Eating Fermented: Health Benefits of LAB-Fermented Foods
Abstract
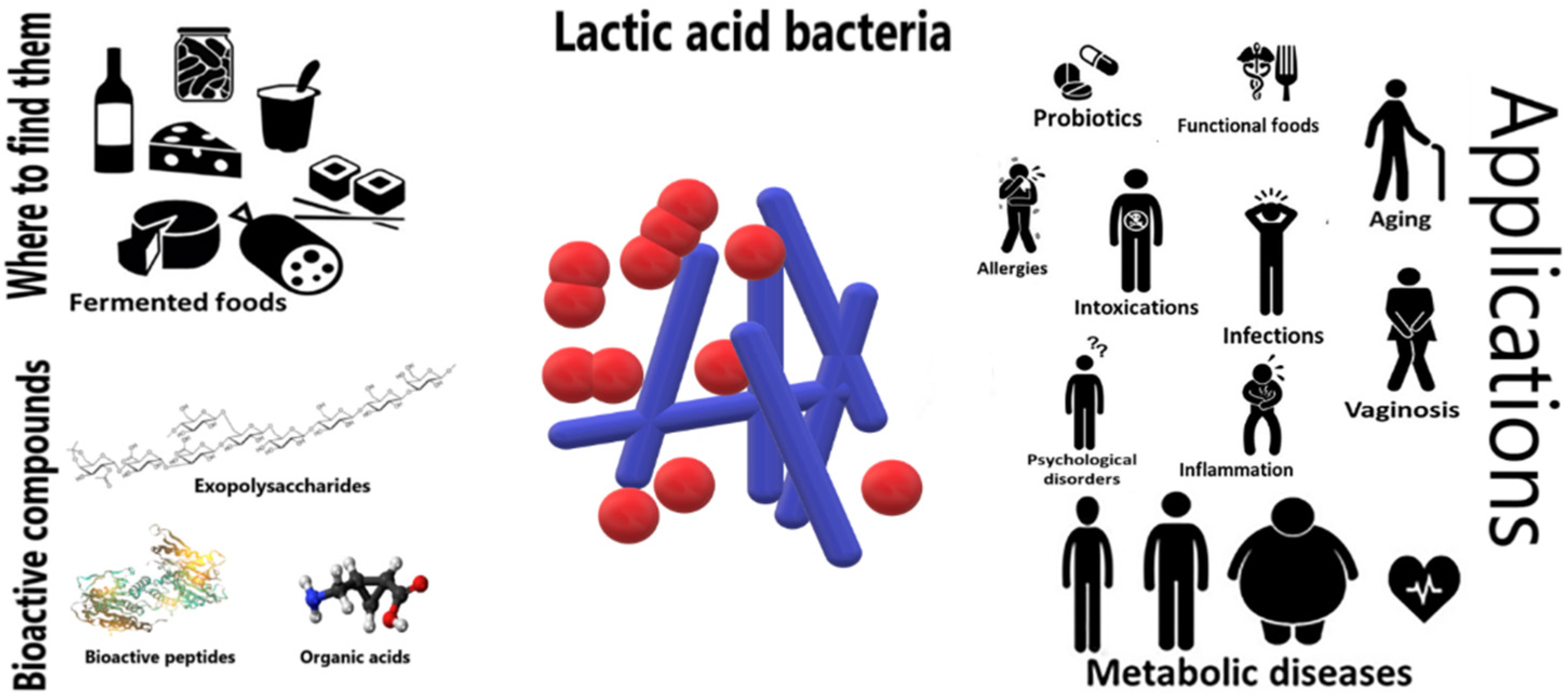
:1. Introduction
1.1. Lactic Acid Bacteria as Probiotics
1.2. Health Effects of Probiotics
1.3. Health Effects of Foods Fermented by LAB
2. Health-Related Effects of Different LAB Fermented Foods
2.1. Fermented Dairy Products
2.1.1. Yoghurt
2.1.2. Kefir
2.1.3. Cheese
2.2. Vegetable Fermented Products
2.2.1. Sauerkraut
2.2.2. Kimchi
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varzakas, T. Microbiology of Fermented Foods and Beverages. Foods 2020, 9, 1660. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, L. Lactic Acid Bacteria: Classific ation and Physiology. In Lactic Acid Bacteria Microbiological and Functional Aspects; Salmine, S., Von Wright, A., Ouwehand, A., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2004; pp. 195–196. ISBN 0-8247-5332-1. [Google Scholar]
- Schillinger, U.; Holzapfel, W. Lactic acid bacteria. In Food Spoilage Microorganisms; de W Blackburn, C., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2006; pp. 541–567. ISBN 978-1-85573-966-6. [Google Scholar]
- Narvhus, J.A.; Axelsson, L. Lactic acid bacteria. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 3465–3472. [Google Scholar]
- Bao, Y.; Wang, Z.; Zhang, Y.; Zhang, J.; Wang, L.; Dong, X.; Su, F.; Yao, G.; Wang, S.; Zhang, H. Effect of Lactobacillus plantarum P-8 on lipid metabolism in hyperlipidemic rat model. Eur. J. Lipid Sci. Technol. 2012, 114, 1230–1236. [Google Scholar] [CrossRef]
- Liceaga, A.M. Postbiotics and paraprobiotics: From concepts to applications. Food Res. Int. 2020, 136, 109502. [Google Scholar] [CrossRef]
- Orisakwe, O.E.; Amadi, C.N.; Frazzoli, C.; Dokubo, A. Nigerian foods of probiotics relevance and chronic metal exposure: A systematic review. Environ. Sci. Pollut. Res. 2020, 27, 19285–19297. [Google Scholar] [CrossRef] [PubMed]
- Langella, P.; Guarner, F.; Martín, R. Editorial: Next-generation probiotics: From commensal bacteria to novel drugs and food supplements. Front. Microbiol. 2019, 10, 1973. [Google Scholar] [CrossRef] [Green Version]
- Ljungh, Å.; Wadström, T. Lactic Acid Bacteria as Probiotics. Curr. Issues Intest. Microbiol. 2006, 7, 73–90. [Google Scholar]
- Vizoso Pinto, M.G.; Franz, C.M.A.P.; Schillinger, U.; Holzapfel, W.H. Lactobacillus spp. with in vitro probiotic properties from human faeces and traditional fermented products. Int. J. Food Microbiol. 2006, 109, 205–214. [Google Scholar] [CrossRef]
- Martín, R.; Langella, P. Emerging health concepts in the probiotics field: Streamlining the definitions. Front. Microbiol. 2019, 10, 1047. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.L.; Shu, C.C.; Lai, W.F.; Tzeng, C.M.; Lai, H.C.; Lu, C.C. Investiture of next generation probiotics on amelioration of diseases–Strains do matter. Med. Microecol. 2019, 1–2, 100002. [Google Scholar] [CrossRef]
- Sagheddu, V.; Guidesi, E.; Galletti, S.; Elli, M. Selection and Characterization Criteria of Probiotics Intended for Human Use from the Past to the Future. Food Sci. Nutr. Stud. 2019, 3, 73. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Elinav, E. Probiotics in the next-generation sequencing era. Gut Microbes 2020, 11, 77–93. [Google Scholar] [CrossRef] [PubMed]
- van Pijkeren, J.-P.; Barrangou, R. Genome Editing of Food-Grade Lactobacilli to Develop Therapeutic Probiotics. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef] [Green Version]
- Morovic, W.; Budinoff, C.R. Epigenetics: A New Frontier in Probiotic Research. Trends Microbiol. 2021, 29, 117–126. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef]
- Peredo-Lovillo, A.; Romero-Luna, H.E.; Jiménez-Fernández, M. Health promoting microbial metabolites produced by gut microbiota after prebiotics metabolism. Food Res. Int. 2020, 136, 109473. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food. 2002, pp. 1–11. Available online: https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (accessed on 10 August 2021).
- Conte, M.; Porpora, M.; Nigro, F.; Nigro, R.; Budelli, A.L.; Barone, M.V.; Nanayakkara, M. Pro-Pre and Postbiotic in Celiac Disease. Appl. Sci. 2021, 11, 8185. [Google Scholar] [CrossRef]
- Sugawara, T.; Sawada, D.; Ishida, Y.; Aihara, K.; Aoki, Y.; Takehara, I.; Takano, K.; Fujiwara, S. Regulatory effect of paraprobiotic Lactobacillus gasseri CP2305 on gut environment and function. Microb. Ecol. Health Dis. 2016, 27, 30259. [Google Scholar] [CrossRef] [PubMed]
- Średnicka, P.; Juszczuk-Kubiak, E.; Wójcicki, M.; Akimowicz, M.; Roszko, M. Probiotics as a biological detoxification tool of food chemical contamination: A review. Food Chem. Toxicol. 2021, 153, 112306. [Google Scholar] [CrossRef]
- Rau, M.; Rehman, A.; Dittrich, M.; Groen, A.K.; Hermanns, H.M.; Seyfried, F.; Beyersdorf, N.; Dandekar, T.; Rosenstiel, P.; Geier, A. Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease. United Eur. Gastroenterol. J. 2018, 6, 1496–1507. [Google Scholar] [CrossRef]
- den Besten, G.; Lange, K.; Havinga, R.; van Dijk, T.H.; Gerding, A.; van Eunen, K.; Müller, M.; Groen, A.K.; Hooiveld, G.J.; Bakker, B.M.; et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am. J. Physiol.-Gastrointest. Liver Physiol. 2013, 305, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef] [Green Version]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Cox, S.; Rossi, M.; Whelan, K. Fermented foods: Definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gille, D.; Schmid, A.; Walther, B.; Vergères, G. Fermented food and non-communicable chronic diseases: A review. Nutrients 2018, 10, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef] [PubMed]
- La Fata, G.; Weber, P.; Mohajeri, M.H. Probiotics and the Gut Immune System: Indirect Regulation. Probiotics Antimicrob. Proteins 2018, 10, 11–21. [Google Scholar] [CrossRef]
- Qi, D.; Nie, X.L.; Zhang, J.J. The effect of probiotics supplementation on blood pressure: A systemic review and meta-analysis. Lipids Health Dis. 2020, 19, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, X.; Yang, W.; Zhu, Q.; Zhang, G.; Zhang, X.; Liu, L.; Li, X.; Hussain, M.; Ni, C.; Jiang, X. Proteolysis and ACE-inhibitory peptide profile of Cheddar cheese: Effect of digestion treatment and different probiotics. LWT 2021, 145, 111295. [Google Scholar] [CrossRef]
- Korcz, E.; Varga, L. Exopolysaccharides from lactic acid bacteria: Techno-functional application in the food industry. Trends Food Sci. Technol. 2021, 110, 375–384. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; López, P.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Suárez, A.; Margolles, A.; Ruas-Madiedo, P. Immune Modulation Capability of Exopolysaccharides Synthesised by Lactic Acid Bacteria and Bifidobacteria. Probiotics Antimicrob. Proteins 2012, 4, 227–237. [Google Scholar] [CrossRef] [Green Version]
- Lynch, K.M.; Zannini, E.; Coffey, A.; Arendt, E.K. Lactic Acid Bacteria Exopolysaccharides in Foods and Beverages: Isolation, Properties, Characterization, and Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 155–176. [Google Scholar] [CrossRef]
- Holst, O.; Brennan, J.P.; Von Itzstein, M. Microbial Glycobiology, 1st ed.; Academic Press: Cambridge, MA, USA, 2009; ISBN 9780123745460. [Google Scholar]
- Yang, H.; He, M.; Wu, C. Cross protection of lactic acid bacteria during environmental stresses: Stress responses and underlying mechanisms. LWT 2021, 144, 111203. [Google Scholar] [CrossRef]
- Ren, C.; Dokter-Fokkens, J.; Figueroa Lozano, S.; Zhang, Q.; de Haan, B.J.; Zhang, H.; Faas, M.M.; de Vos, P. Lactic Acid Bacteria May Impact Intestinal Barrier Function by Modulating Goblet Cells. Mol. Nutr. Food Res. 2018, 62, 1700572. [Google Scholar] [CrossRef] [Green Version]
- George, F.; Daniel, C.; Thomas, M.; Singer, E.; Guilbaud, A.; Tessier, F.J.; Revol-Junelles, A.M.; Borges, F.; Foligné, B. Occurrence and dynamism of lactic acid bacteria in distinct ecological niches: A multifaceted functional health perspective. Front. Microbiol. 2018, 9, 2899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van De Water, J.; Naiyanetr, P. Yoghurt and Immunity: The Health Benefits of Fermented Milk Products that Contain Lactic Acid Bacteria. In Handbook of Fermented Functional Foods; Farnworth, E., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 71–88. ISBN 978-1-4200-5326-5. [Google Scholar]
- Kabak, B.; Dobson, A.D.W. An introduction to the traditional fermented foods and beverages of Turkey. Crit. Rev. Food Sci. Nutr. 2011, 51, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.C.; Montet, D. Microorganisms and Fermentation of Traditional Foods; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9781482223095. [Google Scholar]
- von Gastrow, L.; Madec, M.N.; Chuat, V.; Lubac, S.; Morinière, C.; Lé, S.; Santoni, S.; Sicard, D.; Valence, F. Microbial diversity associated with gwell, a traditional french mesophilic fermented milk inoculated with a natural starter. Microorganisms 2020, 8, 982. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Zhang, T.; Li, J.; Zhou, Y.; Li, H.; Yu, J. Comparison of backslopping and two-stage fermentation methods for koumiss powder production based on chemical composition and nutritional properties. J. Sci. Food Agric. 2020, 100, 1822–1826. [Google Scholar] [CrossRef]
- World Health Organization; Food and Agriculture Organization of the United Nations. Codex Alimentarus: Milk and Milk Products; World Health Organization: Geneva, Switzerland; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011; ISBN 9789251058374. [Google Scholar]
- Willet, W.C.; Ludwig, D.S. Milk and health. N. Engl. J. Med. 2020, 382, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gallego, C.; Gueimonde, M.; Salminen, S. The role of yogurt in food-based dietary guidelines. Nutr. Rev. 2018, 76, 29–39. [Google Scholar] [CrossRef]
- Sarkar, S. Potentiality of probiotic yoghurt as a functional food—A review. Nutr. Food Sci. 2019, 49, 182–202. [Google Scholar] [CrossRef]
- Korhonen, H. Milk-derived bioactive peptides: From science to applications. J. Funct. Foods 2009, 1, 177–187. [Google Scholar] [CrossRef]
- Fernandez, M.A.; Picard-Deland, E.; Le Barz, M.; Daniel, L.; Marette, A. Yoghurt and Health. In Fermented Foods in Health and Disease Prevention; Frias, J., Martinez-Villaluenga, C., Penas, E., Eds.; Academic Press: London, UK, 2017; pp. 305–327. ISBN 978-0-12-802309-9. [Google Scholar]
- den Hartigh, L.J. Conjugated linoleic acid effects on cancer, obesity, and atherosclerosis: A review of pre-clinical and human trials with current perspectives. Nutrients 2018, 11, 370. [Google Scholar] [CrossRef] [Green Version]
- Thavasuraj, S.; Nithya, V. Evaluation on efficacy of fatty acids and Conjugated Linoleic Acid [CLA] derived from indegenous cow milk against hepato cellular carcinoma: A key and novel approach. Biochem. Cell. Arch. 2020, 20, 3253–3257. [Google Scholar]
- Thavasuraj, S.; Nithya, V.; Vinotha, M.; Chinniah, S.; Archunan, G. Assessment of dosage response of cow milk fat isomers against hepatocellular carcinoma cell lines: A key to novel approach for anticancer. Biochem. Cell. Arch. 2020, 20, 3223–3227. [Google Scholar]
- Abd El-Gawad, A.M.; Abo El-Hassan, D.G.; Aboul-Enein, A.M.; Abdelgayed, S.S.; Aly, S.A.; Esmat, G.; Mostafa, A.A.; Bakr, M.H.; Ali, R.A.; Ayoub, M.A. Anticancer activity of milk fat rich in conjugated linoleic acid against Ehrlich ascites carcinoma cells in female Swiss albino mice. Vet. World 2021, 14, 696–708. [Google Scholar] [CrossRef]
- Kousgaard, S.J.; Nielsen, H.L.; Kirk, K.F.; Thorlacius-Ussing, O. Consumption of yoghurt favours remission after faecal microbiota transplantation for chronic pouchitis. Int. J. Colorectal Dis. 2020, 35, 1955–1958. [Google Scholar] [CrossRef]
- Panel, E.; Nda, A.; Golly, I. Scientific Opinion on the substantiation of health claims related to live yoghurt cultures and improved lactose digestion (ID 1143, 2976) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1763. [Google Scholar] [CrossRef]
- Barengolts, E.; Smith, E.D.; Reutrakul, S.; Tonucci, L.; Anothaisintawee, T. The effect of probiotic yogurt on glycemic control in type 2 diabetes or obesity: A meta-analysis of nine randomized controlled trials. Nutrients 2019, 11, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, C.Y.; Li, Z.M.; Han, B.; Zhang, Z.Y.; Chen, H.L.; Zhang, S.L.; Xu, J.Q.; Mao, Y.Q.; Zhao, Y.P.; Wang, L.S. Diet Consisting of Balanced Yogurt, Fruit, and Vegetables Modifies the Gut Microbiota and Protects Mice against Nonalcoholic Fatty Liver Disease. Mol. Nutr. Food Res. 2019, 63, 1900249. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, C.; Li, R.; Liu, K.; Jin, G.; Ge, R.; Tang, F.; Cui, S. High-altitude Tibetan fermented milk ameliorated cognitive dysfunction by modified gut microbiota in Alzheimer’s disease transgenic mice. Food Funct. 2020, 11, 5308–5319. [Google Scholar] [CrossRef]
- Guo, X.; Long, R.; Kreuzer, M.; Ding, L.; Shang, Z.; Zhang, Y.; Yang, Y.; Cui, G. Importance of Functional Ingredients in Yak Milk-Derived Food on Health of Tibetan Nomads Living Under High-Altitude Stress: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Mikhailidis, D.P.; Sattar, N.; Howard, G.; Graham, I.; Banach, M. Consumption of dairy product and its association with total and cause specific mortality—A population-based cohort study and meta-analysis. Clin. Nutr. 2019, 38, 2833–2845. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Astrup, A.; Lovegrove, J.A.; Gijsbers, L.; Givens, D.I.; Soedamah-Muthu, S.S. Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: Dose–response meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2017, 32, 269–287. [Google Scholar] [CrossRef] [Green Version]
- Farvid, M.S.; Malekshah, A.F.; Pourshams, A.; Poustchi, H.; Sepanlou, S.G.; Sharafkhah, M.; Khoshnia, M.; Farvid, M.; Abnet, C.C.; Kamangar, F.; et al. Dairy food intake and all-cause, cardiovascular disease, and cancer mortality: The Golestan Cohort Study. Am. J. Epidemiol. 2017, 185, 697–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, D.D.; Dias, M.M.S.; Grześkowiak, Ł.M.; Reis, S.A.; Conceição, L.L.; Peluzio, M.D.C.G. Milk kefir: Nutritional, microbiological and health benefits. Nutr. Res. Rev. 2017, 30, 82–96. [Google Scholar] [CrossRef]
- Azizi, N.F.; Kumar, M.R.; Yeap, S.K.; Abdullah, J.O.; Khalid, M.; Omar, A.R.; Osman, M.A.; Mortadza, S.A.S.; Alitheen, N.B. Kefir and its biological activities. Foods 2021, 10, 1210. [Google Scholar] [CrossRef]
- Vinderola, C.G.; Duarte, J.; Thangavel, D.; Perdigón, G.; Farnworth, E.; Matar, C. Immunomodulating capacity of kefir. J. Dairy Res. 2005, 72, 195–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bengoa, A.A.; Iraporda, C.; Garrote, G.L.; Abraham, A.G. Kefir micro-organisms: Their role in grain assembly and health properties of fermented milk. J. Appl. Microbiol. 2019, 126, 686–700. [Google Scholar] [CrossRef] [Green Version]
- Kesenkas, H.; Gursoy, O.; Ozbas, H. Kefir. In Fermented Foods in Health and Disease Prevention; Frias, J., Martinez-Villaluenga, C., Penas, E., Eds.; Academic Press: London, UK, 2017; pp. 339–353. ISBN 978-0-12-802309-9. [Google Scholar]
- Talib, N.; Mohamad, N.E.; Yeap, S.K.; Hussin, Y.; Mubin Aziz, M.N.; Masarudin, M.J.; Sharifuddin, S.A.; Hui, Y.W.; Ho, C.L.; Alitheen, N.B. Isolation and characterization of Lactobacillus spp. From kefir samples in Malaysia. Molecules 2019, 24, 2606. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, K.L.; Gaudino Caputo, L.R.; Tavares Carvalho, J.C.; Evangelista, J.; Schneedorf, J.M. Antimicrobial and healing activity of kefir and kefiran extract. Int. J. Antimicrob. Agents 2005, 25, 404–408. [Google Scholar] [CrossRef]
- Rosa, D.D.; Grześkowiak, L.M.; Ferreira, C.L.L.F.; Fonseca, A.C.M.; Reis, S.A.; Dias, M.M.; Siqueira, N.P.; Silva, L.L.; Neves, C.A.; Oliveira, L.L.; et al. Kefir reduces insulin resistance and inflammatory cytokine expression in an animal model of metabolic syndrome. Food Funct. 2016, 7, 3390–3401. [Google Scholar] [CrossRef]
- Bellikci-Koyu, E.; Sarer-Yurekli, B.P.; Akyon, Y.; Aydin-kose, F.; Karagozlu, C.; Ozgen, A.G.; Brikmann, A.; Nitsche, A.; Ergunay, K.; Yilmaz, E.; et al. Effects of regular kefir consumption on gut microbiota in patients with metabolic syndrome: A parallel-group, randomized, controlled study. Nutrients 2019, 11, 2089. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Kim, H.; Jeong, D.; Kang, I.B.; Chon, J.W.; Kim, H.S.; Song, K.Y.; Seo, K.H. Kefir alleviates obesity and hepatic steatosis in high-fat diet-fed mice by modulation of gut microbiota and mycobiota: Targeted and untargeted community analysis with correlation of biomarkers. J. Nutr. Biochem. 2017, 44, 35–43. [Google Scholar] [CrossRef]
- Veiga, F.M.S.; Graus-Nunes, F.; Rachid, T.L.; Barreto, A.B.; Mandarim-de-Lacerda, C.A.; Souza-Mello, V. Anti-obesogenic effects of WY14643 (PPAR-alpha agonist): Hepatic mitochondrial enhancement and suppressed lipogenic pathway in diet-induced obese mice. Biochimie 2017, 140, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.; Moridnia, A.; Mortazavi, D.; Salehi, M.; Bagheri, M.; Sheikhi, A. Kefir A powerful probiotics with anticancer properties. Med. Oncol. 2017, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rafie, N.; Hamedani, S.G.; Ghiasv, R.; Miraghajani, M. Kefir and cancer: A systematic review of literatures. Arch. Iran. Med. 2015, 18, 852–857. [Google Scholar]
- Miller, I. The gut–brain axis: Historical reflections. Microb. Ecol. Health Dis. 2018, 29, 1542921. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Yun, M.; Oh, Y.J.; Choi, H.J. Mind-altering with the gut: Modulation of the gut-brain axis with probiotics. J. Microbiol. 2018, 56, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Özcan, H.; Oskay, Ü.; Bodur, A.F. Effects of Kefir on Quality of Life and Sleep Disturbances in Postmenopausal Women. Holist. Nurs. Pract. 2019, 33, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jeong, D.; Kim, H.; Seo, K.H. Modern perspectives on the health benefits of kefir in next generation sequencing era: Improvement of the host gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 1782–1793. [Google Scholar] [CrossRef]
- Behera, J.; Ison, J.; Tyagi, S.C.; Tyagi, N. The role of gut microbiota in bone homeostasis. Bone 2020, 135, 115317. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.; Han, K.; Chang, G.R.; Lai, G.; Chang, K.Y.; Lai, J.C.; Lai, C.Y.; Chen, H.L.; Chen, C.M. Kefir Peptides Prevent Estrogen Deficiency-Induced Bone Loss and Modulate the Structure of the Gut Microbiota in Ovariectomized Mice. Nutrients 2020, 12, 3432. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.Y.; Chen, H.L.; Tung, Y.T.; Kao, C.C.; Hu, F.C.; Chen, C.M. Short-Term Effects of Kefir-Fermented Milk Consumption on Bone Mineral Density and Bone Metabolism in a Randomized Clinical Trial of Osteoporotic Patients. PLoS ONE 2015, 10, e0144231. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Tung, Y.T.; Chuang, C.H.; Tu, M.Y.; Tsai, T.C.; Chang, S.Y.; Chen, C.M. Kefir improves bone mass and microarchitecture in an ovariectomized rat model of postmenopausal osteoporosis. Osteoporos. Int. 2015, 26, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.L.; Costabile, A.; Wilbey, R.A.; Grandison, A.S.; Duarte, L.C.; Roseiro, L.B. In vitro evaluation of the fermentation properties and potential prebiotic activity of caprine cheese whey oligosaccharides in batch culture systems. BioFactors 2012, 38, 440–449. [Google Scholar] [CrossRef]
- Vera, C.; Illanes, A. Lactose-Derived Nondigestible Oligosaccharides and Other High Added-Value Products. In Lactose: Structure, Food Industry Applications and Role in Disorders; Illanes, A., Guerrero, C., Vera, C., Wilson, L., Conejeros, R., Scott, F., Eds.; Academic press: London, UK, 2016; pp. 87–105. ISBN 978-0-12-802724-0. [Google Scholar]
- McSweneey, P.L.H. Biochemistry of cheese ripening. Int. J. Dairy Technol. 2004, 57, 127–144. [Google Scholar] [CrossRef]
- Santiago-López, L.; Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Liceaga, A.M.; González-Córdova, A.F. Invited review: Bioactive compounds produced during cheese ripening and health effects associated with aged cheese consumption. J. Dairy Sci. 2018, 101, 3742–3757. [Google Scholar] [CrossRef] [Green Version]
- Bottari, B.; Levante, A.; Bancalari, E.; Sforza, S.; Bottesini, C.; Prandi, B.; De Filippis, F.; Ercolini, D.; Nocetti, M.; Gatti, M. The Interrelationship Between Microbiota and Peptides During Ripening as a Driver for Parmigiano Reggiano Cheese Quality. Front. Microbiol. 2020, 11, 581658. [Google Scholar] [CrossRef]
- Trieu, K.; Bhat, S.; Dai, Z.; Leander, K.; Gigante, B.; Qian, F.; Korat, A.V.A.; Sun, Q.; Pan, X.-F.; Laguzzi, F.; et al. Biomarkers of dairy fat intake, incident cardiovascular disease, and all-cause mortality: A cohort study, systematic review, and meta-analysis. PLoS Med. 2021, 18, e1003763. [Google Scholar] [CrossRef]
- Daliri, E.B.M.; Lee, B.H.; Park, B.J.; Kim, S.H.; Oh, D.H. Antihypertensive peptides from whey proteins fermented by lactic acid bacteria. Food Sci. Biotechnol. 2018, 27, 1781–1789. [Google Scholar] [CrossRef]
- Fekete, Á.A.; Givens, D.I.; Lovegrove, J.A. Casein-derived lactotripeptides reduce systolic and diastolic blood pressure in a meta-analysis of randomised clinical trials. Nutrients 2015, 7, 659–681. [Google Scholar] [CrossRef] [Green Version]
- Crippa, G.; Zabzuni, D.; Bravi, E.; Piva, G.; De Noni, I.; Bighi, E.; Rossi, F. Randomized, double blind placebo-controlled pilot study of the antihypertensive effects of Grana Padano, D.O.P. cheese consumption in mild-Moderate hypertensive subjects. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7573–7581. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moyano, S.; Gonçalves dos Santos, M.T.P.; Galván, A.I.; Merchán, A.V.; González, E.; de Guía Córdoba, M.; Benito, M.J. Screening of autochthonous lactic acid bacteria strains from artisanal soft cheese: Probiotic characteristics and prebiotic metabolism. LWT 2019, 114, 108388. [Google Scholar] [CrossRef]
- Arshad, F.-A.; Mehmood, R.; Hussain, S.; Annus Khan, M.; Khan, M.S. Lactobacilli as Probiotics and their Isolation from Different Sources. Br. J. Res. 2018, 5. [Google Scholar] [CrossRef]
- Bajic, S.S.; Djokic, J.; Dinic, M.; Veljovic, K.; Golic, N.; Mihajlovic, S.; Tolinacki, M. GABA-producing natural dairy isolate from artisanal zlatar cheese attenuates gut inflammation and strengthens gut epithelial barrier in vitro. Front. Microbiol. 2019, 10, 527. [Google Scholar] [CrossRef] [PubMed]
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain-Raspaud, S.; Trotin, B.; Naliboff, B.; et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013, 144, 1394–1401.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boricha, A.A.; Shekh, S.L.; Pithva, S.P.; Ambalam, P.S.; Manuel Vyas, B.R. In vitro evaluation of probiotic properties of Lactobacillus species of food and human origin. LWT 2019, 106, 201–208. [Google Scholar] [CrossRef]
- Mancini, A.; Carafa, I.; Franciosi, E.; Nardin, T.; Bottari, B.; Larcher, R.; Tuohy, K.M. In vitro probiotic characterization of high GABA producing strain Lactobacilluas brevis DSM 32386 isolated from traditional “wild” Alpine cheese. Ann. Microbiol. 2019, 69, 1435–1443. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Martini, S.; Solieri, L. Bioprospecting for bioactive peptide production by lactic acid bacteria isolated from fermented dairy food. Fermentation 2019, 5, 96. [Google Scholar] [CrossRef] [Green Version]
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health benefits of lactic acid bacteria (Lab) fermentates. Nutrients 2020, 12, 1679. [Google Scholar] [CrossRef]
- Linares, D.M.; Gómez, C.; Renes, E.; Fresno, J.M.; Tornadijo, M.E.; Ross, R.P.; Stanton, C. Lactic Acid Bacteria and Bifidobacteria with Potential to Design Natural Biofunctional Health-Promoting Dairy Foods. Front. Microbiol. 2017, 8, 846. [Google Scholar] [CrossRef]
- García-Burgos, M.; Moreno-Fernández, J.; Alférez, M.J.M.; Díaz-Castro, J.; López-Aliaga, I. New perspectives in fermented dairy products and their health relevance. J. Funct. Foods 2020, 72, 104059. [Google Scholar] [CrossRef]
- Liu, D.; Tong, C. Bacterial community diversity of traditional fermented vegetables in China. LWT-Food Sci. Technol. 2017, 86, 40–48. [Google Scholar] [CrossRef]
- Lorn, D.; Nguyen, T.K.C.; Ho, P.H.; Tan, R.; Licandro, H.; Waché, Y. Screening of lactic acid bacteria for their potential use as aromatic starters in fermented vegetables. Int. J. Food Microbiol. 2021, 350, 109242. [Google Scholar] [CrossRef]
- Varzakas, T.; Zakynthinos, G.; Proestos, C.; Radwanska, M. Fermented Vegetables. In Minimally Processed Refrigerated Fruits and Vegetables; Yildiz, F., Wiliey, R., Eds.; Springer Science: New York, NY, USA, 2017; pp. 537–584. ISBN 978-1-4939-7018-6. [Google Scholar]
- Bousquet, J.; Anto, J.M.; Czarlewski, W.; Haahtela, T.; Fonseca, S.C.; Iaccarino, G.; Blain, H.; Vidal, A.; Sheikh, A.; Akdis, C.A.; et al. Cabbage and fermented vegetables: From death rate heterogeneity in countries to candidates for mitigation strategies of severe COVID-19. Allergy Eur. J. Allergy Clin. Immunol. 2021, 76, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Peñas, E.; Martinez-Villaluenga, C.; Frias, J. Sauerkraut: Production, Composition, and Health benefits. In Fermented Foods in Health and Disease Prevention; Frias, J., Martinez-Villaluenga, C., Peñas, E., Eds.; Academic Press: London, UK, 2017; pp. 557–570. [Google Scholar]
- He, G.Q.; Liu, T.J.; Sadiq, F.A.; Gu, J.S.; Zhang, G.H. Insights into the microbial diversity and community dynamics of Chinese traditional fermented foods from using high-throughput sequencing approaches. J. Zhejiang Univ. Sci. B 2017, 18, 289–302. [Google Scholar] [CrossRef]
- Cai, W.; Tang, F.; Wang, Y.; Zhang, Z.; Xue, Y.; Zhao, X.; Guo, Z.; Shan, C. Bacterial diversity and flavor profile of Zha-Chili, a traditional fermented food in China. Food Res. Int. 2021, 141, 110112. [Google Scholar] [CrossRef]
- Mallappa, R.H.; Balasubramaniam, C.; Nataraj, B.H.; Ramesh, C.; Kadyan, S.; Pradhan, D.; Muniyappa, S.K.; Grover, S. Microbial diversity and functionality of traditional fermented milk products of India: Current scenario and future perspectives. Int. Dairy J. 2021, 114, 104941. [Google Scholar] [CrossRef]
- Orgeron, R.P., II; Corbin, A.; Scott, B. Sauerkraut: A Probiotic Superfood. Funct. Foods Health Dis. 2016, 6, 536. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, E.S.; Garnås, E.; Jensen, K.J.; Hansen, L.H.; Olsen, P.S.; Ritz, C.; Krych, L.; Nielsen, D.S. Lacto-fermented sauerkraut improves symptoms in IBS patients independent of product pasteurisation-a pilot study. Food Funct. 2018, 9, 5323–5335. [Google Scholar] [CrossRef]
- Peñas, E.; Martinez-Villaluenga, C.; Frias, J.; Sánchez-Martínez, M.J.; Pérez-Corona, M.T.; Madrid, Y.; Cámara, C.; Vidal-Valverde, C. Se improves indole glucosinolate hydrolysis products content, Se-methylselenocysteine content, antioxidant capacity and potential anti-inflammatory properties of sauerkraut. Food Chem. 2012, 132, 907–914. [Google Scholar] [CrossRef] [Green Version]
- Sawicka, H. Glucosinolates As Natural Plant Substances-Structural and Application Aspects: A Review. J. Cell Tissue Res. 2020, 20, 6919–6928. [Google Scholar]
- Raak, C.; Ostermann, T.; Boehm, K.; Molsberger, F. Regular Consumption of Sauerkraut and Its Effect on Human Health: A Bibliometric Analysis. Glob. Adv. Health Med. 2014, 3, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Stiemsma, L.T.; Nakamura, R.E.; Nguyen, J.G.; Michels, K.B. Does Consumption of Fermented Foods Modify the Human Gut Microbiota? J. Nutr. 2020, 150, 1680–1692. [Google Scholar] [CrossRef]
- Liu, L.; Du, P.; Zhang, G.; Mao, X.; Zhao, Y.; Wang, J.; Duan, C.; Li, C.; Li, X. Residual nitrite and biogenic amines of traditional northeast sauerkraut in China. Int. J. Food Prop. 2017, 20, 2448–2455. [Google Scholar] [CrossRef] [Green Version]
- Park, S.E.; Kwon, S.J.; Cho, K.M.; Seo, S.H.; Kim, E.J.; Unno, T.; Bok, S.H.; Park, D.H.; Son, H.S. Intervention with kimchi microbial community ameliorates obesity by regulating gut microbiota. J. Microbiol. 2020, 58, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Lee, W.H.; Seo, H.; Oh, J.Y.; Lee, D.Y.; Kim, S.J.; Hahm, K.B. Microbiota changes with fermented kimchi contributed to either the amelioration or rejuvenation of helicobacter pylori-associated chronic atrophic gastritis. J. Clin. Biochem. Nutr. 2021, 69, 96–110. [Google Scholar] [CrossRef]
- Park, J.M.; Han, Y.M.; Oh, J.Y.; Lee, D.Y.; Choi, S.H.; Kim, S.J.; Hahm, K.B. Fermented kimchi rejuvenated precancerous atrophic gastritis via mitigating Helicobacter pylori-associated endoplasmic reticulum and oxidative stress. J. Clin. Biochem. Nutr. 2021, 69, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.H.; Noh, J.S.; Han, J.S.; Kim, H.J.; Han, E.S.; Song, Y.O. Kimchi, a Fermented Vegetable, improves serum lipid profiles in healthy young adults: Randomized clinical trial. J. Med. Food 2013, 16, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Sekhon, S.S.; Ko, J.H.; Kim, H.C.; Kim, S.Y.; Won, K.; Ahn, J.Y.; Lee, K.; Kim, Y.H. Lactic acid bacteria isolated from kimchi to evaluate anti-obesity effect in high fat diet-induced obese mice. Toxicol. Environ. Health Sci. 2018, 10, 11–16. [Google Scholar] [CrossRef]
- An, S.Y.; Lee, M.S.; Jeon, J.Y.; Ha, E.S.; Kim, T.H.; Yoon, J.Y.; Ok, C.O.; Lee, H.K.; Hwang, W.S.; Choe, S.J.; et al. Beneficial effects of fresh and fermented kimchi in prediabetic individuals. Ann. Nutr. Metab. 2013, 63, 111–119. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Prasanth, M.I.; Chaiyasut, C. A mini review on antidiabetic properties of fermented foods. Nutrients 2018, 10, 1973. [Google Scholar] [CrossRef] [Green Version]
- Henry, C.J. Functional foods. Eur. J. Clin. Nutr. 2010, 64, 657–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.M.; Park, J.M.; Jeong, M.; Yoo, J.H.; Kim, W.H.; Shin, S.P.; Ko, W.J.; Hahm, K.B. Dietary, non-microbial intervention to prevent Helicobacter pylori-associated gastric diseases. Ann. Transl. Med. 2015, 3, 122. [Google Scholar] [CrossRef]
- Han, Y.M.; Kang, E.A.; Park, J.M.; Oh, J.Y.; Lee, D.Y.; Choi, S.H.; Hahm, K.B. Dietary intake of fermented kimchi prevented colitis-associated cancer. J. Clin. Biochem. Nutr. 2020, 67, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Park, K.Y. Clinical trials of kimchi intakes on the regulation of metabolic parameters and colon health in healthy Korean young adults. J. Funct. Foods 2018, 47, 325–333. [Google Scholar] [CrossRef]
- Seong, G.; Lee, S.; Min, Y.W.; Jang, Y.S.; Kim, H.S.; Kim, E.J.; Park, S.Y.; Kim, C.H.; Chang, D.K. Effect of heat-killed lactobacillus casei dkgf7 on a rat model of irritable bowel syndrome. Nutrients 2021, 13, 568. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.G.; Min, Y.W.; Lee, C.; Hong, S.N.; Won, J.Y.; Jang, J.A.; Kim, C.H.; Chang, D.K. Effects of Novel Probiotics in a Murine Model of Irritable Bowel Syndrome. Korean J. Gastroenterol. 2020, 75, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Graudal, N.; Jürgens, G. Conflicting Evidence on Health Effects Associated with Salt Reduction Calls for a Redesign of the Salt Dietary Guidelines. Prog. Cardiovasc. Dis. 2018, 61, 20–26. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef]
| Families | Genera Frequently Found in Foods | CO2 from Glucose | Growth at 10 | Growth at 45 | Growth in 6.5% NaCl | Growth in 18% NaCl | Growth at pH 4.4 | Growth at pH 9.6 | |
|---|---|---|---|---|---|---|---|---|---|
| Carnobacteriaceae | Rods | Carnobacterium | - | + | - | - | - | - | - |
| Enterococcaceae | Cocci | Enterococcus | - | + | + | + | - | + | + |
| Tetragenococcus | |||||||||
| Streptococcaceae | Streptococcus | - | + | - | + | + | - | + | |
| Lactococcus | - | - | +/- * | - | - | - | - | ||
| Lactobacillaceae | Cocci | Leuconostoc | + | + | - | +/- * | - | +/- * | - |
| Oenococcus | + | + | - | +/- * | - | +/- * | - | ||
| Pediococcus | - | + | +/- * | +/- * | - | + | - | ||
| Rods | Lactobacillus | +/- * | +/- * | +/- * | +/- * | - | +/- * | - | |
| Lacticaseibacillus | - | + | +/- * | +/- * | - | + | - | ||
| Lactiplantibacillus | - | + | +/- * | + | - | + | - | ||
| Furfurilactibacillus | + | + | - | + | +/- * | + | - | ||
| Fructilactibacillus | + | - | + | + | - | + | - | ||
| Levilactobacillus | + | + | - | + | +/- * | + | - | ||
| Limosilactobacillus | + | - | +/- * | +/- * | +/- * | + | +/- * | ||
| Latilactobacillus | - | +/- * | +/- * | + | +/- * | + | - | ||
| Lentilactobacillus | + | + | + | + | - | + | +/- * | ||
| Weissella | + | + | - | +/- * | - | +/- * | - |
| Health Effects | Specific Effects | Fermented Food | Microorganisms | Reference |
|---|---|---|---|---|
| Reduce initiation and progression of cronic disease: | food ingredients, including living microbial cells | Lactobacillus and Lactococcus genera | [99] | |
| Musculoskeletal disorders | ||||
| Cardiovascular diseases | ||||
| Mental health pathologies | ||||
| Type 2 diabetes | ||||
| Production of Bioactive peptides: | Milk-derived foods (Fermented milks, Cheese, yoghurt, kefir) | Lactobacillus and Lactococcus genera | [99] | |
| Satiety regulation | ||||
| Antimicrobial | ||||
| Anti-carcinogenic | ||||
| Anti-thrombotic | ||||
| Mineral absorption | ||||
| Hypotensive | ||||
| Anti-inflammatory | ||||
| Stress relief | ||||
| Aids relaxation and sleep | ||||
| Reduces symptoms of psoriasis | ||||
| ACE-inhibitors | ||||
| Amelioration of glucose metabolism | LAB-fermented foods, especially fermented milks | GRAS Lactic acid bacteria | [100] | |
| Amelioration of glucose intollerance symptoms | LAB-fermented foods, especially fermented milks | GRAS Lactic acid bacteria | [100] | |
| Reduce severity of infections | LAB-fermented foods, especially fermented milks | GRAS Lactic acid bacteria | [100] | |
| Reduce burden of IBS | LAB-fermented foods, especially fermented milks | GRAS Lactic acid bacteria | [100] | |
| Anti-anxiety effect | LAB-fermented foods, especially fermented milks | GRAS Lactic acid bacteria | [100] | |
| Reduction of serum cholesterol level | [101] | |||
| Production of B’s group vitamines | Fermented milks, Yoghurts, Fermented Soymilk, Kefir | L. casei, Bifidobacterium infantis, L. plantarum... | [101] | |
| Production of GABA | [101] | |||
| Antidiabetic, blood pressure | Fermented milk, Fermented soy milk, Yoghurt | L. casei Shirota, S. salivarius, L. plantarum, L. brevis | ||
| Production of conjugated linoleic acid | [101] | |||
| Cholesterol lowering | Cheddar cheese, Buffalo cheese, Fermented buffalo milk, Yoghurt | L. lactis, L. rhamnosus, S. thermophilus, B. bifidum | [101] | |
| Exopolysaccharides production | [101] | |||
| Immunostimulatory | Yoghurt, Cheddar cheese, Turkish cheese, Kefir, Fermented ice-cream | L. bulgaricus, L. mucosae, P. freudenreichii, L. lactis, B. longum | ||
| Hypocholesterolemic | ||||
| Microbiota modulation | ||||
| Immune modulation | ||||
| Bacteriocines production | Camembert/Semihard cheese, Cheddar, Yoghurt, Munster cheese | L. lactis, L. acidophilus, P. acidilactici | [101] | |
| Alleviate constipation | Yoghurt | B. animalis subsp lactis DN-173010, L. casei subsp Shirota | [102] | |
| Reduce eczemas | fermented milk | [102] | ||
| Antibiotic-associated diarrhea | Fermented drink, yoghurt | Lactobacillus casei DN-114001 | [102] | |
| Prevention of pediatric diarrhea | Fermented drink, yoghurt | Lactobacillus casei DN-114002 | [102] | |
| Prevention and help healing from respiratory infections | Fermented drink, yoghurt | Lactobacillus casei DN-114003 | [102] | |
| Fights infections | [102] | |||
| H. pylori infection | Fermented oat gruel in fruit drink | L. plantarum 299v (DSM9843) | ||
| Clostridium difficile infection | Fermented drink | L. acidophilus CL1285 + L. casei Lbc80r + L. rhamnosus CLR2 | ||
| Improves microbiota | Yoghurt | L. acidophilus + B. animalis subsp lactis | [102] | |
| Health Effect | Specific Effect | Fermented Food | Microorganisms | References |
|---|---|---|---|---|
| Antioxidant | ||||
| Carotenoids modified by fermentation | Kimchi and Sauerkraut, Soybean, | W. koreensis, L. brevis, Leu. gelidum | [132] | |
| Reduction of cronic diseases | Tomato Juice, Leek, Carrots, | Leu. mesenteroides, L. plantarum, W. Confusa, | ||
| Cardiovascular disease | Fennels, Onions, Pomegranate | L. delbrueckii subsp. lactis, B. thermophilum | [132] | |
| Cancer | Pear juice, Pineapple juice, Apple, | |||
| Diabetes | Quince, Grape, Kiwifruit | |||
| Alzheimer | ||||
| Cataracts | ||||
| Age-related functional declines | ||||
| Hypoglycemic | [132] | |||
| Anti-inflammatory | [132] | |||
| Hypolipidemic | [132] | |||
| Immunomodulatory | ||||
| Anti-microbic | Lactobacillus and Lactococcus genera | [132,133] | ||
| Eliminate H. pylori | [133] | |||
| Reduction of anti-nutritional compounds | Fermented legumes and cereals | Lb. plantarum and other LAB | ||
| Increase of nutritional density | Every fermented vegetable | Generic LAB | ||
| Breakdown of complex carbohydrates | ||||
| proteolysis | ||||
| Glucosinolates breakdown | [132] | |||
| Increase antioxidant activity | Brassica vegetables | Generic LAB | ||
| Ameliorate metabolic syndrome | Brassica vegetables | Generic LAB | ||
| Production of SCFA | Every fermented vegetable | Generic LAB | [132] | |
| Anti obesogenic effect | Every fermented vegetable | Generic LAB | ||
| Lower obesity incidence | ||||
| Direct anti-obesogenic effect | ||||
| Prebiotic effect | Production of EPS | W.confusa and W. hellenica, Lactobacillus, Lactococcus, Leuconostoc, Pediococcus and Weissella, | [132] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellone, V.; Bancalari, E.; Rubert, J.; Gatti, M.; Neviani, E.; Bottari, B. Eating Fermented: Health Benefits of LAB-Fermented Foods. Foods 2021, 10, 2639. https://doi.org/10.3390/foods10112639
Castellone V, Bancalari E, Rubert J, Gatti M, Neviani E, Bottari B. Eating Fermented: Health Benefits of LAB-Fermented Foods. Foods. 2021; 10(11):2639. https://doi.org/10.3390/foods10112639
Chicago/Turabian StyleCastellone, Vincenzo, Elena Bancalari, Josep Rubert, Monica Gatti, Erasmo Neviani, and Benedetta Bottari. 2021. "Eating Fermented: Health Benefits of LAB-Fermented Foods" Foods 10, no. 11: 2639. https://doi.org/10.3390/foods10112639
APA StyleCastellone, V., Bancalari, E., Rubert, J., Gatti, M., Neviani, E., & Bottari, B. (2021). Eating Fermented: Health Benefits of LAB-Fermented Foods. Foods, 10(11), 2639. https://doi.org/10.3390/foods10112639






