Implication of Row Orientation Changes on Fruit Parameters of Vitis vinifera L. cv. Riesling in Steep Slope Vineyards
Abstract
:1. Introduction
2. Materials and Methods
2.1. Berry Sampling and Processing
2.1.1. Berry Juice Analyses
2.1.2. Berry Skin Polyphenol Analysis
2.2. Canopy Density Measurements
2.3. Cluster Light Interception
2.4. Leaf Nutritional Status
2.5. Scoring of Sunburn Damage
2.6. Statistical Analyses
3. Results
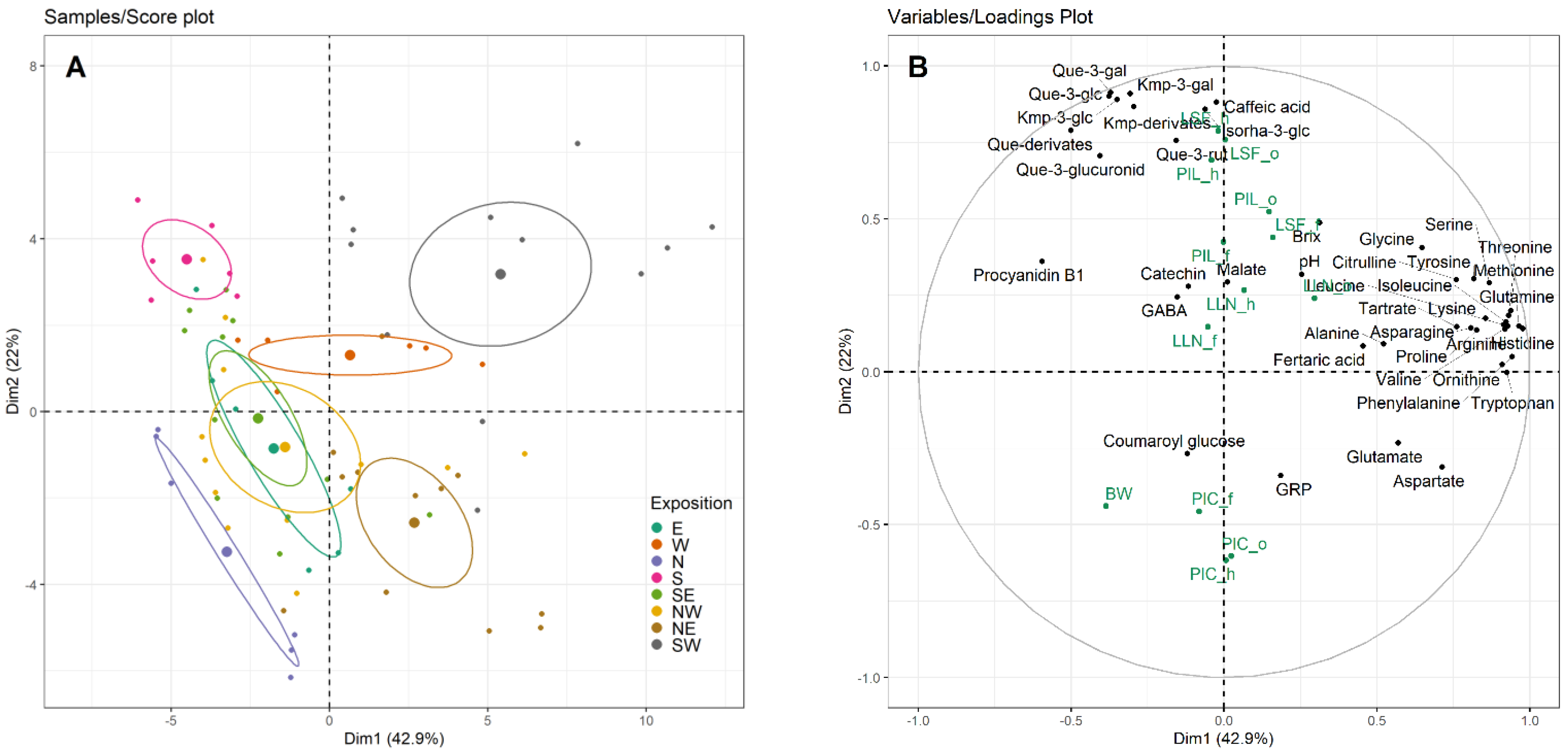
3.1. Berry Composition
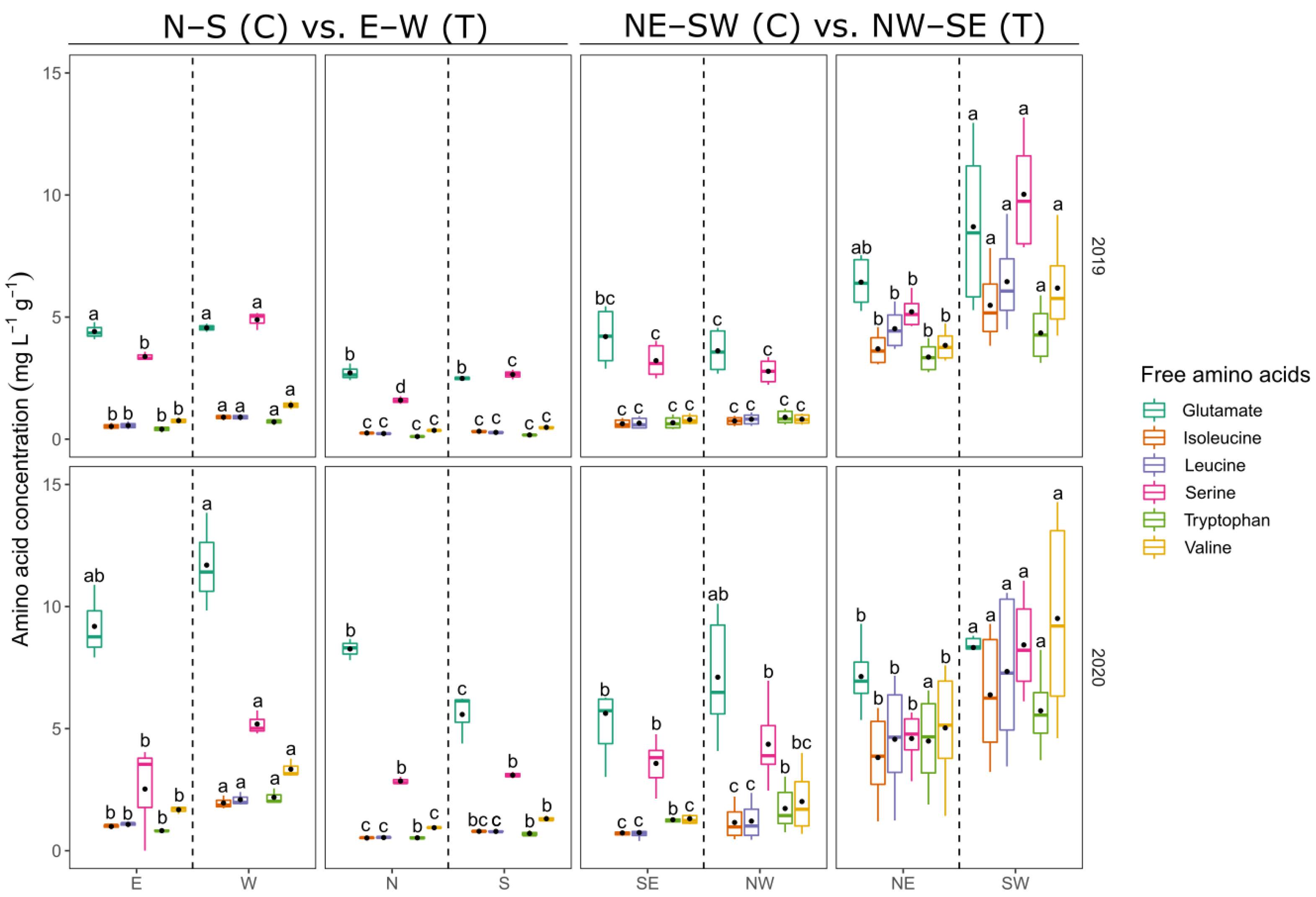
3.1.1. Berry Juice Composition
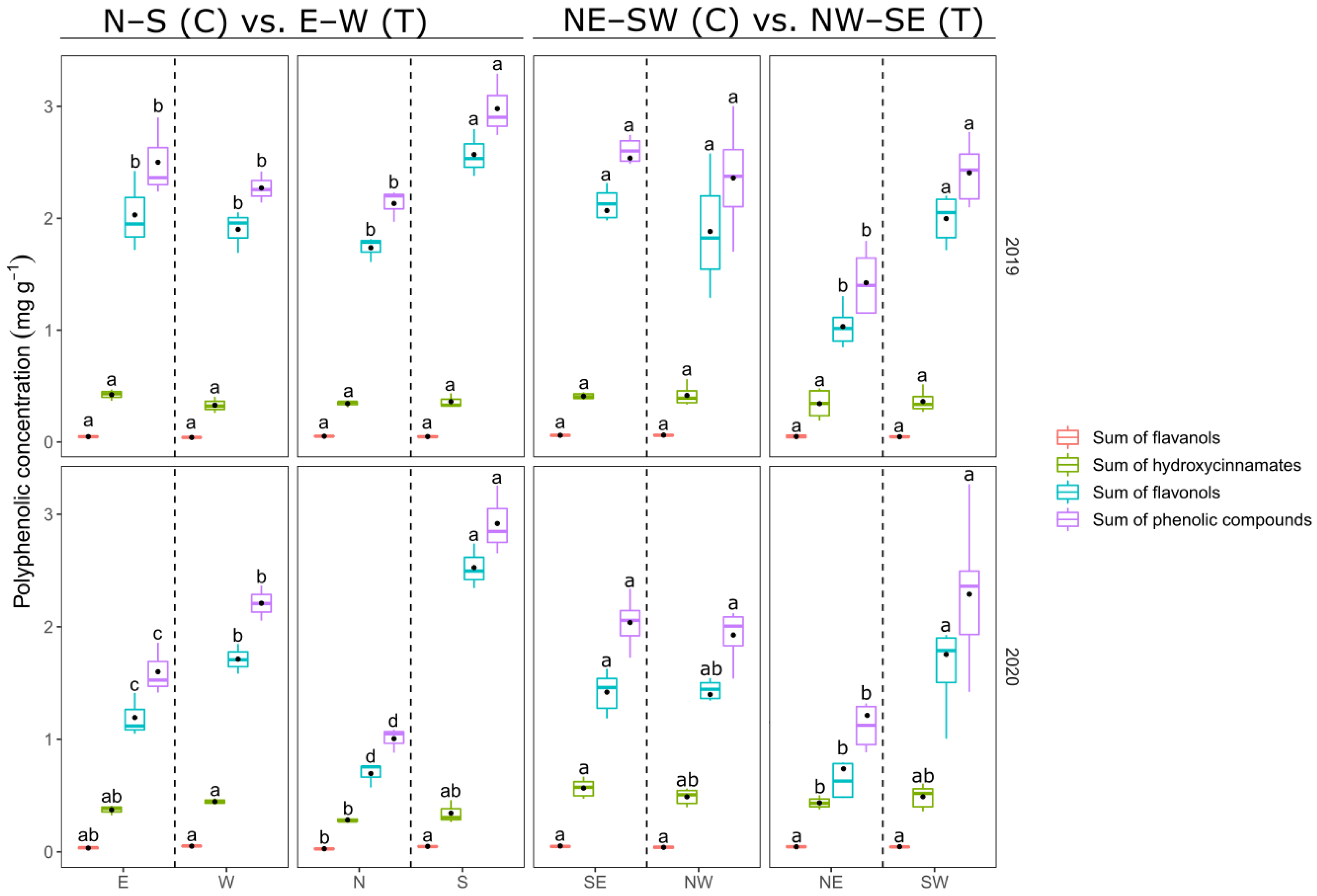
3.1.2. Berry Skin Polyphenol Content
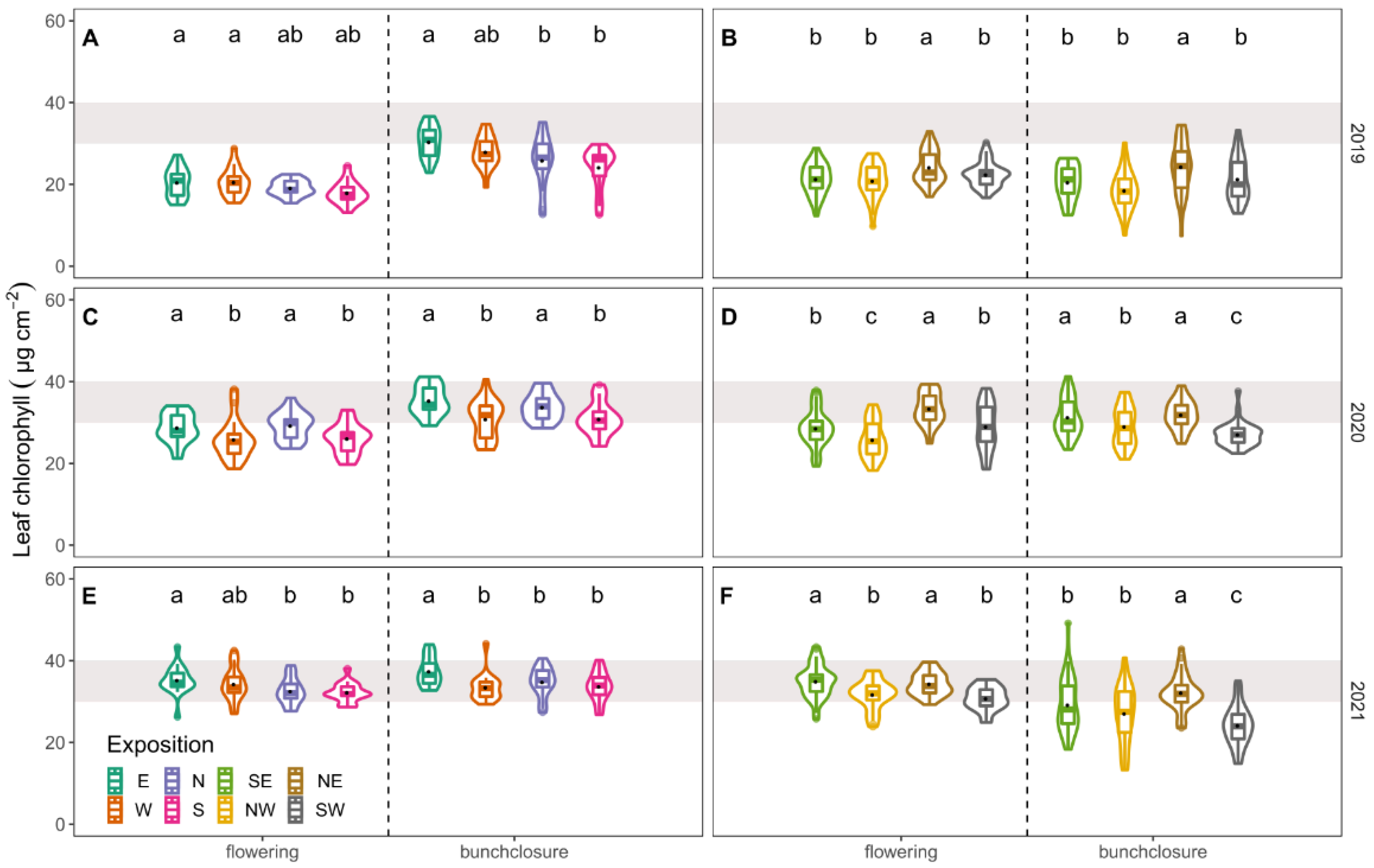
3.2. Leaf Nitrogen Content
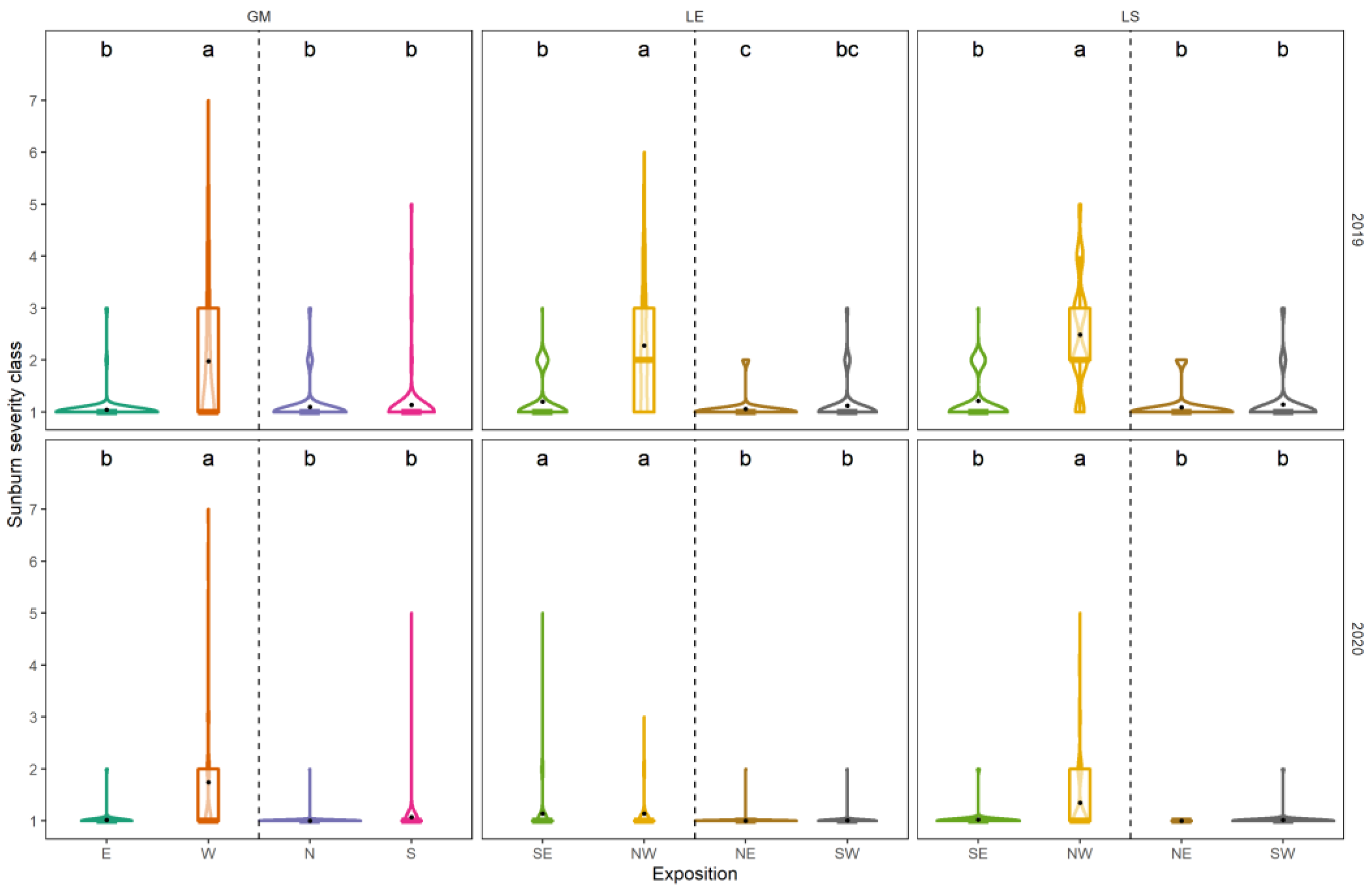
3.3. Sunburn Damage
4. Discussion
4.1. Berry Parameters
4.1.1. Berry Juice Parameters
4.1.2. Berry Skin Parameters
4.2. Leaf Nitrogen
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gambetta, J.M.; Holzapfel, B.P.; Stoll, M.; Friedel, M. Sunburn in Grapes: A Review. Front. Plant Sci. 2020, 11, 604691. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, Y.-Y. Grape phytochemicals and associated health benefits. Crit. Rev. Food Sci. Nutr. 2013, 53, 1202–1225. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.-T.; Correia, C.; Moriondo, M.; Leolini, L.; Dibari, C.; Costafreda-Aumedes, S.; et al. A Review of the Potential Climate Change Impacts and Adaptation Options for European Viticulture. Appl. Sci. 2020, 10, 3092. [Google Scholar] [CrossRef]
- OIV. Focus OIV 2017: Distribution of the World’s Grapevine Varieties; OIV: Paris, France, 2017. [Google Scholar]
- Friedel, M.; Frotscher, J.; Nitsch, M.; Hofmann, M.; Bogs, J.; Stoll, M.; Dietrich, H. Light promotes expression of monoterpene and flavonol metabolic genes and enhances flavour of winegrape berries (Vitis vinifera L. cv. Riesling). Aust. J. Grape Wine Res. 2016, 22, 409–421. [Google Scholar] [CrossRef]
- Brandt, M.; Scheidweiler, M.; Rauhut, D.; Patz, C.-D.; Will, F.; Zorn, H.; Stoll, M. The influence of temperature and solar radiation on phenols in berry skin and maturity parameters of Vitis vinifera L. cv. Riesling. OENO One 2019, 53, 2. [Google Scholar] [CrossRef]
- Friedel, M.; Stoll, M.; Patz, C.D.; Will, F.; Dietrich, H. Impact of light exposure on fruit composition of white ‘Riesling’ grape berries (Vitis vinifera L.). Vitis 2015, 54, 107–116. [Google Scholar] [CrossRef]
- Schultz, H.R.; Löhnertz, O.; Bettner, W.; Balo, B.; Linsenmeier, A.; Jähnisch, A.; Müller, M.; Gaubatz, B.; Varadi, G. Is grape composition affected by current levels of UV-B radiation. Vitis 1998, 37, 191–192. [Google Scholar]
- Keller, M.; Torres-Martinez, N. Does UV radiation affect winegrape composition? Acta Hortic. 2004, 313–319. [Google Scholar] [CrossRef]
- Gregan, S.M.; Wargent, J.J.; Liu, L.; Shinkle, J.; Hofmann, R.; Winfield, C.; Trought, M.; Jordan, B. Effects of solar ultraviolet radiation and canopy manipulation on the biochemical composition of Sauvignon Blanc grapes. Aust. J. Grape Wine Res. 2012, 18, 227–238. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Torres, N.; Hilbert, G.; Richard, T.; Sánchez-Díaz, M.; Delrot, S.; Aguirreolea, J.; Pascual, I.; Gomès, E. Ultraviolet-B radiation modifies the quantitative and qualitative profile of flavonoids and amino acids in grape berries. Phytochemistry 2014, 102, 106–114. [Google Scholar] [CrossRef]
- Wang, L.; Harada, J.; Endo, Y.; Hisamoto, M.; Saito, F.; Okuda, T. Diurnal Changes in Amino Acid Concentrations in Riesling and Chardonnay Grape Juices and a Possible Role of Sunlight. Am. J. Enol. Vitic. 2014, 65, 435–442. [Google Scholar] [CrossRef]
- Stines, A.P.; Grubb, J.; Gockowiak, H.; Henschke, P.A.; Høj, P.B.; Heeswijck, R. Proline and arginine accumulation in developing berries of Vitis vinifera L. in Australian vineyards: Influence of vine cultivar, berry maturity and tissue type. Aust. J. Grape Wine Res. 2000, 6, 150–158. [Google Scholar] [CrossRef]
- Guan, L.; Wu, B.; Hilbert, G.; Li, S.; Gomès, E.; Delrot, S.; Dai, Z. Cluster shading modifies amino acids in grape (Vitis vinifera L.) berries in a genotype- and tissue-dependent manner. Food Res. Int. 2017, 98, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Spayd, S.E.; Andersen-Bagge, J. Free Amino Acid Composition of Grape Juice from 12 Vitis vinifera Cultivars in Washington. Am. J. Enol. Vitic. 1996, 47, 389. [Google Scholar]
- Alem, H.; Rigou, P.; Schneider, R.; Ojeda, H.; Torregrosa, L. Impact of agronomic practices on grape aroma composition: A review. J. Sci. Food Agric. 2019, 99, 975–985. [Google Scholar] [CrossRef]
- Marais, J.; van Wyk, C.J.; Rapp, A. Effect of Sunlight and Shade on N orisoprenoid Levels in Maturing Weisser Riesling and Chenin blanc Grapes and Weisser Riesling Wines. S. Afr. J. Enol. Vitic. 2017, 13, 23–32. [Google Scholar] [CrossRef]
- Meyers, J.M.; Sacks, G.L.; Vanden Heuvel, J.E. Glycosylated Aroma Compound Responses in ‘Riesling’ Wine Grapes to Cluster Exposure and Vine Yield. HortTechnology 2013, 23, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Gerdes, S.M.; Winterhalter, P.; Ebeler, S.E. Effect of Sunlight Exposure on Norisoprenoid Formation in White Riesling Grapes. In Food Flavor; Tamura, H., Ebeler, S.E., Kubota, K., Takeoka, G.R., Eds.; American Chemical Society: Washington, DC, USA, 2008; pp. 262–272. ISBN 9780841274112. [Google Scholar]
- Daniel, M.A.; Capone, D.L.; Sefton, M.A.; Elsey, G.M. Riesling acetal is a precursor to 1,1,6-trimethyl-1,2-dihydronaphthalene (TDN) in wine. Aust. J. Grape Wine Res. 2009, 15, 93–96. [Google Scholar] [CrossRef]
- Smart, R.E. Sunlight Interception by Vineyards. Am. J. Enol. Vitic. 1973, 24, 141. [Google Scholar]
- Hunter, J.J.; Volschenk, C.G.; Zorer, R. Vineyard row orientation of Vitis vinifera L. cv. Shiraz/101-14 Mgt: Climatic profiles and vine physiological status. Agric. For. Meteorol. 2016, 228–229, 104–119. [Google Scholar] [CrossRef]
- Strack, T.; Schmidt, D.; Stoll, M. Impact of steep slope management system and row orientation on canopy microclimate. Comparing terraces to downslope vineyards. Agric. For. Meteorol. 2021, 307, 108515. [Google Scholar] [CrossRef]
- Grifoni, D.; Carreras, G.; Zipoli, G.; Sabatini, F.; Dalla Marta, A.; Orlandini, S. Row orientation effect on UV-B, UV-A and PAR solar irradiation components in vineyards at Tuscany, Italy. Int. J. Biometeorol. 2008, 52, 755–763. [Google Scholar] [CrossRef]
- Hoppmann, D.; Schaller, K.; Stoll, M. Terroir: Wetter, Klima und Boden im Weinbau, 2nd ed.; Aktualisierte Auflage; Ulmer: Stuttgart, Germany, 2017; ISBN 978-3800103508. [Google Scholar]
- Jagoutz, H. Bewässerungsbedarf von Reben in der Vergangenheit und in der Zukunft. In Deutsches Weinbau-Jahrbuch 2006; Schruft, G., Ed.; Eugen Ulmer KG: Stuttgart, Germany, 2005; pp. 43–49. ISBN 978-3-8001-4900-X. [Google Scholar]
- Coombe, B.G. Growth Stages of the Grapevine: Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Dukes, B.C.; Butzke, C.E. Rapid Determination of Primary Amino Acids in Grape Juice Using an o-Phthaldialdehyde/N-Acetyl-L-Cysteine Spectrophotometric Assay. Am. J. Enol. Vitic. 1998, 49, 125. [Google Scholar]
- Krause, B.; Löhnertz, O. Saccharopin und Pipecolinsäure: Diagnostische Biomarker in der klassischen Aminosäureanalytik. MTA Dialog. 2017, 18, 316–321. [Google Scholar] [CrossRef]
- Smart, R.E.; Robinson, M. Sunlight into Wine: A Handbook for Winegrape Canopy Management; Winetitles Media: Ashford, Australia, 1991; ISBN 1875130101. [Google Scholar]
- Cerovic, Z.G.; Ghozlen, N.B.; Milhade, C.; Obert, M.; Debuisson, S.; Le Moigne, M. Nondestructive diagnostic test for nitrogen nutrition of grapevine (Vitis vinifera L.) based on dualex leaf-clip measurements in the field. J. Agric. Food Chem. 2015, 63, 3669–3680. [Google Scholar] [CrossRef]
- EPPO. EPPO Database on PP1 Standards. Available online: https://pp1.eppo.int/standards/PP1-031-3 (accessed on 1 November 2021).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Soft. 2008, 25. [Google Scholar] [CrossRef] [Green Version]
- De Mendiburu, F.; Yaseen, M. Agricolae: Statistical Procedures for Agricultural Research. Available online: https://cran.r-project.org/package=agricolae (accessed on 1 November 2021).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [Green Version]
- Smart, R.E. Principles of Grapevine Canopy Microclimate Manipulation with Implications for Yield and Quality. A Review. Am. J. Enol. Vitic. 1985, 36, 230. [Google Scholar]
- Reshef, N.; Walbaum, N.; Agam, N.; Fait, A. Sunlight Modulates Fruit Metabolic Profile and Shapes the Spatial Pattern of Compound Accumulation within the Grape Cluster. Front. Plant Sci. 2017, 8, 70. [Google Scholar] [CrossRef] [Green Version]
- Du Plessis, K.; Young, P.R.; Eyéghé-Bickong, H.A.; Vivier, M.A. The Transcriptional Responses and Metabolic Consequences of Acclimation to Elevated Light Exposure in Grapevine Berries. Front. Plant Sci. 2017, 8, 1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, G.E.; Gaudillere, J.-P.; Pieri, P.; Hilbert, G.; Maucourt, M.; Deborde, C.; Moing, A.; Rolin, D. Microclimate influence on mineral and metabolic profiles of grape berries. J. Agric. Food Chem. 2006, 54, 6765–6775. [Google Scholar] [CrossRef]
- Hunter, J.J.; Volschenk, C.G.; Bonnardot, V. Linking grapevine row orientation to a changing climate in South Africa. In Proceedings of the 60th German Grape and Wine Congress, International IVIF Congress, Stuttgart, Germany, 24–27 March 2010; pp. 60–70. [Google Scholar]
- Friedel, M. Microclimatic Influences on Grape Quality. Ph.D. Dissertation, Universitätsbibliothek, Gießen, Germany, 2018. [Google Scholar]
- Hunter, J.J.; Volschenk, C.G.; Booyse, M. Vineyard row orientation and grape ripeness level effects on vegetative and reproductive growth characteristics of Vitis vinifera L. cv. Shiraz/101-14 Mgt. Eur. J. Agron. 2017, 84, 47–57. [Google Scholar] [CrossRef]
- Hunter, J.K.; Tarricone, L.; Volschenk, C.; Giacalone, C.; Melo, M.S.; Zorer, R. Grapevine physiological response to row orientation-induced spatial radiation and microclimate changes. OENO One 2020, 54, 411–433. [Google Scholar] [CrossRef]
- Friedel, M.; Patz, C.-D.; Stoll, M. Impact of row orientation on berry quality parameters White Riesling (Vitis vinifera L.). In Proceedings of the 17th International Symposium GiESCO, Asti-Alba, Italy, 29 August–2 September 2011; Novello, V., Bovio, M., Cavalletto, S., Eds.; Le Progès Agricole et Viticole: Asti-Alba, Italy, 2011; pp. 327–330. [Google Scholar]
- Pieri, P.; Zott, K.; Gomès, E.; Hilbert, G. Nested effects of berry half, berry and bunch microclimate on biochemical composition in grape. OENO One 2016, 50, 23. [Google Scholar] [CrossRef]
- Minnaar, P.P.; Jolly, N.P.; Ntushelo, N.S. Effect of Grapevine Canopy Side on Selected Sensory Attributes of Pinotage and Cabernet Sauvignon Wines. S. Afr. J. Enol. Vitic. 2020, 41. [Google Scholar] [CrossRef]
- Lee, S.-H.; Seo, M.-J.; Riu, M.; Cotta, J.P.; Block, D.E.; Dokoozlian, N.K.; Ebeler, S.E. Vine Microclimate and Norisoprenoid Concentration in Cabernet Sauvignon Grapes and Wines. Am. J. Enol. Vitic. 2007, 58, 291. [Google Scholar]
- Chorti, E.; Theocharis, S.; Boulokostas, K.; Kallithraka, S.; Kotseridis, Y.; Koundouras, S. Row Orientation and Defoliation Effects on Grape Composition of Vitis vinifera L. Agiorgitiko in Nemea (Greece). E3S Web Conf. 2018, 50, 1039. [Google Scholar] [CrossRef] [Green Version]
- Shellie, K.C. Interactive Effects of Deficit Irrigation and Berry Exposure Aspect on Merlot and Cabernet Sauvignon in an Arid Climate. Am. J. Enol. Vitic. 2011, 62, 462–470. [Google Scholar] [CrossRef]
- Crépin, L.; Truong, N.M.; Bloem, A.; Sanchez, I.; Dequin, S.; Camarasa, C. Management of Multiple Nitrogen Sources during Wine Fermentation by Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2017, 83, e02617-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobert, A.; Tourdot-Maréchal, R.; Sparrow, C.; Morge, C.; Alexandre, H. Influence of nitrogen status in wine alcoholic fermentation. Food Microbiol. 2019, 83, 71–85. [Google Scholar] [CrossRef]
- Gregan, S.M.; Winefield, C.; Jordan, B. Amino acid metabolism and accumulation in ‘Sauvignon Blanc’ grapes–Investigating berry composition in response to canopy manipulation. Acta Hortic. 2017, 9–14. [Google Scholar] [CrossRef]
- Bahr, C.; Schmidt, D.; Friedel, M.; Kahlen, K. Leaf removal effects on light absorption in virtual Riesling canopies (Vitis vinifera L.). Silico Plants 2021. [Google Scholar] [CrossRef]
- Arrizabalaga-Arriazu, M.; Gomès, E.; Morales, F.; Irigoyen, J.J.; Pascual, I.; Hilbert, G. High Temperature and Elevated Carbon Dioxide Modify Berry Composition of Different Clones of Grapevine (Vitis vinifera L.) cv. Tempranillo. Front. Plant Sci. 2020, 11, 603687. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, C.; Sadras, V.O.; Hancock, R.D.; Soole, K.L.; Ford, C.M. Metabolic effects of elevated temperature on organic acid degradation in ripening Vitis vinifera fruit. J. Exp. Bot. 2014, 65, 5975–5988. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.E.; Gaudillere, J.-P.; van Leeuwen, C.; Hilbert, G.; Maucourt, M.; Deborde, C.; Moing, A.; Rolin, D. 1H NMR metabolite fingerprints of grape berry: Comparison of vintage and soil effects in Bordeaux grapevine growing areas. Anal. Chim. Acta 2006, 563, 346–352. [Google Scholar] [CrossRef]
- Gambetta, G.A.; Herrera, J.C.; Dayer, S.; Feng, Q.; Hochberg, U.; Castellarin, S.D. The physiology of drought stress in grapevine: Towards an integrative definition of drought tolerance. J. Exp. Bot. 2020, 71, 4658–4676. [Google Scholar] [CrossRef] [PubMed]
- Herrera, J.C.; Hochberg, U.; Degu, A.; Sabbatini, P.; Lazarovitch, N.; Castellarin, S.D.; Fait, A.; Alberti, G.; Peterlunger, E. Grape Metabolic Response to Postveraison Water Deficit Is Affected by Interseason Weather Variability. J. Agric. Food Chem. 2017, 65, 5868–5878. [Google Scholar] [CrossRef] [PubMed]
- Reshef, N.; Fait, A.; Agam, N. Grape berry position affects the diurnal dynamics of its metabolic profile. Plant Cell Environ. 2019, 42, 1897–1912. [Google Scholar] [CrossRef]
- Sweetman, C.; Deluc, L.G.; Cramer, G.R.; Ford, C.M.; Soole, K.L. Regulation of malate metabolism in grape berry and other developing fruits. Phytochemistry 2009, 70, 1329–1344. [Google Scholar] [CrossRef]
- Rienth, M.; Torregrosa, L.; Sarah, G.; Ardisson, M.; Brillouet, J.-M.; Romieu, C. Temperature desynchronizes sugar and organic acid metabolism in ripening grapevine fruits and remodels their transcriptome. BMC Plant Biol. 2016, 16, 164. [Google Scholar] [CrossRef]
- Reshef, N.; Agam, N.; Fait, A. Grape Berry Acclimation to Excessive Solar Irradiance Leads to Repartitioning between Major Flavonoid Groups. J. Agric. Food Chem. 2018, 66, 3624–3636. [Google Scholar] [CrossRef]
- Smart, R.E.; Sinclair, T.R. Solar heating of grape berries and other spherical fruits. Agric. Meteorol. 1976, 17, 241–259. [Google Scholar] [CrossRef]
- Webb, L.; Whiting, J.; Watt, A.; Hill, T.; Wigg, F.; Dunn, G.; Needs, S.; Barlow, E.W.R. Managing Grapevines through Severe Heat: A Survey of Growers after the 2009 Summer Heatwave in South-eastern Australia. J. Wine Res. 2010, 21, 147–165. [Google Scholar] [CrossRef]
- Schultz, H.R. Sommer 98: Verbrennungserscheinungen an den Trauben: Einfacher Sonnenbrand oder Umweltschaden? Der Dtsch. Weinbau 1999, 3, 12–15. [Google Scholar]
- Teixeira, A.; Eiras-Dias, J.; Castellarin, S.D.; Gerós, H. Berry phenolics of grapevine under challenging environments. Int. J. Mol. Sci. 2013, 14, 18711–18739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banilas, G.; Korkas, E.; Englezos, V.; Nisiotou, A.A.; Hatzopoulos, P. Genome-wide analysis of the heat shock protein 90 gene family in grapevine (Vitis vinifera L.). Aust. J. Grape Wine Res. 2012, 18, 29–38. [Google Scholar] [CrossRef]
- Rocheta, M.; Becker, J.D.; Coito, J.L.; Carvalho, L.; Amâncio, S. Heat and water stress induce unique transcriptional signatures of heat-shock proteins and transcription factors in grapevine. Funct. Integr. Genom. 2014, 14, 135–148. [Google Scholar] [CrossRef] [Green Version]
- Dixon, R.A.; Paiva, N.L. Stress-Induced Phenylpropanoid Metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Manela, N.; Oliva, M.; Ovadia, R.; Sikron-Persi, N.; Ayenew, B.; Fait, A.; Galili, G.; Perl, A.; Weiss, D.; Oren-Shamir, M. Phenylalanine and tyrosine levels are rate-limiting factors in production of health promoting metabolites in Vitis vinifera cv. Gamay Red cell suspension. Front. Plant Sci. 2015, 6, 538. [Google Scholar] [CrossRef]
- Grechi, I.; Vivin, P.; Hilbert, G.; Milin, S.; Robert, T.; Gaudillère, J.-P. Effect of light and nitrogen supply on internal C:N balance and control of root-to-shoot biomass allocation in grapevine. Environ. Exp. Bot. 2007, 59, 139–149. [Google Scholar] [CrossRef]
- Flexas, J.; Hendrickson, L.; Chow, W.S. Photoinactivation of photosystem II in high light-acclimated grapevines. Funct. Plant Biol. 2001, 28, 755. [Google Scholar] [CrossRef]
- Cartechini, A.; Palliotti, A. Effect of Shading on Vine Morphology and Productivity and Leaf Gas Exchange Characteristics in Grapevines in the Field. Am. J. Enol. Vitic. 1995, 46, 227–234. [Google Scholar]
- Luo, Y.Y.; Li, R.X.; Jiang, Q.S.; Bai, R.; Duan, D. Changes in the chlorophyll content of grape leaves could provide a physiological index for responses and adaptation to UV-C radiation. Nord. J. Bot. 2019, 37, 2101. [Google Scholar] [CrossRef]
- Steele, M.R.; Gitelson, A.A.; Rundquist, D.C. A Comparison of Two Techniques for Nondestructive Measurement of Chlorophyll Content in Grapevine Leaves. Agron. J. 2008, 100, 779–782. [Google Scholar] [CrossRef] [Green Version]
- Taskos, D.G.; Koundouras, S.; Stamatiadis, S.; Zioziou, E.; Nikolaou, N.; Karakioulakis, K.; Theodorou, N. Using active canopy sensors and chlorophyll meters to estimate grapevine nitrogen status and productivity. Precis. Agric. 2015, 16, 77–98. [Google Scholar] [CrossRef]
- Chanishvili, S.S.; Badridze, G.S.; Barblishvili, T.F.; Dolidze, M.D. Defoliation, Photosynthetic Rates, and Assimilate Transport in Grapevine Plants. Russ. J. Plant Physiol. 2005, 52, 448–453. [Google Scholar] [CrossRef]
- Li, Y.-M.; Forney, C.; Bondada, B.; Leng, F.; Xie, Z.-S. The Molecular Regulation of Carbon Sink Strength in Grapevine (Vitis vinifera L.). Front. Plant Sci. 2020, 11, 606918. [Google Scholar] [CrossRef]
| Vintage | Site | Treatment | Exposition | TSS (°Brix g−1) | TTA (g L−1 g−1) | Tartaric Acid (g L−1 g−1) | Malic Acid (g L−1 g−1) | pH | N-OPA (g L−1 g−1) | Berry Weight (g) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | GM | C | E | 22.50 ± 0.44 a | 12.00 ± 0.67 a | 9.46 ± 0.52 a | 3.18 ± 0.20 a | 2.90 ± 0.00 b | 60.06 ± 3.97 b | 0.88 ± 0.02 c |
| 2019 | GM | C | W | 22.57 ± 0.45 a | 10.49 ± 0.20 b | 8.63 ± 0.14 b | 2.57 ± 0.04 b | 3.07 ± 0.06 a | 75.51 ± 7.94 a | 0.87 ± 0.01 c |
| 2019 | GM | T | N | 18.73 ± 0.25 c | 7.44 ± 0.27 d | 5.28 ± 0.12 d | 2.37 ± 0.15 b | 2.9 ± 0.00 b | 26.71 ± 1.89 c | 1.12 ± 0.02 a |
| 2019 | GM | T | S | 20.12 ± 0.26 b | 8.24 ± 0.12 c | 6.20 ± 0.10 c | 2.26 ± 0.09 b | 2.97 ± 0.06 b | 32.92 ± 0.77 c | 1.02 ± 0.01 b |
| 2019 | LE | C | NW | 15.94 ± 0.14 d | 5.96 ± 0.09 c | 4.22 ± 0.07 c | 1.82 ± 0.03 c | 2.87 ± 0.06 c | 40.93 ± 1.29 c | 1.20 ± 0.01 a |
| 2019 | LE | C | SE | 16.65 ± 0.30 c | 6.24 ± 0.25 c | 4.53 ± 0.17 c | 1.83 ± 0.10 c | 2.90 ± 0.00 c | 42.85 ± 1.04 c | 1.18 ± 0.02 a |
| 2019 | LE | T | NE | 24.11 ± 0.51 b | 13.33 ± 0.30 b | 10.28 ± 0.29 b | 4.06 ± 0.20 b | 3.17 ± 0.06 b | 182.57 ± 19.8 b | 0.77 ± 0.01 b |
| 2019 | LE | T | SW | 28.22 ± 0.25 a | 17.33 ± 0.6 a | 13.9 ± 0.61 a | 5.08 ± 0.07 a | 3.27 ± 0.06 a | 304.90 ± 33.19 a | 0.67 ± 0.01 c |
| 2019 | LS | C | NW | 19.19 ± 0.04 c | 8.03 ± 0.04 c | 5.92 ± 0.07 c | 2.38 ± 0.01 c | 2.93 ± 0.06 ab | 63.32 ± 2.25 c | 1.00 ± 0.00 b |
| 2019 | LS | C | SE | 18.76 ± 0.12 c | 7.41 ± 0.07 c | 5.57 ± 0.06 c | 2.19 ± 0.02 c | 3.00 ± 0.00 a | 56.09 ± 3.99 c | 1.06 ± 0.01 a |
| 2019 | LS | T | NE | 22.06 ± 0.67 b | 10.39 ± 0.51 b | 8.11 ± 0.44 b | 2.69 ± 0.14 b | 2.90 ± 0.00 b | 113.60 ± 1.72 b | 0.86 ± 0.02 c |
| 2019 | LS | T | SW | 25.26 ± 0.68 a | 13.91 ± 0.86 a | 11.15 ± 0.79 a | 3.35 ± 0.15 a | 3.00 ± 0.00 a | 171.79 ± 10.11 a | 0.75 ± 0.02 d |
| 2020 | GM | C | E | 17.51 ± 0.08 c | 6.98 ± 0.11 a | 6.95 ± 0.10 a | 0.93 ± 0.07 b | 3.07 ± 0.06 b | 54.58 ± 2.04 b | 1.14 ± 0.01 b |
| 2020 | GM | C | W | 18.77 ± 0.13 a | 7.18 ± 0.26 a | 7.09 ± 0.26 a | 0.69 ± 0.02 c | 3.20 ± 0.00 a | 73.13 ± 2.16 a | 1.08 ± 0.02 c |
| 2020 | GM | T | N | 17.79 ± 0.06 c | 7.13 ± 0.00 a | 6.42 ± 0.05 b | 1.42 ± 0.05 a | 3.00 ± 0.00 c | 38.11 ± 0.75 c | 1.17 ± 0.00 a |
| 2020 | GM | T | S | 18.24 ± 0.37 b | 6.88 ± 0.15 a | 6.78 ± 0.22 a | 0.93 ± 0.02 b | 3.10 ± 0.00 b | 36.37 ± 2.24 c | 1.17 ± 0.02 a |
| 2020 | LE | C | NW | 14.53 ± 0.13 d | 4.65 ± 0.09 d | 4.09 ± 0.05 d | 0.98 ± 0.05 c | 2.90 ± 0.00 b | 27.57 ± 1.1 c | 1.42 ± 0.01 a |
| 2020 | LE | C | SE | 15.79 ± 0.27 c | 5.57 ± 0.16 c | 4.89 ± 0.14 c | 1.18 ± 0.05 b | 2.90 ± 0.00 b | 30.05 ± 1.91 c | 1.32 ± 0.02 b |
| 2020 | LE | T | NE | 17.72 ± 0.11 b | 9.61 ± 0.22 b | 8.34 ± 0.24 b | 2.23 ± 0.03 a | 2.97 ± 0.06 a | 76.95 ± 2.98 b | 1.02 ± 0.01 c |
| 2020 | LE | T | SW | 19.19 ± 0.44 a | 11.15 ± 0.66 a | 10.00 ± 0.59 a | 2.37 ± 0.14 a | 3.00 ± 0.00 a | 119.24 ± 6.2 a | 0.95 ± 0.02 d |
| 2020 | LS | C | NW | 15.58 ± 0.21 d | 5.21 ± 0.15 d | 4.81 ± 0.15 d | 0.95 ± 0.03 b | 2.90 ± 0.00 b | 45.60 ± 3.70 c | 1.29 ± 0.02 a |
| 2020 | LS | C | SE | 16.73 ± 0.34 c | 5.98 ± 0.23 c | 5.60 ± 0.22 c | 1.05 ± 0.05 b | 3.00 ± 0.00 a | 52.65 ± 2.97 c | 1.22 ± 0.02 b |
| 2020 | LS | T | NE | 18.77 ± 0.24 b | 10.82 ± 0.13 b | 9.70 ± 0.11 b | 2.16 ± 0.03 a | 2.87 ± 0.06 b | 98.13 ± 6.48 b | 0.96 ± 0.01 c |
| 2020 | LS | T | SW | 20.71 ± 0.43 a | 13.05 ± 0.74 a | 12.25 ± 0.66 a | 2.27 ± 0.12 a | 2.90 ± 0.00 b | 136.67 ± 15.3 a | 0.89 ± 0.02 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strack, T.; Stoll, M. Implication of Row Orientation Changes on Fruit Parameters of Vitis vinifera L. cv. Riesling in Steep Slope Vineyards. Foods 2021, 10, 2682. https://doi.org/10.3390/foods10112682
Strack T, Stoll M. Implication of Row Orientation Changes on Fruit Parameters of Vitis vinifera L. cv. Riesling in Steep Slope Vineyards. Foods. 2021; 10(11):2682. https://doi.org/10.3390/foods10112682
Chicago/Turabian StyleStrack, Timo, and Manfred Stoll. 2021. "Implication of Row Orientation Changes on Fruit Parameters of Vitis vinifera L. cv. Riesling in Steep Slope Vineyards" Foods 10, no. 11: 2682. https://doi.org/10.3390/foods10112682
APA StyleStrack, T., & Stoll, M. (2021). Implication of Row Orientation Changes on Fruit Parameters of Vitis vinifera L. cv. Riesling in Steep Slope Vineyards. Foods, 10(11), 2682. https://doi.org/10.3390/foods10112682






