Enhance Fruit Ripening Uniformity and Accelerate the Rutab Stage by Using ATP in ‘Zaghloul’ Dates during the Shelf Life
Abstract
:1. Introduction
2. Materials and Methods
2.1. Date Fruits and Samples
2.2. ATP: Exogenous Treatment Protocol
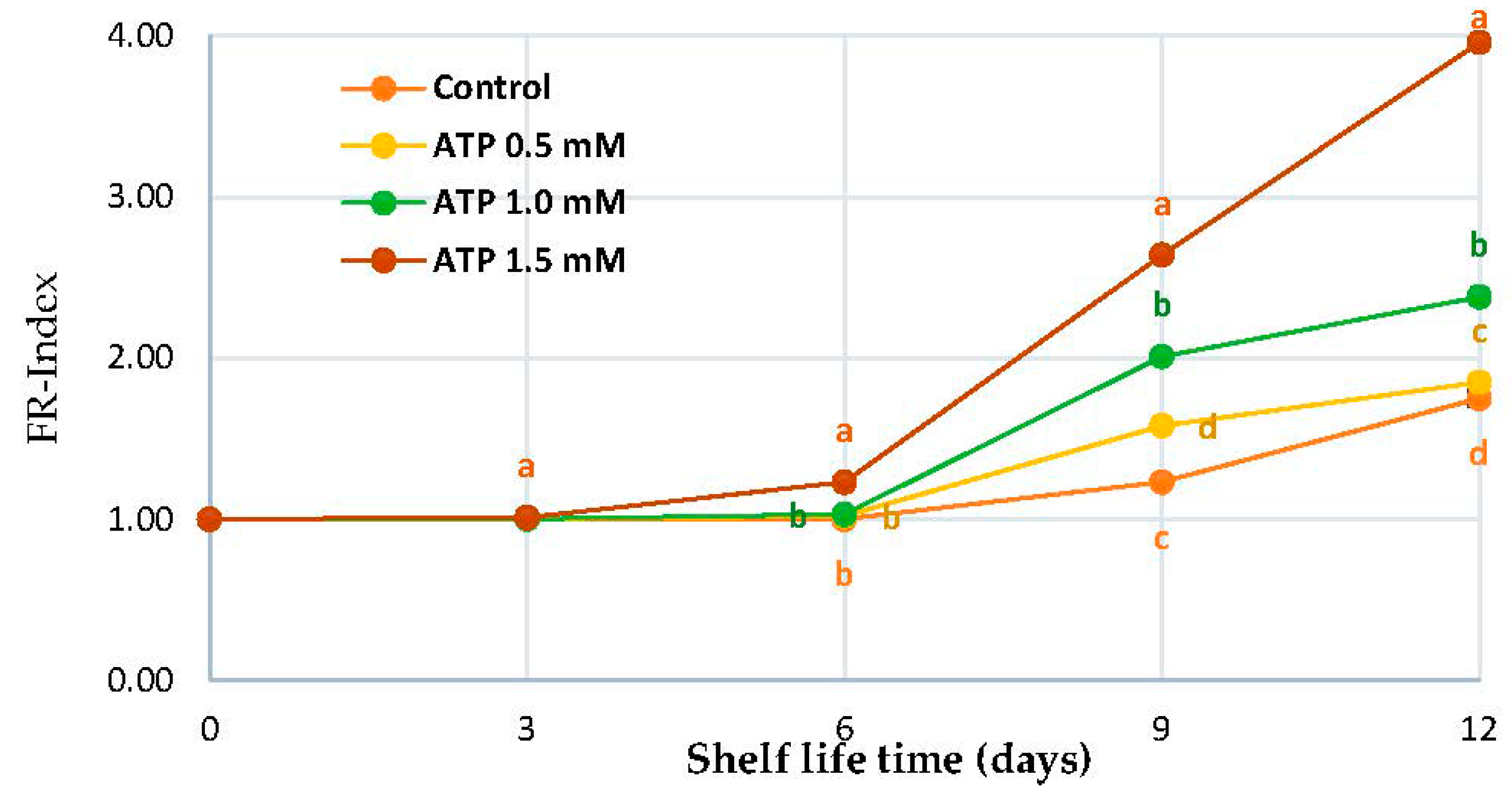
2.3. Fruit Rutab Index
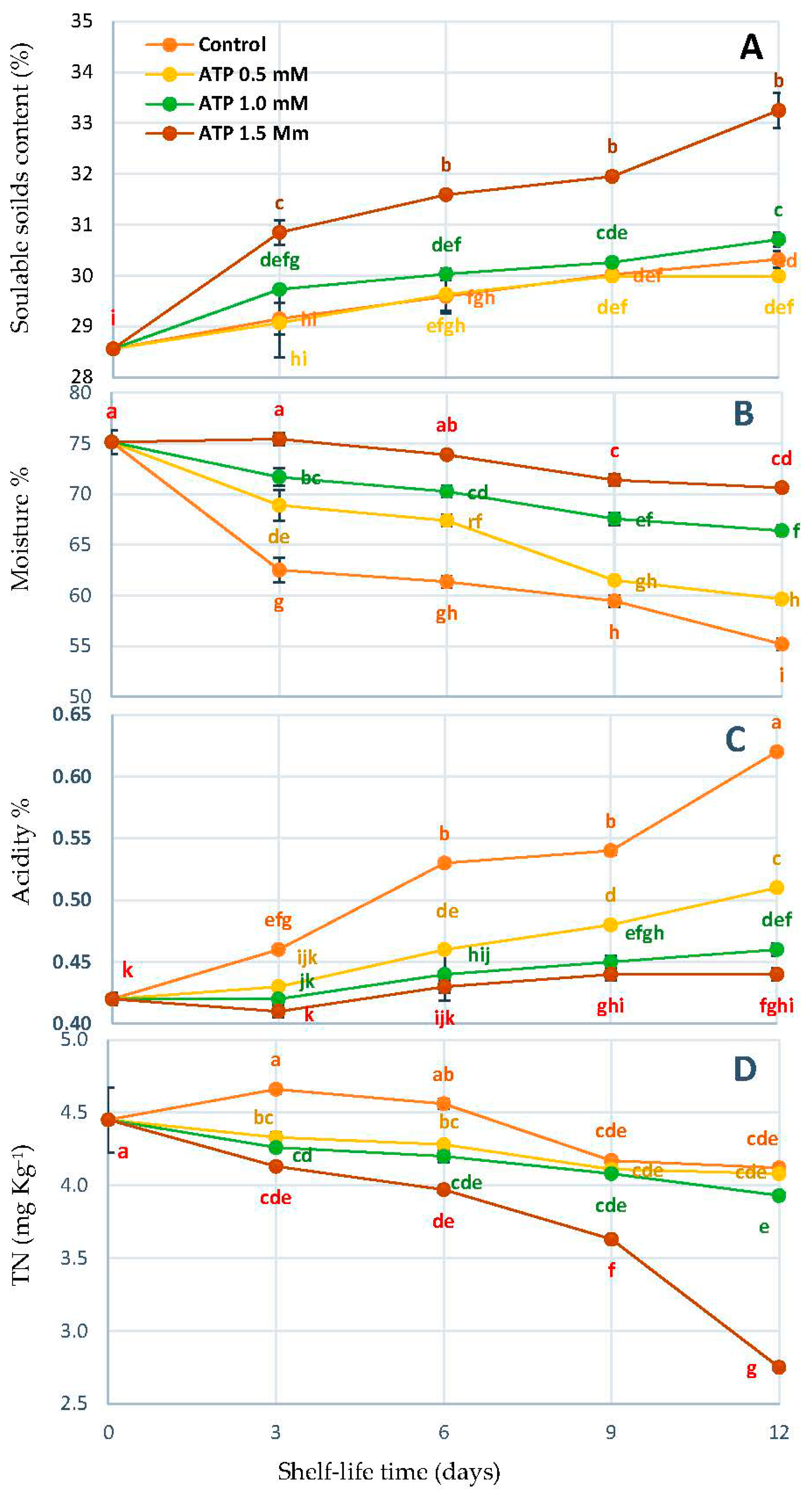
2.4. Soluble Solid Content (SSC%), Acidity (TA%), Moisture (%), and Tannins Content (TN)
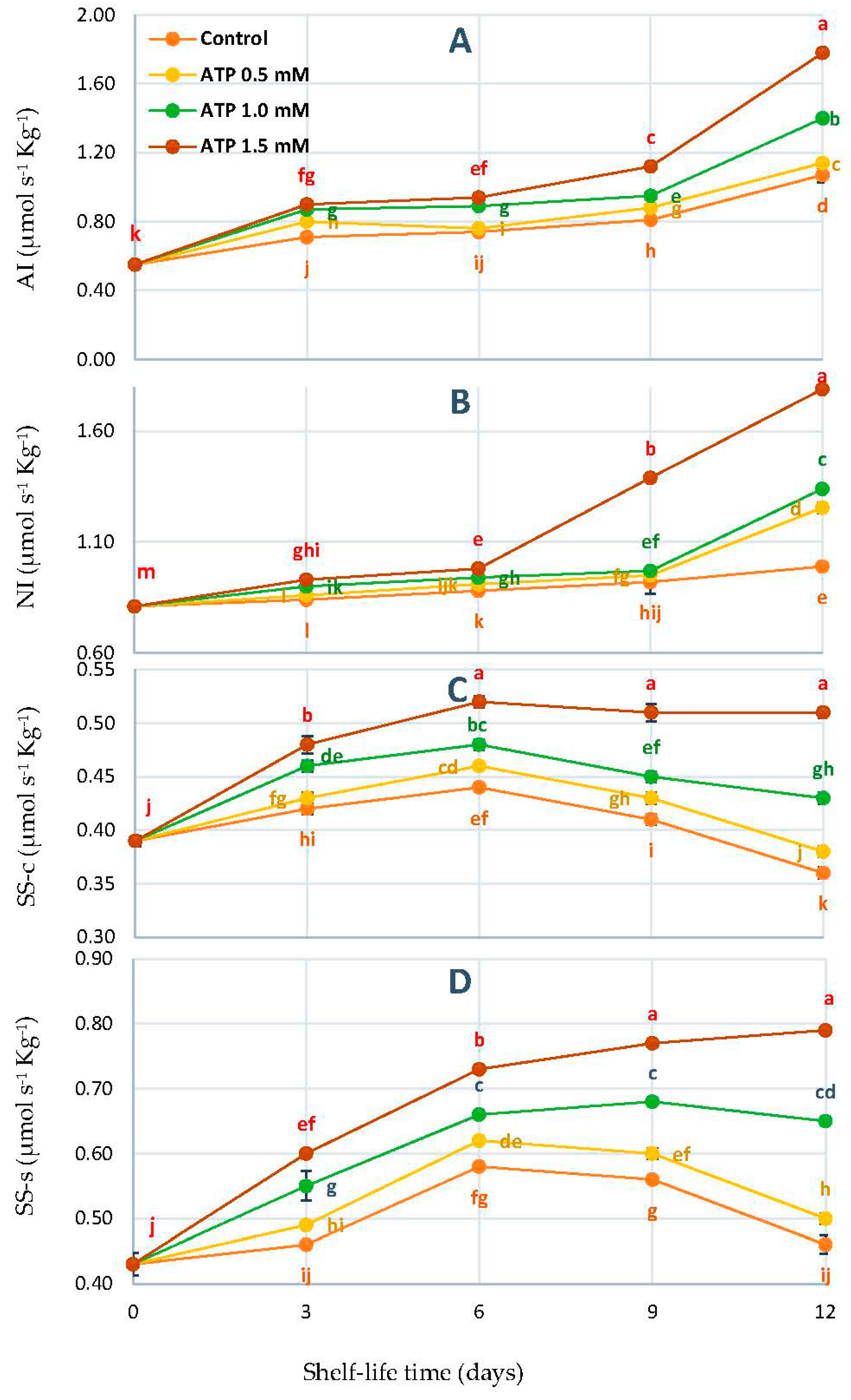
2.5. Sucrose Metabolic: Enzyme Activities Monitoring
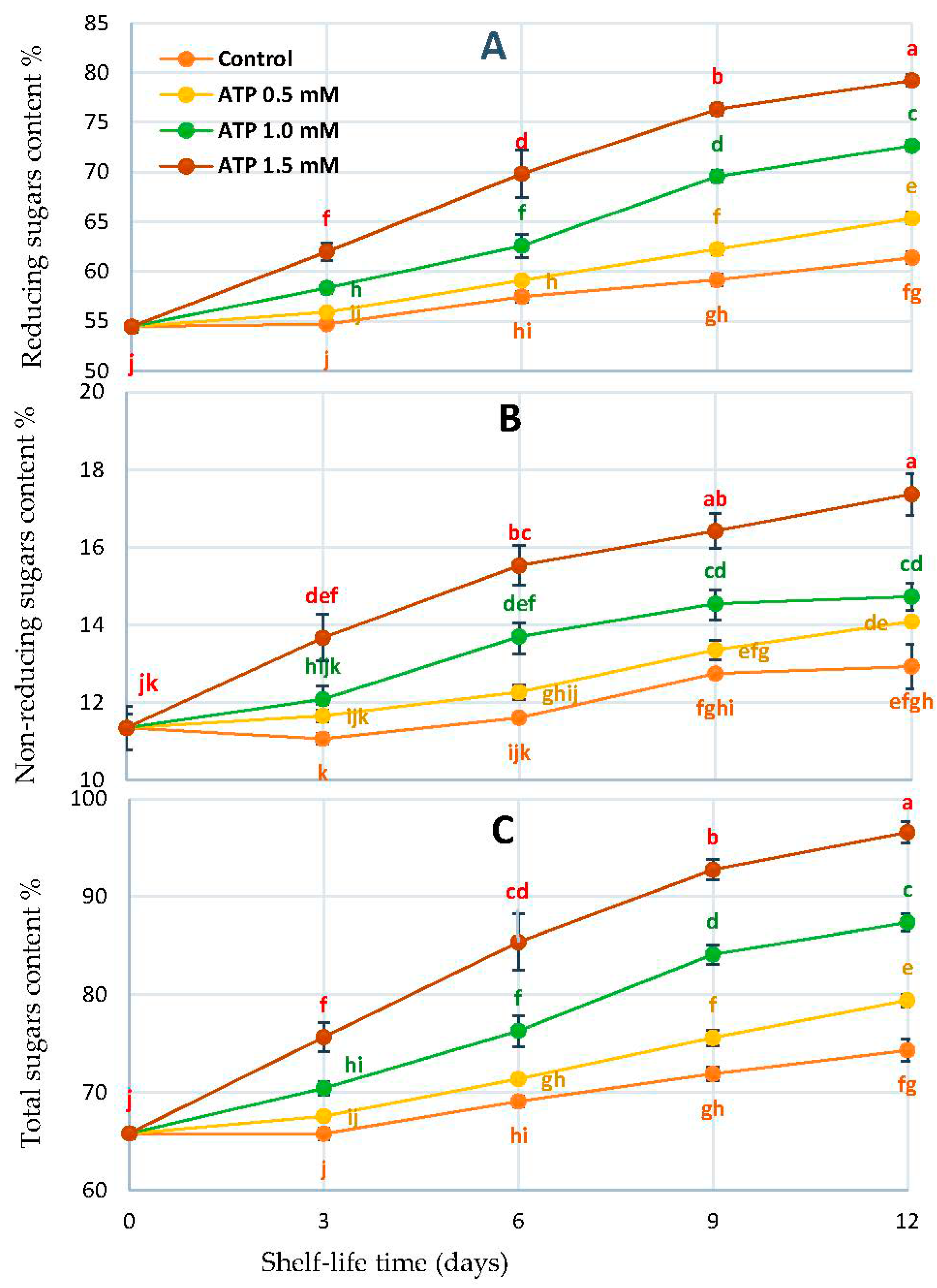
2.6. Sugars Content Profile Accumulation
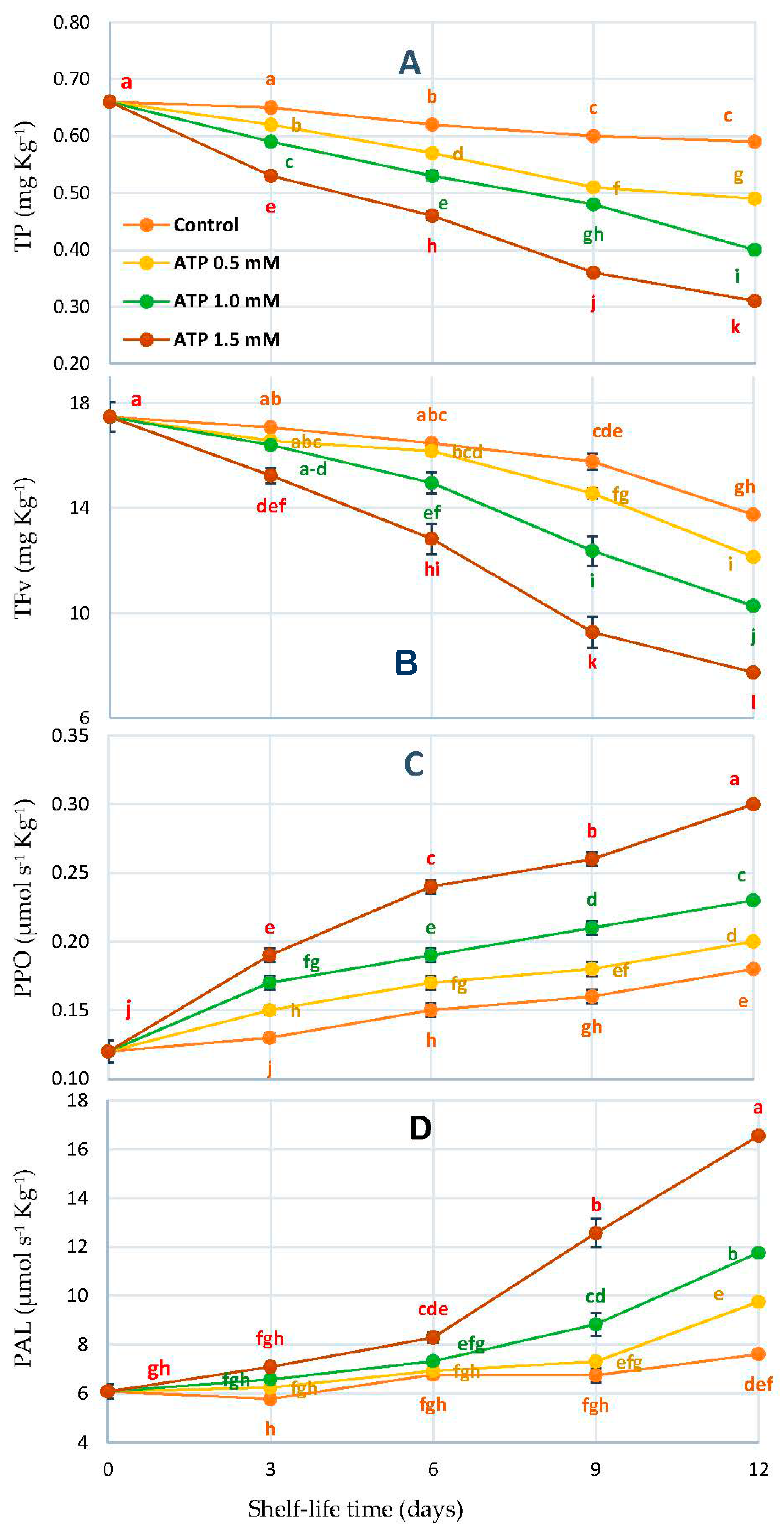
2.7. Total Phenol (TP), Flavonoids (TFv), Polyphenol Oxidase (PPO), and Phenylalanine Ammonia-Lyase (PAL)
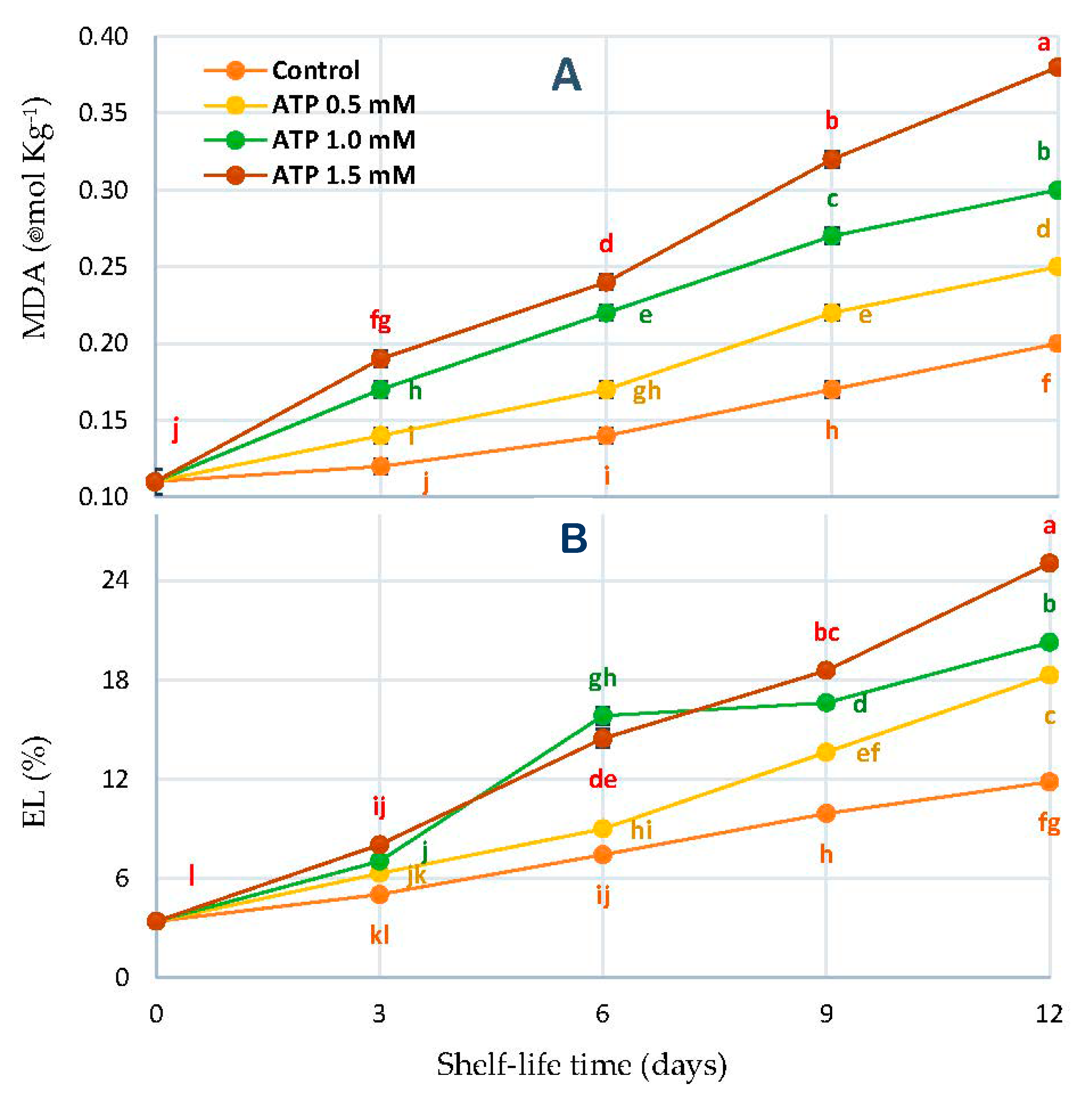
2.8. Malondialdehyde Concentration (MDA) and Electrolyte Leakage Percentage (EL%)
2.9. Statistical Analysis
3. Results
3.1. FR-Index
3.2. Change in Content SSC%, Moisture%, TA%, and TN%
3.3. Changes in Acid Invertase (AI), Neutral Invertase (NI), Sucrose Synthase-Cleavage (SS-c), and Sucrose Synthase-Synthesis (SS-s) Activities
3.4. Fruit Sugar Profile
3.5. Total Phenol (TP), Flavonoids (TFv), Polyphenol Oxidase (PPO), and Phenylalanine Ammonia-Lyase (PAL)
3.6. Malondialdehyde Concentration (MDA) and Electrolyte Leakage Percentage (EL%)
3.7. Multivariate Analysis of Date Parameters
3.8. Modelling and Validation
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Botes, A.; Zaid, A. The economic importance of date production and international trade. Available online: http://www.fao.org/43/y4360e/y4360e4307.htm (accessed on 28 September 2021).
- Hong, Y.J.; Tomás-Barberán, F.A.; Kader, A.A.; Mitchell, A.E. The flavonoid glycosides and procyanidin composition of Deglet Noor dates (Phoenix dactylifera). J. Agric. Food Chem. 2006, 54, 2405–2411. [Google Scholar] [CrossRef]
- Al-Mssallem, M.Q.; Alqurashi, R.M.; Al-Khayri, J.M. Bioactive Compounds of Date Palm (Phoenix dactylifera L.). In Bioactive Compounds in Underutilized Fruits and Nuts; Murthy, H.N., Bapat, V.A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–15. [Google Scholar]
- EAM-Statistics. Agriculture Directorates of Governorates; Economic Affairs Sector: Cairo, Eygpt, 2015; pp. 1–450. [Google Scholar]
- FAOSTAT. Source FAOSTAT. 2019. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 28 September 2021).
- Glasner, B.; Botes, A.; Zaid, A.; Emmens, J. Date Harvesting, Packing House Management, and Marketing Aspects; Zaid, A., Arias, E.J., Eds.; FAO: Rome, Italy, 1999; pp. 177–198. [Google Scholar]
- Vayalil, P.K. Antioxidant and antimutagenic properties of aqueous extract of date fruit (Phoenix dactylifera L. Arecaceae). J. Agric. Food Chem. 2002, 50, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Embarek, G.; Kokkalou, E.; Kefalas, P. Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera). Food Chem. 2005, 89, 411–420. [Google Scholar] [CrossRef]
- Brady, C.J. Fruit ripening. Annu. Rev. Plant Physiol. 1987, 38, 155–178. [Google Scholar] [CrossRef]
- Barreveld, W.H.; Date palm products. FAO Agric. Servaces Bull. 1993. Available online: http://www.fao.org/docrep/t0681e/t0681e0600.htm (accessed on 28 September 2021).
- Cosme Silva, G.M.; Silva, W.S.; Medeiros, D.B.; Salvador, A.R.; Cordeiro, M.H.M.; da Silva, N.M.; Santana, D.B.; Mizobutsi, G.P. The chitosan affects severely the carbon metabolism in mango (Mangifera indica L. cv. Palmer) fruit during storage. Food Chem. 2017, 237, 372–378. [Google Scholar] [CrossRef]
- Moustafa, A.A.; Seif, S.A. Effect of ethrel and GA treatments on yield and fruit quality of seewy date palms, grown in El-Fayoum governorate. In Proceedings of the 3rd Symposium on the Date Palm in Saudi Arabia, Alahsa, Saudi Arabia, 17–20 January 1996; pp. 379–388. [Google Scholar]
- El-Hamady, M.M.; Al-Maghrabi, M.A.; Basha, M.A. Effect of ethephon treatment on fruit thinning and quality of seleg and meneify date palm cultivars. Annal Agric. Sci. Cairo 1992, 37, 531–538. [Google Scholar]
- Al-Khateeb, A.A.; Al-Khateeb, S.A.; Okawara, R.; Al-Abdoulhady, I.A. Promotive Effects of 5-Aminolevulinic Acid (5-ALA) on Fruit Yield and Quality of Date Palm cv., Khalas. J. Biol. Sci. 2006, 6, 1118–1121. [Google Scholar] [CrossRef] [Green Version]
- Huo, K.; Lu, W.; Li, X.; Chen, P. Recent progress in understanding extracellular ATP signal molecule in plants. Bull. Bot. 2014, 49, 618–625. [Google Scholar] [CrossRef]
- Tanaka, K.; Gilroy, S.; Jones, A.M.; Stacey, G. Extracellular ATP signaling in plants. Trends Cell Biol. 2010, 20, 601–608. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Chen, F.; Lai, S.; Tao, J.; Yang, H.; Jiao, Z. Effects of calcium treatment and low temperature storage on cell wall polysaccharide nanostructures and quality of postharvest apricot (Prunus armeniaca). Food Chem. 2017, 225, 87–97. [Google Scholar] [CrossRef]
- Yi, C.; Qu, H.X.; Jiang, Y.M.; Shi, J.; Duan, X.W.; Joyce, D.C.; Li, Y.B. ATP-induced changes in energy status and membrane integrity of harvested litchi fruit and its relation to pathogen resistance. J. Phytopathol. 2010, 156, 365–371. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Li, G.; Zhou, X.; Fu, W.; Jiang, Y.; Liu, T.; Ji, S. Exogenous ATP alleviated aroma fading by regulating LOX pathway and fatty acids synthesis in ‘Nanguo’ pears after refrigeration. Sci. Hortic. 2018, 240, 522–529. [Google Scholar] [CrossRef]
- Chen, M.; Lin, H.; Zhang, S.; Lin, Y.; Chen, Y.; Lin, Y. Effects of adenosine triphosphate (ATP) treatment on postharvest physiology, quality and storage behavior of longan fruit. Food Bioprocess. Technol. 2015, 8, 971–982. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, Y.; He, Z.; Liu, Q.; Lai, S.; Yang, H. Effect of exogenous ATP on the postharvest properties and pectin degradation of mung bean sprouts (Vigna radiata). Food Chem. 2018, 251, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lin, H.; Lin, Y.; Lin, Y.; Hung, Y.; Chen, Y.; Wang, H.; Shi, J. Energy status regulates disease development and respiratory metabolism of Lasiodiplodia theobromae (Pat.) Griff. & Maubl.-infected longan fruit. Food Chem. 2017, 231, 238–246. [Google Scholar] [CrossRef]
- Sun, B.; Ricardo-da-Silva, J.M.; Spranger, I. Critical Factors of Vanillin Assay for Catechins and Proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, J.; Peng, T.; Lu, H. Experimental Investigation on Added Mass Coefficient of a Truss Spar Subjected to Vortex-Induced Motions. Proceedings of ASME 2012 31st International Conference on Ocean. Offshore and Arctic Engineering, Rio de Janeiro, Brazil, 1–6 January 2012; Volume 5, pp. 589–596. [Google Scholar]
- Sun, Z.; Li, Y.; Zhou, J.; Zhu, S. Effects of exogenous nitric oxide on contents of soluble sugars and related enzyme activities in ‘Feicheng’ peach fruit. J. Sci. Food Agric. 2011, 91, 1795–1800. [Google Scholar] [CrossRef]
- Solomakhin, A.A.; Blanke, M.M. Mechanical flower thinning improves the fruit quality of apples. J. Sci. Food Agric. 2010, 90, 735–741. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Paramesha, D.; Keerthishree, M.; Chethan, P.H. Effect of CaCl2 and KMnO4 on shelf life of fig fruits. Int. J. Agric. Sci. 2017, 9, 4170–4176. [Google Scholar]
- Jiang, Y.; Joyce, D.; Jiang, W.; Lu, W. Effects of Chilling Temperatures on Ethylene Binding by Banana Fruit. Plant Growth Regul. 2004, 43, 109–115. [Google Scholar] [CrossRef]
- Ke, D.; Salveit, M.E. The effect of calcium and auxin on russet spotting and phenylalanine ammonia lyase activity in iceberg lettuce. HortiScience 1986, 21, 1169–1171. [Google Scholar]
- Hoff, J.E.; Singleton, K.I. A method for determination of tannins in foods by means of immobilized protein. Food Policy Nutr. Div. 1977, 42, 1566–1569. [Google Scholar] [CrossRef]
- Iturbe-Ormaetxe, I.; Escuredo, P.R.; Arrese-Igor, C.; Becana, M. Oxidative Damage in Pea Plants Exposed to Water Deficit or Paraquat. Plant. Physiol. 1998, 116, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Lo’ay, A.A.; Dawood, H.D. Tolerance of ‘Baladi’ mandarin fruits to cold storage by postharvest pectin/PVA blend with ascorbic acid treatment. Sci. Hortic. 2019, 256, 108637. [Google Scholar] [CrossRef]
- Fikry, M.; Yusof, Y.A.; Al-Awaadh, A.M.; Rahman, R.A.; Chin, N.L.; Mousa, E.; Chang, L.S. Kinetics modelling of the colour, hardness, grinding energy consumption and oil yield changes during the conventional roasting of palm date seeds. Food Sci. Technol. Res. 2019, 25, 351–362. [Google Scholar] [CrossRef]
- Sadka, A.; Shlizerman, L.; Kamara, I.; Blumwald, E. Primary Metabolism in Citrus Fruit as Affected by Its Unique Structure. Front. Plant. Sci. 2019, 10, 1167. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Ge, Y.; Li, C.; Gao, X.; Tang, Q.; Li, X.; Wei, M.; Chen, Y. Effect of exogenous ATP treatment on sucrose metabolism and quality of Nanguo pear fruit. Sci. Hortic. 2019, 249, 71–76. [Google Scholar] [CrossRef]
- Serrano, M.; Pretel, M.T.; Botella, M.A.; Amorós, A. Physicochemical Changes during Date Ripening Related to Ethylene Production. Food Sci. Technol. Int. 2001, 7, 31–36. [Google Scholar] [CrossRef]
- Awad, M.A. Increasing the rate of ripening of date palm fruit (Phoenix Dactylifera L.) cv. Helali by preharvest and postharvest treatments. Postharvest Biol. Technol. 2007, 43, 121–127. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Y.; Liu, C.; Zheng, Y.; Xu, M.; Wu, N.; Sheng, J.; Shen, L. Characterization of WRKY transcription factors in Solanum lycopersicum reveals collinearity and their expression patterns under cold treatment. Biochem. Biophys. Res. Commun. 2015, 464, 962–968. [Google Scholar] [CrossRef]
- Li, L.; Lv, F.-Y.; Guo, Y.-Y.; Wang, Z.-Q. Respiratory pathway metabolism and energy metabolism associated with senescence in postharvest Broccoli (Brassica oleracea L. var. italica) florets in response to O2/CO2 controlled atmospheres. Postharvest Biol. Technol. 2016, 111, 330–336. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, Y.; Lin, H.; Ritenour, M.A.; Shi, J.; Zhang, S.; Chen, Y.; Wang, H. Hydrogen peroxide-induced pericarp browning of harvested longan fruit in association with energy metabolism. Food Chem. 2017, 225, 31–36. [Google Scholar] [CrossRef]
- Bolouri-Moghaddam, M.; Le Roy, K.; Xiang, L.; Rolland, F.; Van den Ende, W. Sugar signalling and antioxidant network connections in plant cells. FEBS J. 2010, 277, 2022–2037. [Google Scholar] [CrossRef]
- Tsutsui, T.; Nakano, A.; Ueda, T. The Plant-Specific RAB5 GTPase ARA6 is Required for Starch and Sugar Homeostasis in Arabidopsis thaliana. Plant. Cell Physiol. 2015, 56, 1073–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Wu, G.; Hao, C.; Yu, H.; Tan, L. Transcriptome and selected metabolite analyses reveal points of sugar metabolism in jackfruit (Artocarpus heterophyllus Lam.). Plant. Sci. 2016, 248, 45–56. [Google Scholar] [CrossRef]
- Shao, X.; Zhu, Y.; Cao, S.; Wang, H.; Song, Y. Soluble Sugar Content and Metabolism as Related to the Heat-Induced Chilling Tolerance of Loquat Fruit During Cold Storage. Food Bioprocess. Tech. 2013, 6, 3490–3498. [Google Scholar] [CrossRef]
- Wang, K.; Shao, X.; Gong, Y.; Zhu, Y.; Wang, H.; Zhang, X.; Yu, D.; Yu, F.; Qiu, Z.; Lu, H. The metabolism of soluble carbohydrates related to chilling injury in peach fruit exposed to cold stress. Postharvest Biol. Technol. 2013, 86, 53–61. [Google Scholar] [CrossRef]
- Cao, S.; Yang, Z.; Zheng, Y. Sugar metabolism in relation to chilling tolerance of loquat fruit. Food Chem. 2013, 136, 139–143. [Google Scholar] [CrossRef]
- Velasco, R.; Zharkikh, A.; Affourtit, J.; Dhingra, A.; Cestaro, A.; Kalyanaraman, A.; Fontana, P.; Bhatnagar, S.K.; Troggio, M.; Pruss, D.; et al. The genome of the domesticated apple (Malus × domestica Borkh.). Nat. Genet. 2010, 42, 833–839. [Google Scholar] [CrossRef]
- Yuan, H.; Wei, Y.; Li, X.; Li, J.; Wang, A. Differences in sugar accumulation and the related gene expression in fruit development between’ Nanguo’ and its mutant’ Nanhong’ pears. J. Fruit Sci. 2017, 34, 534–540. [Google Scholar] [CrossRef]
- AlFaris, N.A.; AlTamimi, J.Z.; AlGhamdi, F.A.; Albaridi, N.A.; Alzaheb, R.A.; Aljabryn, D.H.; Aljahani, A.H.; AlMousa, L.A. Total phenolic content in ripe date fruits (Phoenix dactylifera L.): A systematic review and meta-analysis. Saudi J. Biol. Sci. 2021, 28, 3566–3577. [Google Scholar] [CrossRef]
- Shahdadi, F.; Mirzaei, H.O.; Daraei, G.A. Study of phenolic compound and antioxidant activity of date fruit as a function of ripening stages and drying process. J. Food Sci. Technol. 2015, 52, 1814–1819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, F.; Huang, Z.; Li, D.; Wang, H.; Xu, X.; Jiang, Y.; Qu, H. Phenolic components, antioxidant enzyme activities and anatomic structure of longan fruit pericarp following treatment with adenylate triphosphate. Sci. Hortic. 2014, 180, 6–13. [Google Scholar] [CrossRef]
- He, X.; Li, L.; Sun, J.; Li, C.; Sheng, J.; Zheng, F.; Li, J.; Liu, G.; Ling, D.; Tang, Y. Adenylate quantitative method analyzing energy change in postharvest banana (Musa acuminate L.) fruits stored at different temperatures. Sci. Hortic. 2017, 219, 118–124. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Dawood, H.D. Chilling injury, fruit color maturity stages, and antioxidant enzyme activities of lemon ′baladi CV′ fruits under cold storage stress. Sci. Hortic. 2019, 257, 108676. [Google Scholar] [CrossRef]

| FR-Index | SSC% | Moisture% | TA% | TN | AI | NI | SS-s | SS-c | RS | NRS | Total S | TP | TFv | PPO | PAL | MDA | EL% | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FR-Index | * 1.0000 | |||||||||||||||||
| SSC% | 0.7652 | 1.0000 | ||||||||||||||||
| Moisture% | −0.0422 | −0.0338 | 1.0000 | |||||||||||||||
| TA% | 0.8632 | 0.9046 | 0.1080 | 1.0000 | ||||||||||||||
| TN | −0.8413 | −0.8795 | 0.0230 | −0.9038 | 1.0000 | |||||||||||||
| AI | 0.8673 | 0.8480 | −0.1505 | 0.8648 | −0.8920 | 1.0000 | ||||||||||||
| NI | 0.9423 | 0.8176 | −0.0538 | 0.8906 | −0.8666 | 0.9396 | 1.0000 | |||||||||||
| SS-s | 0.6699 | 0.8524 | 0.0308 | 0.8515 | −0.7842 | 0.6962 | 0.6798 | 1.0000 | ||||||||||
| SS-c | 0.3772 | 0.6987 | 0.2425 | 0.6745 | −0.6011 | 0.4327 | 0.4350 | 0.8572 | 1.0000 | |||||||||
| RS | 0.8557 | 0.8906 | 0.0016 | 0.9443 | −0.8712 | 0.8781 | 0.8796 | 0.8746 | 0.6277 | 1.0000 | ||||||||
| NRS | 0.7900 | 0.8887 | 0.0450 | 0.9199 | −0.8069 | 0.8248 | 0.8202 | 0.8637 | 0.6365 | 0.9471 | 1.0000 | |||||||
| Total S | 0.8498 | 0.8978 | 0.0104 | 0.9474 | −0.8657 | 0.8749 | 0.8752 | 0.8799 | 0.6349 | 0.9979 | 0.9658 | 1.0000 | ||||||
| TP | −0.8574 | −0.8942 | −0.0061 | −0.9295 | 0.8679 | −0.8907 | −0.8990 | −0.8826 | −0.6529 | −0.9795 | −0.9441 | −0.9808 | 1.0000 | |||||
| TFv | −0.8798 | −0.8618 | 0.0839 | −0.9084 | 0.8312 | −0.9115 | −0.9148 | −0.7891 | −0.4959 | −0.9660 | −0.9500 | −0.9710 | 0.9577 | 1.0000 | ||||
| PPO | 0.8124 | 0.9323 | −0.0302 | 0.9399 | −0.8801 | 0.9014 | 0.8705 | 0.8871 | 0.6704 | 0.9663 | 0.9659 | 0.9745 | −0.9688 | −0.9586 | 1.0000 | |||
| PAL | 0.9424 | 0.8347 | 0.0128 | 0.9342 | −0.8816 | 0.9173 | 0.9746 | 0.7431 | 0.4997 | 0.9209 | 0.8664 | 0.9178 | −0.9172 | −0.9390 | 0.9000 | 1.0000 | ||
| MDA | 0.8703 | 0.8791 | −0.0647 | 0.9128 | −0.8741 | 0.9236 | 0.8996 | 0.8515 | 0.5629 | 0.9721 | 0.9446 | 0.9749 | −0.9801 | −0.9743 | 0.9702 | 0.9228 | 1.0000 | |
| EL% | 0.8714 | 0.8383 | −0.1864 | 0.8778 | −0.8670 | 0.9284 | 0.9029 | 0.7837 | 0.4617 | 0.9479 | 0.8929 | 0.9449 | −0.9508 | −0.9590 | 0.9298 | 0.9088 | 0.9737 | 1.0000 |
| Properties | Linear Model (Y = a ± bX) * | ||
|---|---|---|---|
| a (p-Value) | b (p-Value) | R2 | |
| FR-Index | 0.459 (0.00) | 0.252 (0.064) | 0.836 |
| SSC% | 29.15 (0.00) | 0.349 (0.00) | 0.886 |
| Moisture% | 75.92 (0.00) | −0.436 (0.00) | 0.729 |
| TA% | 0.418 (0.00) | 0.016 (0.00) | 0.95 |
| TN | 4.75 (0.00) | −0.151 (0.00) | 0.892 |
| AI | 0.526 (0.00) | 0.089 (0.00) | 0.874 |
| NI | 0.695 (0.00) | 0.081 (0.00) | 0.892 |
| SS-s | 0.49 (0.00) | 0.029 (0.00) | 0.872 |
| SS-c | 0.43 (0.00) | 0.009 (0.00) | 0.588 |
| RS | 55.6 (0.00) | 2.13 (0.00) | 0.94 |
| NRS | 11.9 (0.00) | 0.493(0.00) | 0.848 |
| Total S | 67.49 (0.00) | 2.624 (0.00) | 0.936 |
| TP | 0.639 (0.00) | 0.029 (0.00) | 0.971 |
| TFv | 17.59 (0.00) | 0.848 (0.00) | 0.948 |
| PPO | 0.138 (0.00) | 0.0141 (0.00) | 0.942 |
| PAL | 4.89 (0.00) | 0.886 (0.00) | 0.906 |
| MDA | 0.116 (0.00) | 0.0218 (0.00) | 0.978 |
| EL% | 3.14 (0.00) | 0.79 (0.00) | 0.984 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo’ay, A.A.; Elgammal, R.E.; Alhaithloul, H.A.S.; Alghanem, S.M.; Fikry, M.; Abdein, M.A.; Hikal, D.M. Enhance Fruit Ripening Uniformity and Accelerate the Rutab Stage by Using ATP in ‘Zaghloul’ Dates during the Shelf Life. Foods 2021, 10, 2641. https://doi.org/10.3390/foods10112641
Lo’ay AA, Elgammal RE, Alhaithloul HAS, Alghanem SM, Fikry M, Abdein MA, Hikal DM. Enhance Fruit Ripening Uniformity and Accelerate the Rutab Stage by Using ATP in ‘Zaghloul’ Dates during the Shelf Life. Foods. 2021; 10(11):2641. https://doi.org/10.3390/foods10112641
Chicago/Turabian StyleLo’ay, A. A., Rania E. Elgammal, Haifa A. S. Alhaithloul, Suliman M. Alghanem, Mohammad Fikry, Mohamed A. Abdein, and Dalia M. Hikal. 2021. "Enhance Fruit Ripening Uniformity and Accelerate the Rutab Stage by Using ATP in ‘Zaghloul’ Dates during the Shelf Life" Foods 10, no. 11: 2641. https://doi.org/10.3390/foods10112641
APA StyleLo’ay, A. A., Elgammal, R. E., Alhaithloul, H. A. S., Alghanem, S. M., Fikry, M., Abdein, M. A., & Hikal, D. M. (2021). Enhance Fruit Ripening Uniformity and Accelerate the Rutab Stage by Using ATP in ‘Zaghloul’ Dates during the Shelf Life. Foods, 10(11), 2641. https://doi.org/10.3390/foods10112641









