Microbiological Quality Assessment of Chicken Thigh Fillets Using Spectroscopic Sensors and Multivariate Data Analysis
Abstract
:1. Introduction
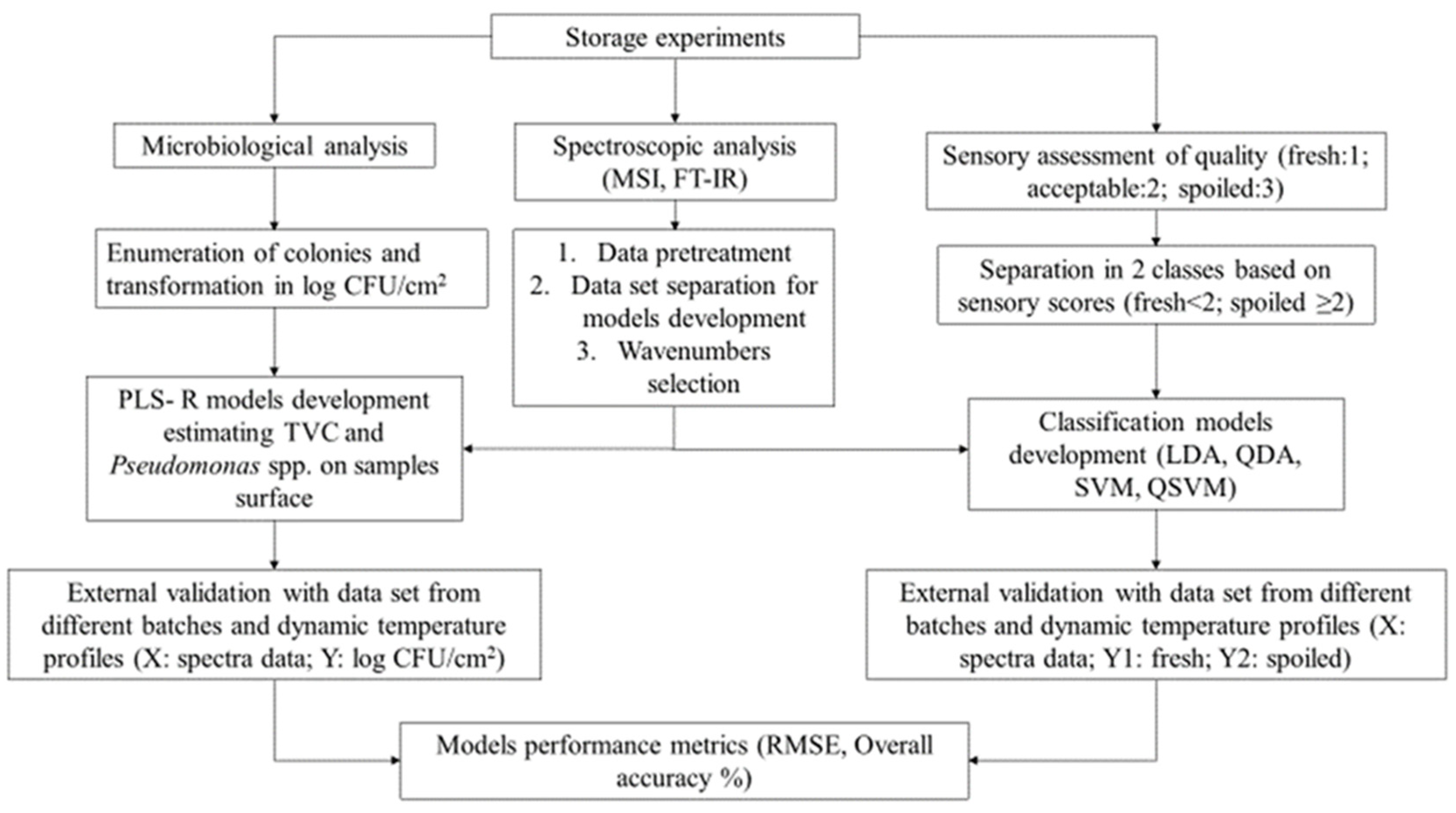
2. Materials and Methods
2.1. Experimental Design
2.2. Microbiological Analysis and Sensory Evaluation
2.3. Spectra Acquisition
2.4. Data Pre-Processing and Analysis
3. Results and Discussion
3.1. Microbiological Analysis and Sensory Evaluation
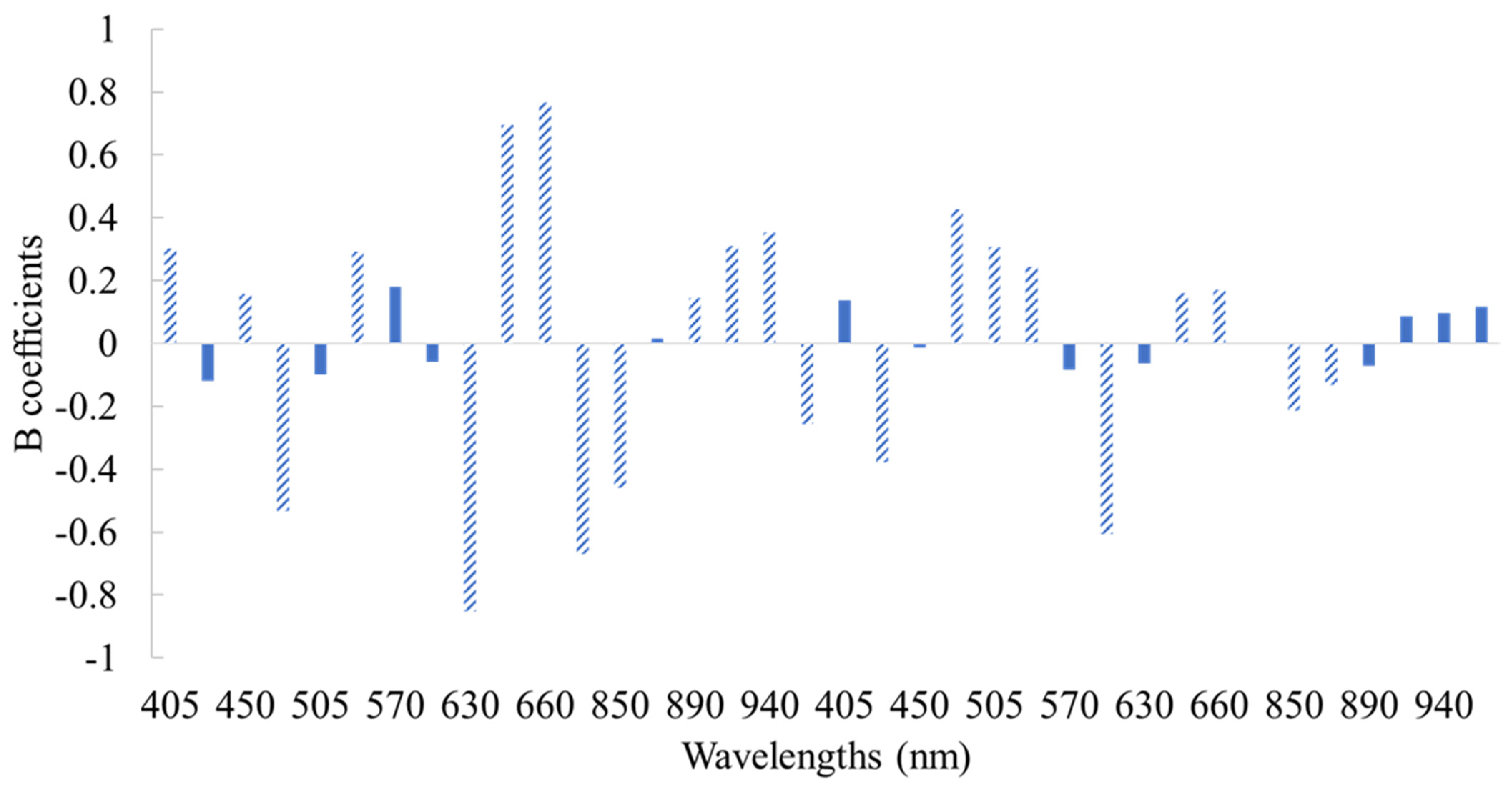
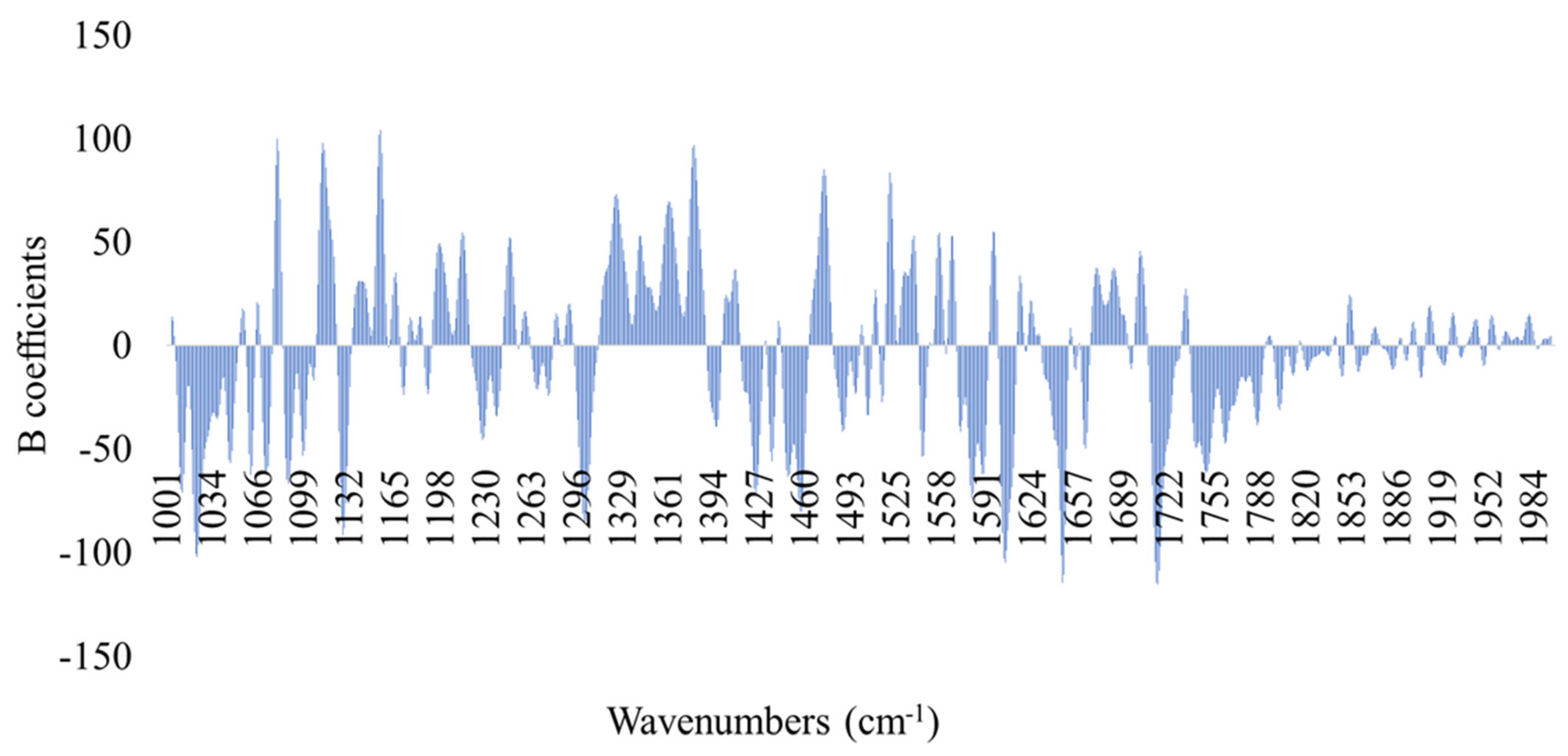
3.2. Correlation of Microbiological Data to Spectral Information
3.3. Classification Models for the Assessment of Spoilage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Technical Platform on the Measurement and Reduction of Food Loss and Waste. Available online: http://www.fao.org/platform-food-loss-waste/en/ (accessed on 4 October 2021).
- FAO. Gateway to Poultry Production and Products. Available online: http://www.fao.org/poultry-production-products/en/ (accessed on 4 October 2021).
- Tsakanikas, P.; Karnavas, A.; Panagou, E.Z.; Nychas, G.J. A machine learning workflow for raw food spectroscopic classification in a future industry. Sci. Rep. 2020, 10, 11212. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, F.; Lyndgaard, C.B.; Sørensen, K.M.; Engelsen, S.B. Process analytical technology in the food industry. Trends Food Sci. Technol. 2013, 31, 27–35. [Google Scholar] [CrossRef]
- Cullen, P.J.; O’Donnell, C.P.; Fagan, C.C. Process Analytical Technology for the Food Industry, 1st ed.; Springer: New York, NY, USA, 2014; pp. 1–5. [Google Scholar] [CrossRef]
- Nychas, G.J.E.; Panagou, E.Z.; Mohareb, F. Novel approaches for food safety management and communication. Curr. Opin. Food Sci. 2016, 12, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Panagou, E.Z.; Papadopoulou, O.; Carstensen, J.M.; Nychas, G.J.E. Potential of multispectral imaging technology for rapid and non-destructive determination of the microbiological quality of beef filets during aerobic storage. Int. J. Food Microbiol. 2014, 174, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Xie, A.; Sun, D.W.; Zeng, X.A.; Liu, D. Applications of hyperspectral imaging in chicken meat safety and quality detection and evaluation: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1287–1301. [Google Scholar] [CrossRef] [PubMed]
- Alamprese, C.; Amigo, J.M.; Casiraghi, E.; Engelsen, S.B. Identification and quantification of turkey meat adulteration in fresh, frozen-thawed and cooked minced beef by FT-NIR spectroscopy and chemometrics. Meat Sci. 2016, 121, 175–181. [Google Scholar] [CrossRef]
- Dissing, B.S.; Papadopoulou, O.S.; Tassou, C.; Ersbøll, B.K.; Carstensen, J.M.; Panagou, E.Z.; Nychas, G.J. Using multispectral imaging for spoilage detection of pork meat. Food Bioproc. Technol. 2013, 6, 2268–2279. [Google Scholar] [CrossRef]
- Pu, H.; Kamruzzaman, M.; Sun, D.W. Selection of feature wavelengthFigires for developing multispectral imaging systems for quality, safety and authenticity of muscle foods-a review. Trends Food Sci. Technol. 2015, 45, 86–104. [Google Scholar] [CrossRef]
- Kutsanedzie, F.Y.; Guo, Z.; Chen, Q. Advances in nondestructive methods for meat quality and safety monitoring. Food Rev. Int. 2019, 35, 536–562. [Google Scholar] [CrossRef]
- Feng, Y.Z.; Sun, D.W. Determination of total viable count (TVC) in chicken breast fillets by near-infrared hyperspectral imaging and spectroscopic transforms. Talanta 2013, 105, 244–249. [Google Scholar] [CrossRef]
- Feng, Y.Z.; Sun, D.W. Near-infrared hyperspectral imaging in tandem with partial least squares regression and genetic algorithm for non-destructive determination and visualization of Pseudomonas loads in chicken fillets. Talanta 2013, 109, 74–83. [Google Scholar] [CrossRef]
- Ye, X.; Iino, K.; Zhang, S. Monitoring of bacterial contamination on chicken meat surface using a novel narrowband spectral index derived from hyperspectral imagery data. Meat Sci. 2016, 122, 25–31. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Makino, Y.; Oshita, S. Rapid and non-destructive detection of chicken adulteration in minced beef using visible near-infrared hyperspectral imaging and machine learning. J. Food Eng. 2016, 170, 8–15. [Google Scholar] [CrossRef]
- Fengou, L.C.; Tsakanikas, P.; Nychas, G.J.E. Rapid detection of minced pork and chicken adulteration in fresh, stored and cooked ground meat. Food Control 2021, 125, 108002. [Google Scholar] [CrossRef]
- Yang, C.C.; Chao, K.; Chen, Y.R.; Early, H.L. Systemically diseased chicken identification using multispectral images and region of interest analysis. Comput. Electron. Agric. 2005, 49, 255–271. [Google Scholar] [CrossRef]
- Nakariyakul, S.; Casasent, D.P. Fast feature selection algorithm for poultry skin tumor detection in hyperspectral data. J. Food Eng. 2009, 94, 358–365. [Google Scholar] [CrossRef]
- Spyrelli, E.D.; Doulgeraki, A.I.; Argyri, A.A.; Tassou, C.C.; Panagou, E.Z.; Nychas, G.J.E. Implementation of Multispectral Imaging (MSI) for Microbiological Quality Assessment of Poultry Products. Microorganisms 2020, 8, 552. [Google Scholar] [CrossRef] [Green Version]
- Ellis, D.I.; Broadhurst, D.; Kell, D.B.; Rowland, J.J.; Goodacre, R. Rapid and quantitative detection of the microbial spoilage of meat by Fourier transform infrared spectroscopy and machine learning. Appl. Environ. Microbiol. 2002, 68, 2822–2828. [Google Scholar] [CrossRef] [Green Version]
- Alexandrakis, D.; Downey, G.; Scannell, A.G. Rapid non-destructive detection of spoilage of intact chicken breast muscle using near-infrared and Fourier transform mid-infrared spectroscopy and multivariate statistics. Food Bioproc. Technol. 2012, 5, 33–65. [Google Scholar] [CrossRef]
- Argyri, A.A.; Jarvis, R.M.; Wedge, D.; Xu, Y.; Panagou, E.Z.; Goodacre, R.; Nychas, G.J.E. A comparison of Raman and FT-IR spectroscopy for the prediction of meat spoilage. Food Control 2013, 29, 461–470. [Google Scholar] [CrossRef]
- Grewal, M.K.; Jaiswal, P.; Jha, S.N. Detection of poultry meat specific bacteria using FTIR spectroscopy and chemometrics. J. Food Sci. Technol. 2015, 52, 3859–3869. [Google Scholar] [CrossRef] [Green Version]
- Candoğan, K.; Altuntas, E.G.; İğci, N. Authentication and quality assessment of meat products by fourier-transform infrared (FTIR) spectroscopy. Food Eng. Rev. 2021, 13, 66–91. [Google Scholar] [CrossRef]
- Vasconcelos, H.; Saraiva, C.; de Almeida, J.M. Evaluation of the spoilage of raw chicken breast fillets using Fourier transform infrared spectroscopy in tandem with chemometrics. Food Bioproc. Technol. 2014, 7, 2330–2341. [Google Scholar] [CrossRef]
- Berrueta, L.A.; Alonso-Salces, R.M.; Héberger, K. Supervised pattern recognition in food analysis. J. Chromatogr. A 2007, 1158, 196–214. [Google Scholar] [CrossRef]
- Jiménez-Carvelo, A.M.; González-Casado, A.; Bagur-González, M.G.; Cuadros-Rodríguez, L. Alternative data mining/machine learning methods for the analytical evaluation of food quality and authenticity–A review. Food Res. Int. 2019, 122, 25–39. [Google Scholar] [CrossRef]
- Friedman, J.; Hastie, T.; Tibshirani, R. The Elements of Statistical Learning, 2nd ed.; Springer: New York, NY, USA, 2009; pp. 101–132. [Google Scholar] [CrossRef]
- Ropodi, A.I.; Pavlidis, D.E.; Mohareb, F.; Panagou, E.Z.; Nychas, G.J. Multispectral image analysis approach to detect adulteration of beef and pork in raw meats. Food Res. Int. 2015, 67, 12–18. [Google Scholar] [CrossRef]
- Kumar, Y.; Karne, S.C. Spectral analysis: A rapid tool for species detection in meat products. Trends Food Sci Technol. 2017, 62, 59–67. [Google Scholar] [CrossRef]
- Luts, J.; Ojeda, F.; Van de Plas, R.; De Moor, B.; Van Huffel, S.; Suykens, J.A. A tutorial on support vector machine-based methods for classification problems in chemometrics. Anal. Chim. Acta 2010, 665, 129–145. [Google Scholar] [CrossRef]
- Chen, Q.; Cai, J.; Wan, X.; Zhao, J. Application of linear/non-linear classification algorithms in discrimination of pork storage time using Fourier transform near infrared (FT-NIR) spectroscopy. LWT-Food Sci. Technol. 2011, 44, 2053–2058. [Google Scholar] [CrossRef]
- Ropodi, A.I.; Panagou, E.Z.; Nychas, G.J. Data mining derived from food analyses using non-invasive/non-destructive analytical techniques; determination of food authenticity, quality & safety in tandem with computer science disciplines. Trends Food Sci. Technol. 2016, 50, 11–25. [Google Scholar] [CrossRef]
- Estelles-Lopez, L.; Ropodi, A.; Pavlidis, D.; Fotopoulou, J.; Gkousari, C.; Peyrodie, A.; Panagou, E.Z.; Nychas, G.-J.E.; Mohareb, F. An automated ranking platform for machine learning regression models for meat spoilage prediction using multi-spectral imaging and metabolic profiling. Food Res. Int. 2017, 99, 206–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaafreh, S.; Valler, O.; Kreyenschmidt, J.; Günther, K.; Kaul, P. In vitro discrimination and classification of Microbial Flora of Poultry using two dispersive Raman spectrometers (microscope and Portable Fiber-Optic systems) in tandem with chemometric analysis. Talanta 2019, 202, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Fengou, L.C.; Mporas, I.; Spyrelli, E.; Lianou, A.; Nychas, G.J. Estimation of the Microbiological Quality of Meat using Rapid and Non-Invasive Spectroscopic Sensors. IEEE Access 2020, 8, 106614–106628. [Google Scholar] [CrossRef]
- Vaikousi, H.; Biliaderis, C.G.; Koutsoumanis, K.P. Applicability of a microbial Time Temperature Indicator (TTI) for monitoring spoilage of modified atmosphere packed minced meat. Int. J. Food Microbiol. 2009, 133, 272–278. [Google Scholar] [CrossRef]
- Hutchison, M.L.; Walters, L.D.; Avery, S.M.; Reid, C.A.; Wilson, D.; Howell, M.; Johnston, A.M.; Buncic, S.A. A comparison of wet-dry swabbing and excision sampling methods for microbiological testing of bovine, porcine, and ovine carcasses at red meat slaughterhouses. J. Food Prot. 2005, 68, 2155–2162. [Google Scholar] [CrossRef]
- Lytou, A.; Panagou, E.Z.; Nychas, G.J.E. Development of a predictive model for the growth kinetics of aerobic microbial population on pomegranate marinated chicken breast fillets under isothermal and dynamic temperature conditions. Food Microbiol. 2016, 55, 25–31. [Google Scholar] [CrossRef]
- Carstensen, J.M.; Hansen, J.F. An Apparatus and a Method of Recording an Image of an Object. Patent Family EP1051660 Issued in 2003. Available online: https://orbit.dtu.dk/en/publications/an-apparatus-and-a-method-of-recording-an-image-of-an-object (accessed on 4 October 2021).
- Tsakanikas, P.; Pavlidis, D.; Nychas, G.J. High throughput multispectral image processing with applications in food science. PLoS ONE 2015, 10, e0140122. [Google Scholar] [CrossRef]
- Rinnan, A.; Berg, F.; Engelsen, B. Review of the most common pre-processing techniques for near-infrared spectra. Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Engel, J.; Gerretzen, J.; Szymańska, E.; Jansen, J.J.; Downey, G.; Blanchet, L.; Buydens, L.M. Breaking with trends in pre- processing? Trends Anal. Chem. 2013, 50, 96–106. [Google Scholar] [CrossRef]
- Qin, J.; Chao, K.; Kim, M.S.; Lu, R.; Burks, T.F. Hyperspectral and multispectral imaging for evaluating food safety and quality. J. Food Eng. 2013, 118, 157–171. [Google Scholar] [CrossRef]
- Tsakanikas, P.; Pavlidis, D.; Panagou, E.; Nychas, G.J. Exploiting multispectral imaging for non-invasive contamination assessment and mapping of meat samples. Talanta 2016, 161, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Choi, H.H.; Moon, C.S.; Mun, C.W. Comparison of k-nearest neighbor, quadratic discriminant and linear discriminant analysis in classification of electromyogram signals based on the wrist-motion directions. Curr. Appl. Phys. 2011, 11, 740–745. [Google Scholar] [CrossRef]
- Osuna, E.; Freund, R.; Girosi, F. An improved training algorithm for support vector machines. In Neural Networks for Signal Processing VII. Proceedings of the 1997 IEEE Signal Processing Society Workshop; IEEE: Piscataway Township, NJ, USA, 1997; pp. 276–285. [Google Scholar] [CrossRef]
- Sokolova, M.; Lapalme, G. A systematic analysis of performance measures for classification tasks. Inf. Process. Manag. 2009, 45, 427–437. [Google Scholar] [CrossRef]
- Márquez, C.; López, M.I.; Ruisánchez, I.; Callao, M.P. FT-Raman and NIR spectroscopy data fusion strategy for multivariate qualitative analysis of food fraud. Talanta 2016, 161, 80–86. [Google Scholar] [CrossRef]
- Doulgeraki, A.I.; Ercolini, D.; Villani, F.; Nychas, G.J.E. Spoilage microbiota associated to the storage of raw meat in different conditions. Int. J. Food Microbiol. 2012, 157, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Rouger, A.; Tresse, O.; Zagorec, M. Bacterial contaminants of poultry meat: Sources, species, and dynamics. Microorganisms 2017, 5, 50. [Google Scholar] [CrossRef]
- Dominguez, S.A.; Schaffner, D.W. Development and validation of a mathematical model to describe the growth of Pseudomonas spp. in raw poultry stored under aerobic conditions. Int. J. Food Microbiol. 2007, 120, 287–295. [Google Scholar] [CrossRef]
- Galarz, L.A.; Fonseca, G.G.; Prentice, C. Predicting bacterial growth in raw, salted, and cooked chicken breast fillets during storage. Food Sci. Technol. Int. 2016, 22, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Gospavic, R.; Kreyenschmidt, J.; Bruckner, S.; Popov, V.; Haque, N. Mathematical modelling for predicting the growth of Pseudomonas spp. in poultry under variable temperature conditions. Int. J. Food Microbiol. 2008, 127, 290–297. [Google Scholar] [CrossRef]
- Raab, V.; Bruckner, S.; Beierle, E.; Kampmann, Y.; Petersen, B.; Kreyenschmidt, J. Generic model for the prediction of remaining shelf life in support of cold chain management in pork and poultry supply chains. Int. J. Netw. Sci 2008, 8, 59–73. [Google Scholar] [CrossRef]
- Wickramasinghe, N.N.; Hlaing, M.M.; Ravensdale, J.T.; Coorey, R.; Chandry, P.S.; Dykes, G.A. Characterization of the biofilm matrix composition of psychrotrophic, meat spoilage pseudomonads. Sci. Rep. 2020, 10, 16457. [Google Scholar] [CrossRef] [PubMed]
- Fengou, L.C.; Lianou, A.; Tsakanikas, P.; Mohareb, F.; Nychas, G.J.E. Detection of Meat Adulteration Using Spectroscopy-Based Sensors. Foods 2021, 10, 861. [Google Scholar] [CrossRef] [PubMed]

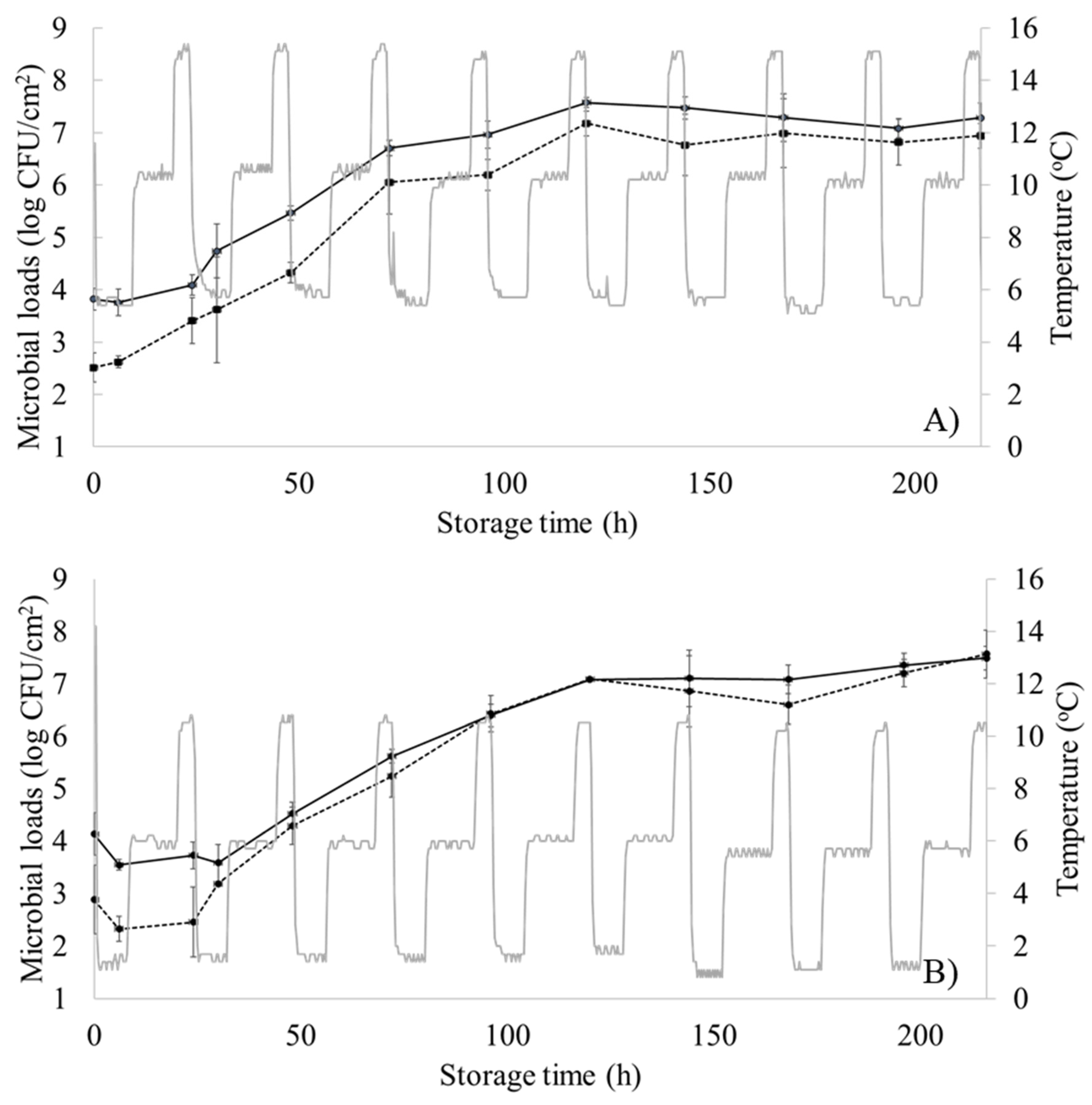



| Temperature (°C) | Storage Time (h) | Odor | TVCs (log CFU/cm2) |
|---|---|---|---|
| 0 | 240 | 2.5 | 6.99 |
| 5 | 96 | 2.3 | 7.08 |
| 10 | 48 | 2.3 | 6.90 |
| 15 | 24 | 2.1 | 7.46 |
| 20 | 24 | 2.5 | 7.40 |
| 25 | 24 | 2.9 | 8.22 |
| 30 | 6 | 2.1 | 5.1 |
| 35 | 12 | 2.2 | 6.84 |
| TVCs average (log CFU/cm2) | 6.99 |
| TVCs | n | LVs | Slope | Offset | r | RMSE |
|---|---|---|---|---|---|---|
| Calibration Full Cross Validation Prediction | 330 | 10 | 0.741 | 1.684 | 0.861 | 0.730 |
| 330 | 10 | 0.726 | 1.787 | 0.840 | 0.779 | |
| 72 | 0.774 | 2.023 | 0.895 | 0.987 | ||
| Pseudomonas spp. | n | LVs | slope | offset | r | RMSE |
| Calibration Full Cross Validation Prediction | 330 | 10 | 0.727 | 1.615 | 0.853 | 0.828 |
| 330 | 10 | 0.711 | 1.714 | 0.830 | 0.886 | |
| 72 | 0.702 | 2.441 | 0.904 | 1.215 |
| TVCs | n | LVs | Slope | Offset | r | RMSE |
|---|---|---|---|---|---|---|
| Calibration Full Cross Validation Prediction | 328 | 10 | 0.732 | 1.747 | 0.856 | 0.734 |
| 328 | 10 | 0.678 | 2.115 | 0.781 | 0.899 | |
| 63 | 0.367 | 4.192 | 0.583 | 1.251 | ||
| Pseudomonas spp. | n | LVs | slope | offset | r | RMSE |
| Calibration Full Cross Validation Prediction | 328 | 10 | 0.719 | 1.669 | 0.849 | 0.838 |
| 328 | 10 | 0.660 | 2.033 | 0.762 | 1.037 | |
| 63 | 0.282 | 4.152 | 0.514 | 1.589 |
| Model | Procedure | O/P | Fresh | Spoiled | Overall | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|---|
| LDA | FCV | Fresh | 98 | 67 | 330 | 59.4 | 73.3 |
| Spoiled | 44 | 121 | 73.3 | 59.4 | |||
| Overall accuracy (%) | 66.4 | ||||||
| Prediction | Fresh | 19 | 6 | 72 | 76.0 | 63.8 | |
| Spoiled | 17 | 30 | 63.8 | 76.0 | |||
| Overall accuracy (%) | 68.1 | ||||||
| QDA | Procedure | O/P | Fresh | Spoiled | Overall | Sensitivity (%) | Specificity (%) |
| FCV | Fresh | 99 | 73 | 330 | 57.6 | 72.8 | |
| Spoiled | 43 | 115 | 72.8 | 57.6 | |||
| Overall accuracy (%) | 64.8 | ||||||
| Prediction | Fresh | 22 | 8 | 72 | 73.3 | 66.7 | |
| Spoiled | 14 | 28 | 66.7 | 73.3 | |||
| Overall accuracy (%) | 69.4 | ||||||
| SVM | Procedure | O/P | Fresh | Spoiled | Overall | Sensitivity (%) | Specificity (%) |
| FCV | Fresh | 130 | 17 | 330 | 88.4 | 93.4 | |
| Spoiled | 12 | 171 | 93.4 | 88.4 | |||
| Overall accuracy (%) | 91.2 | ||||||
| Prediction | Fresh | 34 | 2 | 72 | 94.4 | 94.4 | |
| Spoiled | 2 | 34 | 94.4 | 94.4 | |||
| Overall accuracy (%) | 94.4 | ||||||
| QSVM | Procedure | O/P | Fresh | Spoiled | Overall | Sensitivity (%) | Specificity (%) |
| FCV | Fresh | 123 | 24 | 330 | 83.7 | 89.6 | |
| Spoiled | 19 | 164 | 89.6 | 83.7 | |||
| Overall accuracy (%) | 87.0 | ||||||
| Prediction | Fresh | 32 | 2 | 72 | 94.1 | 89.5 | |
| Spoiled | 4 | 34 | 89.5 | 94.1 | |||
| Overall accuracy (%) | 91.7 | ||||||
| Model | Procedure | O/P | Fresh | Spoiled | Overall | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|---|
| LDA | FCV | Fresh | 118 | 66 | 328 | 64.1 | 84.7 |
| Spoiled | 22 | 122 | 84.7 | 64.1 | |||
| Overall accuracy (%) | 73.2 | ||||||
| Prediction | Fresh | 19 | 8 | 63 | 70.4 | 69.4 | |
| Spoiled | 11 | 25 | 69.4 | 70.4 | |||
| Overall accuracy (%) | 69.8 | ||||||
| QDA | Procedure | O/P | Fresh | Spoiled | Overall | Sensitivity (%) | Specificity (%) |
| FCV | Fresh | 118 | 87 | 328 | 57.6 | 79.7 | |
| Spoiled | 25 | 98 | 79.7 | 57.6 | |||
| Overall accuracy (%) | 65.9 | ||||||
| Prediction | Fresh | 21 | 9 | 63 | 70.0 | 72.7 | |
| Spoiled | 9 | 24 | 72.7 | 70 | |||
| Overall accuracy (%) | 71.4 | ||||||
| SVM | Procedure | O/P | Fresh | Spoiled | Overall | Sensitivity (%) | Specificity (%) |
| FCV | Fresh | 127 | 13 | 328 | 90.7 | 85.1 | |
| Spoiled | 28 | 160 | 85.1 | 90.7 | |||
| Overall accuracy (%) | 87.5 | ||||||
| Prediction | Fresh | 26 | 15 | 63 | 63.4 | 81.8 | |
| Spoiled | 4 | 18 | 81.8 | 63.4 | |||
| Overall accuracy (%) | 69.8 | ||||||
| QSVM | Procedure | O/P | Fresh | Spoiled | Overall | Sensitivity (%) | Specificity (%) |
| FCV | Fresh | 122 | 26 | 328 | 82.4 | 90.0 | |
| Spoiled | 18 | 162 | 90 | 82.4 | |||
| Overall accuracy (%) | 86.6 | ||||||
| Prediction | Fresh | 24 | 19 | 63 | 55.8 | 70.0 | |
| Spoiled | 6 | 14 | 70.0 | 55.8 | |||
| Overall accuracy (%) | 60.3 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spyrelli, E.D.; Papachristou, C.K.; Nychas, G.-J.E.; Panagou, E.Z. Microbiological Quality Assessment of Chicken Thigh Fillets Using Spectroscopic Sensors and Multivariate Data Analysis. Foods 2021, 10, 2723. https://doi.org/10.3390/foods10112723
Spyrelli ED, Papachristou CK, Nychas G-JE, Panagou EZ. Microbiological Quality Assessment of Chicken Thigh Fillets Using Spectroscopic Sensors and Multivariate Data Analysis. Foods. 2021; 10(11):2723. https://doi.org/10.3390/foods10112723
Chicago/Turabian StyleSpyrelli, Evgenia D., Christina K. Papachristou, George-John E. Nychas, and Efstathios Z. Panagou. 2021. "Microbiological Quality Assessment of Chicken Thigh Fillets Using Spectroscopic Sensors and Multivariate Data Analysis" Foods 10, no. 11: 2723. https://doi.org/10.3390/foods10112723
APA StyleSpyrelli, E. D., Papachristou, C. K., Nychas, G.-J. E., & Panagou, E. Z. (2021). Microbiological Quality Assessment of Chicken Thigh Fillets Using Spectroscopic Sensors and Multivariate Data Analysis. Foods, 10(11), 2723. https://doi.org/10.3390/foods10112723







