 1 | 4.9 | 49.8 | [19] |
| Switzerland | 16 | 93.8 | <1 | 4.6 | 15.9 | [47] |
| Estonia | 33 | 12.1 | 9 | 35 | 62 | [79] |
| USA | 85 | 28.2 | 15 | 92.4 | 342 | [92] |
| Several European Countries | 186 | 12.9 | nd | nd | nd | [93] |
| Country | Matrix | Number of Samples | Detection Frequency (%) | Minimum (μg/kg) | Mean (μg/kg) | Maximum (μg/kg) | References |
|---|---|---|---|---|---|---|---|
| Switzerland | Fruit juice | 11 | 100 | 0.5 | 1.9 | 3.5 | [47] |
| France | Fruit | 6 | 0 | <5 | <5 | <5 | [91] |
| China | Fruit | 15 | 6.7 | 20 | 20 | 20 | [84] |
| Several European Countries | Pear | 627 | 1.0 | nd | nd | nd | [93] |
| Orange | 625 | 0.8 | nd | nd | nd | ||
| Apple | 340 | 0.3 | nd | nd | nd | ||
| Strawberry | 308 | 0.3 | nd | nd | nd | ||
| Blackberry | 68 | 4.4 | nd | nd | nd | ||
| Lime | 58 | 5.2 | nd | nd | nd | ||
| Raisin | 48 | 2.1 | nd | nd | nd | ||
| Walnut | 14 | 7.1 | nd | nd | nd | ||
| Portugal | Orange | 11 | 0 | <100 | <100 | <100 | [94] |
| Pear | 13 | 0 | <100 | <100 | <100 |
| Country | Matrix | Number of Samples | Detection Frequency (%) | Minimum (μg/kg) | Mean (μg/kg) | Maximum (μg/kg) | References |
|---|---|---|---|---|---|---|---|
| Switzerland | Breakfast Cereals | 10 | 80 | <1 | 50.8 | 291 | [47] |
| Wheat | 18 | 88.9 | <1 | 134.9 | 421 | ||
| Snacks | 11 | 36.4 | <1 | 3.7 | 17.9 | ||
| Bread | 10 | 70 | <1 | 6.9 | 45.8 | ||
| Wheat Flower | 28 | 28.6 | <1 | 10.6 | 133 | ||
| Pseudo cereals | 3 | 0 | <1 | <1 | <1 | ||
| Other cereals | 13 | 15.4 | <1 | 1.2 | 12.4 | ||
| Italy | Wheat flower | 4 | 0 | <30 | <30 | <30 | [86] |
| Wheat seeds | 1 | 100 | 243,000 | 243,000 | 243,000 | ||
| France | Breakfast Cereals | 2 | 100 | 6 | 20 | 34 | [91] |
| Several European Countries | Wheat | 676 | 9.0 | nd | nd | nd | [93] |
| Rye | 534 | 3.4 | nd | nd | nd | ||
| Rice | 266 | 0.4 | nd | nd | nd | ||
| Oat | 61 | 4.9 | nd | nd | nd | ||
| Barley | 51 | 23.5 | nd | nd | nd | ||
| Linseeds | 48 | 16.7 | nd | nd | nd | ||
| Pseudo cereals | 45 | 8.9 | nd | nd | nd |
| Country | Matrix | Number of Samples | Detection Frequency (%) | Minimum (μg/kg) | Mean (μg/kg) | Maximum (μg/kg) | References |
|---|---|---|---|---|---|---|---|
| Ghana | Yam | 68 | 20.5 | <120 | <120 | <120 | [13] |
| Switzerland | Potato and vegetables | 10 | 30 | <1 | 1.3 | 7.7 | [47] |
| France | Vegetables | 14 | 0 | <5 | <5 | <5 | [91] |
| Italy | Vegetables | 83 | 18.1 | 3 | nd | 300 | [82] |
| China | Vegetables | 35 | 0 | <5 | <5 | <5 | [84] |
| Several European Countries | Asparagus | 319 | 0.9 | nd | nd | nd | [93] |
| Pepper | 215 | 0.5 | nd | nd | nd | ||
| Peas | 20 | 25 | nd | nd | nd | ||
| Switzerland | Pulses | 41 | 51.2 | <1 | 173.3 | 2948 | [47] |
| Several European Countries | Dried Lentils | 79 | 41.8 | nd | nd | nd | [93] |
| Country | Matrix | Number of Samples | Detection Frequency (%) | Minimum (μg/kg) | Mean (μg/kg) | Maximum (μg/kg) | References |
|---|---|---|---|---|---|---|---|
| Switzerland | Milk | 3 | 0 | <0.5 | <0.5 | <0.5 | [47] |
| Egg | 1 | 0 | <1 | <1 | <1 | ||
| Meat and Fish | 13 | 23.1 | <1 | 0.8 | 4.9 |
| Country | Matrix | Number of Samples | Detection Frequency (%) | Minimum (μg/kg) | Mean (μg/kg) | Maximum (μg/kg) | References |
|---|---|---|---|---|---|---|---|
| Switzerland | Baby food | 11 | 0 | <1 | <1 | <1 | [47] |
| France | Baby food | 71 | 0 | <2 | <2 | <2 | [91] |
| Italy | Baby food | 15 | 13.3 | 3 | 3 | 3 | [82] |
| Country | Matrix | Number of Samples | Detection Frequency (%) | Minimum (μg/L) | Mean (μg/L) | Maximum (μg/L) | References |
|---|---|---|---|---|---|---|---|
| Switzerland | Surface water | 151 | 0 | <0.02 | <0.02 | <0.02 | [95] |
| Mexico | Groundwater | 29 | 89.7 | <0.05 | 0.94 | 1.70 | [96] |
| Bottled drinking water | 15 | 86.7 | <0.05 | 0.48 | 0.78 | ||
| Germany | Surface water | 39 | 59 | <0.025 | 0.12 | 0.59 | [97] |
| USA | Several types of water | 3732 | 39.4 | <0.02 | nd | 476 | [98] |
| Several European Countries | Surface water | 50,805 | 28.9 | <0.003 | nd | 50 | [99] |
| Groundwater | 36,298 | 1.3 | <0.01 | nd | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, D.; Silva, L.; Duarte, S.; Pena, A.; Pereira, A. Glyphosate Use, Toxicity and Occurrence in Food. Foods 2021, 10, 2785. https://doi.org/10.3390/foods10112785
Soares D, Silva L, Duarte S, Pena A, Pereira A. Glyphosate Use, Toxicity and Occurrence in Food. Foods. 2021; 10(11):2785. https://doi.org/10.3390/foods10112785
Chicago/Turabian StyleSoares, Diogo, Liliana Silva, Sofia Duarte, Angelina Pena, and André Pereira. 2021. "Glyphosate Use, Toxicity and Occurrence in Food" Foods 10, no. 11: 2785. https://doi.org/10.3390/foods10112785
APA StyleSoares, D., Silva, L., Duarte, S., Pena, A., & Pereira, A. (2021). Glyphosate Use, Toxicity and Occurrence in Food. Foods, 10(11), 2785. https://doi.org/10.3390/foods10112785








