Different Withering Times Affect Sensory Qualities, Chemical Components, and Nutritional Characteristics of Black Tea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Black Tea Processing
2.3. Sensory Analysis
2.4. Determination of the Main Components of Black Tea
2.5. Biological Activity Assay
2.6. Statistical Analysis
3. Results
3.1. Effect of Withering Time on Sensory Quality
3.2. Effect of Withering Time on Nonvolatile Compounds
3.3. Effect of Withering Time on the Volatile Compounds
3.4. Effect of Withering Time on Antioxidant Activity, on Inhibitory Capacity against α-amylase and α-glucosidase Activity, and on Inhibitory Capacity against Glucose Uptake in Caco-2 Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, J.; Yu, X.; He, C.; Qiu, A.; Li, Y.; Shu, Q.; Chen, Y.; Ni, D. Withering degree affects flavor and biological activity of black tea: A non-targeted metabolomics approach. LWT-Food Sci. Technol. 2020, 130, 109–535. [Google Scholar] [CrossRef]
- Xia, E.H.; Zhang, H.B.; Sheng, J.; Li, K.; Zhang, Q.J.; Kim, C.; Zhang, Y.; Liu, Y.; Zhu, T.; Li, W.; et al. The Tea Tree Genome Provides Insights into Tea Flavor and Independent Evolution of Caffeine Biosynthesis. Mol. Plant 2017, 10, 866–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gramza, A.; Korczak, J. Tea constituents (Camellia sinensis L.) as antioxidants in lipid systems. Trends Food Sci. Technol. 2005, 16, 351–358. [Google Scholar] [CrossRef]
- Higdon, J.V.; Frei, B. Tea Catechins and Polyphenols: Health Effects, Metabolism, and Antioxidant Functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef]
- Zheng, X.Q.; Li, Q.S.; Xiang, L.P.; Liang, Y.R. Recent advances in volatiles of teas. Molecules 2016, 21, 338. [Google Scholar] [CrossRef] [PubMed]
- Olmez, H.; Yilmaz, A. Changes in chemical constituents and polyphenol oxidase activity of tea leaves with shoot maturity and cold storage. J. Food Process. Preserv. 2010, 34, 653–665. [Google Scholar] [CrossRef]
- Obanda, M.; Owuor, P.O.; Mang’oka, R.; Kavoi, M.M. Changes in thearubigin fractions and theaflavin levels due to variations in processing conditions and their influence on black tea liquor brightness and total colour. Food Chem. 2004, 85, 163–173. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, P.C.; Liu, P.P.; Song, X.W.; Guo, F.; Li, Y.Y.; Ni, D.; Jiang, C. Novel insight into the role of withering process in characteristic flavor formation of teas using transcriptome analysis and metabolite profiling. Food Chem. 2019, 272, 313–322. [Google Scholar] [CrossRef]
- Deb, S.; Jolvis Pou, K.R. A review of withering in the processing of black tea. Biosyst. Eng. 2016, 41, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Qiu, F.F.; Yu, Z.; Ai, Z.; Chen, Y.; Liao, G.; Ni, D. The influence of withering temperature, humidity and wind speed on the quality of black tea. Chin. Tea 2014, 11, 22–25. [Google Scholar]
- Wu, H.; Huang, W.; Chen, Z.; Chen, Z.; Shi, J.; Kong, Q.; Sun, S.; Jiang, X.; Chen, D.; Yan, S. GC–MS-based metabolomic study reveals dynamic changes of chemical compositions during black tea processing. Food Res. Int. 2019, 120, 330–338. [Google Scholar] [CrossRef]
- Obanda, M.; Owuor, P.O.; Mang’oka, R. Changes in the chemical and sensory quality parameters of black tea due to variations of fermentation time and temperature. Food Chem. 2001, 75, 395–404. [Google Scholar] [CrossRef]
- Satoh, T.; Igarashi, M.; Yamada, S.; Takahashi, N.; Watanabe, K. Inhibitory effect of black tea and its combination with acarbose on small intestinal α-glucosidase activity. J. Ethnopharmacol. 2015, 161, 147–155. [Google Scholar] [CrossRef]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of Dietary Polyphenols on Carbohydrate Metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Gachhui, R.; Sil, P.C. Effect of Kombucha, a fermented black tea in attenuating oxidative stress mediated tissue damage in alloxan induced diabetic rats. Food Chem. Toxicol. 2013, 60, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Zeng, W.; Tong, X.; Feng, W.; Chen, Y.; Ni, D. The new insight into the influence of fermentation temperature on quality and bioactivities of black tea. LWT-Food Sci. Technol. 2020, 117, 108–646. [Google Scholar] [CrossRef]
- Qu, F.; Zhu, X.; Ai, Z.; Ai, Y.; Qiu, F.; Ni, D. Effect of different drying methods on the sensory quality and chemical components of black tea. LWT-Food Sci. Technol. 2019, 99, 112–118. [Google Scholar] [CrossRef]
- ISO. ISO14502-1:2005. Determination of Substances Characteristic of Green and Black Tea-Part1: Content of Total Polyphenols in Tea—Calorimetric Method Using Folin-Ciocalteu Reagent. 2005. Available online: https://www.iso.org/standard/31356.html (accessed on 20 March 2021).
- Wang, K.; Chen, Q.; Lin, Y.; Li, S.; Lin, H.; Huang, J.; Liu, Z. Comparison of phenolic compounds and taste of Chinese black tea. Food Sci. Technol. Res. 2014, 20, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Pe, X.; Liang, W.; Zhang, R. Comparison of total flavonoids in tangerine peel with 5 spectrophotometric methods. Lishizhen Med. Mater. Med. Res. 2018, 29, 574–576. [Google Scholar]
- Wang, L.; Xia, W. Study on the determination of tea polysaccharides by anthrone-sulfuric acid method. J. Food Sci. 2005, 26, 185–188. [Google Scholar]
- Yu, X.; Li, Y.; He, C.; Zhou, J.; Chen, Y.; Yu, Z.; Wang, P.; Ni, D. Nonvolatile metabolism in postharvest tea (Camellia sinensis L.) leaves: Effects of different withering treatments on nonvolatile metabolites, gene expression levels, and enzyme activity. Food Chem. 2020, 327, 126992. [Google Scholar] [CrossRef]
- Tanaka, T.; Kouno, I. Oxidation of tea catechins: Chemical structures and reaction mechanism. Food Sci. Technol. Res. 2003, 9, 128–133. [Google Scholar] [CrossRef] [Green Version]
- Dai, W.; Hu, Z.; Xie, D.; Tan, J.; Lin, Z. A novel spatial-resolution targeted metabolomics method in a single leaf of the tea plant (Camellia sinensis). Food Chem. 2019, 311, 126007. [Google Scholar] [CrossRef]
- Ye, Y.; Yan, J.; Cui, J.; Mao, S.; Li, M.; Liao, X.; Tong, H. Dynamic changes in amino acids, catechins, caffeine and gallic acid in green tea during withering. J. Food Compost. Anal. 2018, 66, 98–108. [Google Scholar] [CrossRef]
- Riemersma, R.A.; Rice-Evans, C.A.; Tyrrell, R.M.; Clifford, M.N.; Lean, M.E.J. Tea flavonoids and cardiovascular health. QJM-Int. J. Med. 2001, 94, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Wu, J.; Zhang, Y.; Chen, H.; Wang, Y. Physicochemical characterization of puerh tea polysaccharides and their antioxidant and α-glycosidase inhibition. J. Funct. Foods 2014, 6, 545–554. [Google Scholar] [CrossRef]
- Xiao, R.; Grinstaff, M.W. Chemical synthesis of polysaccharides and polysaccharide mimetics. Prog. Polym. Sci. 2017, 74, 78–116. [Google Scholar] [CrossRef]
- Tomlins, K.I.; Mashingaidze, A. Influence of withering, including leaf handling, on the manufacturing and quality of black teas—A review. Food Chem. 1997, 60, 573–580. [Google Scholar] [CrossRef]
- Bak, L.K.; Schousboe, A.; Waagepetersen, H.S. The glutamate/GABA-glutamine cycle: Aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 2006, 98, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Hu, S.; He, C.; Zhou, J.; Qu, F.; Ai, Z.; Chen, Y.; Ni, D. Chlorophyll Metabolism in Postharvest Tea (Camellia sinensis L.) Leaves: Variations in Color Values, Chlorophyll Derivatives, and Gene Expression Levels under Different Withering Treatments. J. Agric. Food Chem. 2019, 67, 10624–10636. [Google Scholar] [CrossRef]
- Sari, F.; Velioglu, Y.S. Changes in theanine and caffeine contents of black tea with different rolling methods and processing stages. Eur. Food Res. Technol. 2013, 237, 229–236. [Google Scholar] [CrossRef]
- Cheng, S.; Fu, X.; Wang, X.; Liao, Y.; Zeng, L.; Dong, F.; Yang, Z. Studies on the biochemical formation pathway of the amino acid l-theanine in tea (Camellia sinensis) and other plants. J. Agric. Food Chem. 2017, 65, 7210–7216. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, B.; Ye, Y.; Zhu, H. Methylglyoxal-hydroimidazolones (MG-Hs) instead of Nɛ-(carboxymethyl)-L-lysine (CML) is the major advanced glycation end-product during drying process in black tea. Food Chem. 2020, 333, 127499. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Engelhardt, U.H.; Thräne, C.; Maiwald, B.; Stark, J. Determination of flavonol glycosides in green tea, oolong tea and black tea by UHPLC compared to HPLC. Food Chem. 2015, 183, 30–35. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, Y.; Dai, W.; Lv, H.; Mu, B.; Li, P.; Tan, J.; Ni, D.; Lin, Z. Aroma formation and dynamic changes during white tea processing. Food Chem. 2019, 274, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Kosińska, A.; Andlauer, W. Antioxidant Capacity of Tea: Effect of Processing and Storage. In Processing and Impact on Antioxidants in Beverages; Preedy, V., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 109–120. [Google Scholar] [CrossRef]
- Yang, Z.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Wang, D.; Kurasawa, E.; Yamaguchi, Y.; Kubota, K.; Kobayashi, A. Analysis of glycosidically bound aroma precursors in tea leaves. 2. Changes in glycoside contents and glycosidase activities in tea leaves during the black tea manufacturing process. J. Agric. Food Chem. 2001, 49, 1900–1903. [Google Scholar] [CrossRef]
- Gui, J.; Fu, X.; Zhou, Y.; Katsuno, T.; Mei, X.; Deng, R.; Xu, X.; Zhang, L.; Dong, F.; Watanabe, N.; et al. Does enzymatic hydrolysis of glycosidically bound volatile compounds really contribute to the formation of volatile compounds during the oolong tea manufacturing process. J. Agric. Food Chem. 2015, 63, 6905–6914. [Google Scholar] [CrossRef]
- Shahidi, F.; Ho, C.T. Antioxidant measurement and applications: An overview. J. Am. Chem. Soc. 2007, 956, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Leung, L.K.; Su, Y.; Chen, R.; Zhang, Z.; Huang, Y.; Chen, Z.Y. Theaflavins in black tea and catechins in green tea are equally effective antioxidants. J. Nutr. 2001, 131, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Oyedemi, S.O.; Oyedemi, B.O.; Ijeh, I.I.; Ohanyerem, P.E.; Coopoosamy, R.M.; Aiyegoro, O.A. Alpha-Amylase Inhibition and Antioxidative Capacity of Some Antidiabetic Plants Used by the Traditional Healers in Southeastern Nigeria. Sci. World J. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, L.W.; Wong, L.L.; Loo, Y.Y.; Kasapis, S.; Huang, D. Evaluation of Different Teas against Starch Digestibility by Mammalian Glycosidases. J. Agric. Food Chem. 2010, 58, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.; Ai, Z.; Sandoval, D.M.; Suresh, R.; Ellis, P.R.; Yuqiong, C.; Sharp, P.A.; Butterworth, P.J.; Yu, Z.; Corpe, C.P. Inhibition of the facilitative sugar transporters (GLUTs) by tea extracts and catechins. FASEB J. 2020, 34, 9995–10010. [Google Scholar] [CrossRef]
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.-H.; Kruhlak, M.; Levine, M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2017, 21, 366–377. [Google Scholar] [CrossRef] [Green Version]
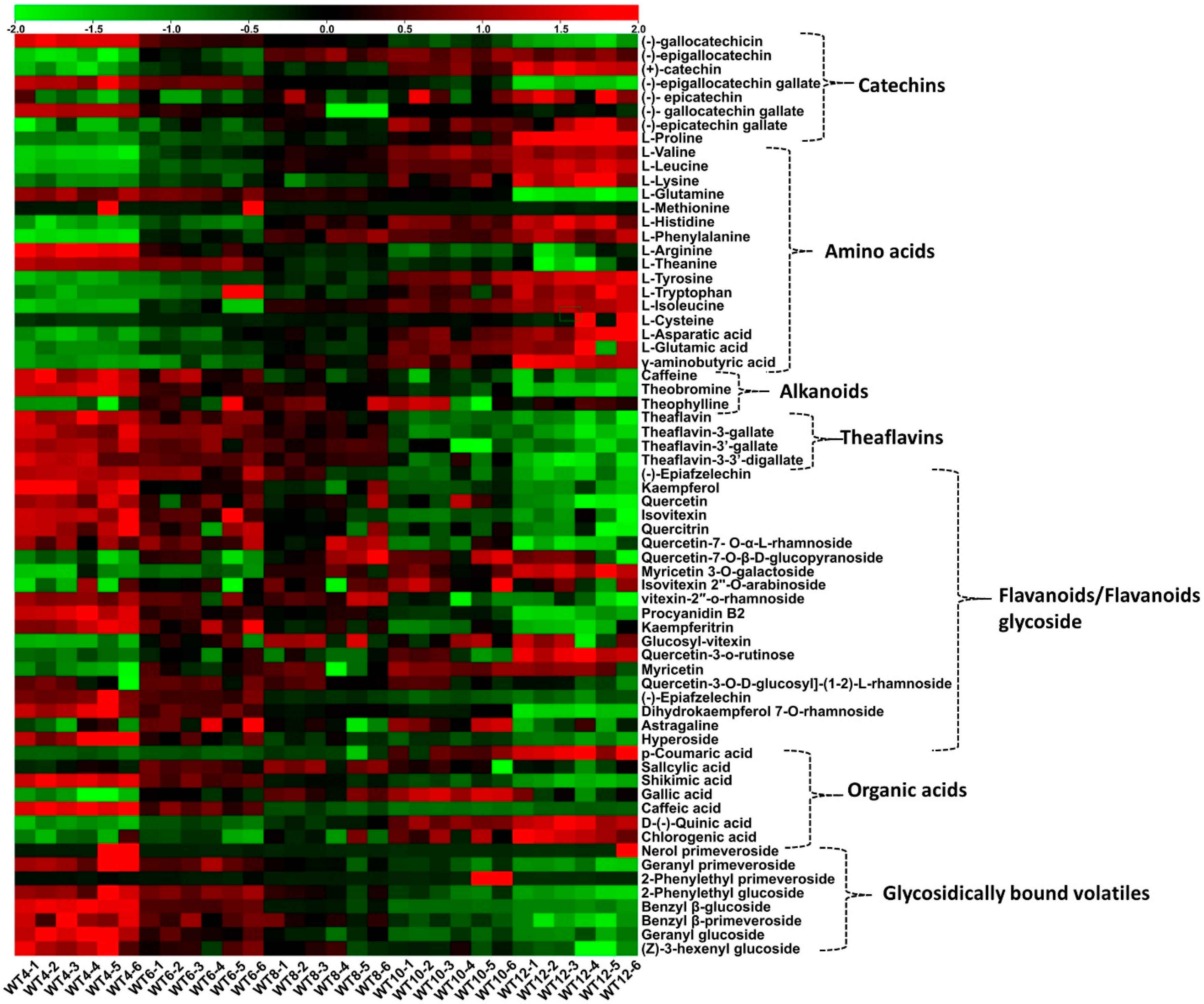
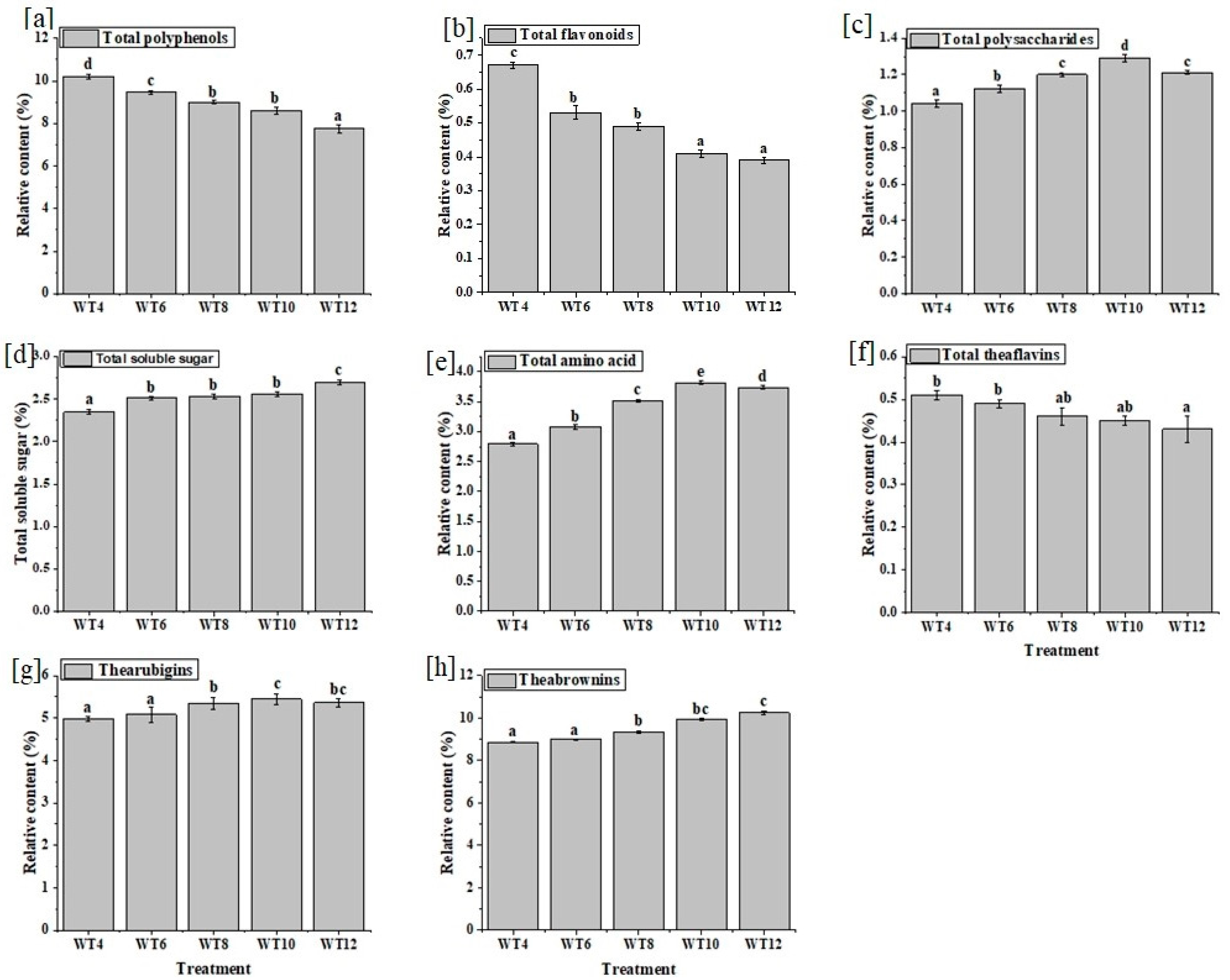
| Treatments | Appearance | Liquor Colour | Aroma | Taste | Infused Leaf | Overall Acceptance | Cumulative Score |
|---|---|---|---|---|---|---|---|
| Professional Evaluation | 20% | 10% | 30% | 30% | 10% | 100% | |
| WT4 | 85.80 ± 0.20 a | 90.83 ± 1.04 a | 79.33 ± 0.58 a | 74.50 ± 1.32 a | 88.83 ± 0.29 a | 81.28 ± 0.40 a | |
| WT6 | 86.97 ± 0.15 b | 90.83 ± 0.58 a | 80.33 ± 0.58 a | 79.33 ± 0.58 b | 88.90 ± 0.17 a | 83.27 ± 0.25 b | |
| WT8 | 87.10 ± 0.26 b | 91.17 ± 0.29 ab | 84.67 ± 0.58 b | 86.00 ± 1.00 d | 90.07 ± 0.12 b | 86.74 ± 0.39 d | |
| WT10 | 88.90 ± 0.36 d | 92.33 ± 0.58 bc | 84.00 ± 1.00 b | 85.50 ± 0.50 d | 90.13 ± 0.15 b | 86.88 ± 0.42 d | |
| WT12 | 88.00 ± 0.20 c | 92.50 ± 0.50 c | 80.33 ± 0.58 a | 82.83 ± 0.76 c | 88.93 ± 0.58 a | 84.63 ± 0.36 c | |
| Customer Evaluation | |||||||
| WT4 | 6.55 ± 0.94 | 5.65 ± 0.87 | 5.80 ± 0.97 | 6.00 ± 1.00 | |||
| WT6 | 6.34 ± 0.93 | 5.52 ± 1.02 | 5.81 ± 1.03 | 6.02 ± 1.09 | |||
| WT8 | 6.43 ± 1.02 | 5.66 ± 1.09 | 6.07 ± 0.97 | 6.06 ± 0.88 | |||
| WT10 | 6.33 ± 0.92 | 5.71 ± 0.92 | 6.00 ± 1.16 | 6.09 ± 0.88 | |||
| WT12 | 6.49 ± 0.92 | 5.52 ± 0.96 | 5.84 ± 0.87 | 5.91 ± 0.91 | |||
| Pr > F | 0.761 | 0.784 | 0.53 | 0.881 | |||
| Significance | No | No | No | No |
| Treatments | Aroma | Liquor Color | Taste | |
|---|---|---|---|---|
| WT4 | Parameter | 0.686 | 0.181 | 1.070 |
| odds ratio | 3.216 | 0.243 | 7.272 | |
| Pr > X2 | 0.072 | 0.621 | 0.008 | |
| WT6 | Parameter | 0.683 | 0.599 | 0.744 |
| odds ratio | 3.319 | 1.908 | 3.135 | |
| Pr > X2 | 0.068 | 0.167 | 0.076 | |
| WT8 | Parameter | 1.00 | 1.203 | 1.682 |
| odds ratio | 3.696 | 5.486 | 5.420 | |
| Pr > X2 | 0.054 | 0.019 | 0.019 | |
| WT10 | Parameter | 1.097 | 1.610 | 1.153 |
| odds ratio | 3.359 | 5.022 | 7.155 | |
| Pr > X2 | 0.066 | 0.025 | 0.007 | |
| WT12 | Parameter | 2.223 | 0.682 | 1.471 |
| odds ratio | 6.431 | 1.663 | 6.554 | |
| Pr > X2 | 0.011 | 0.197 | 0.010 |
| RT | RI | Compound Names | WT4 | WT6 | WT8 | WT10 | WT12 |
|---|---|---|---|---|---|---|---|
| Acids | |||||||
| 6.9 | 990 | Hexanoic acid | 0.46 ± 0.09 a | 0.55 ± 0.08 a | 0.72 ± 0.08 b | 0.63 ± 0.08 b | 0.68 ± 0.07 b |
| 24.16 | 1355 | Geranic acid | 0.16 ± 0.09 ab | 0.17 ± 0.01 ab | 0.25 ± 0.01 b | 0.20 ± 0.02 ab | 0.14 ± 0.01 a |
| 40.41 | 1768 | Tetradecanoic acid | 0.05 ± 0.01 ab | 0.03 ± 0.00 a | 0.14 ± 0.03 c | 0.08 ± 0.03 b | 0.03 ± 0.00 ab |
| Alcohols | |||||||
| 8.43 | 1030 | 1-Hexanol, 2-ethyl- | 0.82 ± 0.21 a | 1.29 ± 0.32 b | 0.51 ± 0.06 a | 2.42 ± 0.25 c | 2.43 ± 0.09 c |
| 8.62 | 1036 | Benzyl alcohol | 3.02 ± 0.23 a | 3.86 ± 0.15 ab | 4.07 ± 0.46 b | 3.85 ± 0.25 ab | 3.07 ± 0.05 a |
| 9.93 | 1074 | Linalool oxide | 1.82 ± 0.10 a | 2.26 ± 0.02 bc | 2.28 ± 0.38 bc | 2.57 ± 0.02 c | 2.01 ± 0.06 ab |
| 10.54 | 1087 | trans-Linalool oxide (furanoid) | 2.25 ± 0.15 a | 2.69 ± 0.11 b | 2.56 ± 0.43 ab | 2.57 ± 0.09 ab | 2.40 ± 0.07 ab |
| 11.06 | 1099 | Linalool | 2.20 ± 0.11 a | 2.73 ± 0.03 b | 2.76 ± 0.02 b | 3.55 ± 0.35 c | 2.53 ± 0.19 ab |
| 11.6 | 1116 | Phenylethyl alcohol | 4.14 ± 0.24 a | 4.60 ± 0.57 b | 5.67 ± 0.77 bc | 6.41 ± 0.29 c | 5.61 ± 0.47 bc |
| 14.41 | 1173 | Linalool oxide II | 1.25 ± 0.31 a | 1.15 ± 0.03 a | 1.36 ± 0.21 a | 1.36 ± 0.23 a | 1.27 ± 0.02 a |
| 15.66 | 1189 | α-Terpineol | 0.30 ± 0.07 a | 0.41 ± 0.00 b | 0.43 ± 0.01 b | 0.43 ± 0.01 b | 0.43 ± 0.01 b |
| 15.82 | 1198 | Bicyclo[3.1.1]hept-2-ene-2-ethanol,6,6-dimethyl- | ND | ND | 0.05 ± 0.01 a | 0.10 ± 0.01 b | 0.08 ± 0.00 ab |
| 17.3 | 1228 | Nerol | 0.63 ± 0.07 a | 0.67 ± 0.01 a | 0.73 ± 0.05 a | 0.76 ± 0.04 b | 0.62 ± 0.02 a |
| 17.59 | 1240 | trans-Isogeraniol | 0.33 ± 0.11 a | 0.33 ± 0.01 a | 0.43 ± 0.04 b | 0.38 ± 0.02 ab | 0.32 ± 0.06 a |
| 18.82 | 1255 | Geraniol | 1.31 ± 0.05 a | 1.64 ± 0.22 b | 2.05 ± 0.03 c | 2.38 ± 0.05 d | 2.02 ± 0.08 c |
| 22.52 | 1317 | trans-Farnesol | 0.48 ± 0.08 a | 0.43 ± 0.01 a | 0.59 ± 0.05 a | 0.75 ± 0.04 b | 0.58 ± 0.03 a |
| 28.02 | 1436 | 1-Dodecanol, 3,7,11-trimethyl- | ND | ND | 0.13 ± 0.01 a | 0.13 ± 0.00 a | 0.14 ± 0.01 a |
| 34.75 | 1564 | trans-Nerolidol | 0.40 ± 0.08 ab | 0.34 ± 0.01 a | 0.39 ± 0.02 ab | 0.53 ± 0.04 b | 0.41 ± 0.05 ab |
| 38.15 | 1632 | 7-epi-cis-Sesquisabinene hydrate | 0.04 ± 0.00 b | 0.02 ± 0.00 a | 0.08 ± 0.01 d | 0.05 ± 0.00 c | 0.04 ± 0.00 b |
| Aldehydes | |||||||
| 5.45 | 943 | 2,4-Hexadienal, (E,E)- | ND | 0.07 ± 0.01 a | 0.09 ± 0.01 b | 0.10 ± 0.00 b | 0.09 ± 0.01 b |
| 6.62 | 962 | Benzaldehyde | 0.21 ± 0.01 a | 0.23 ± 0.00 a | 0.33 ± 0.04 b | 0.34 ± 0.03 b | 0.41 ± 0.05 c |
| 7.49 | 1012 | 2,4-Heptadienal, (E,E)- | 2.31 ± 0.04 a | 3.33 ± 0.56 b | 2.29 ± 0.12 ab | 4.62 ± 0.23 c | 4.27 ± 0.28 c |
| 8.96 | 1045 | Benzeneacetaldehyde | 1.47 ± 0.07 a | 2.03 ± 0.11 b | 2.30 ± 0.18 c | 3.95 ± 0.03 e | 3.13 ± 0.12 d |
| 9.44 | 1060 | 2-Octenal, (E)- | 0.78 ± 0.06 a | 0.82 ± 0.02 a | 1.09 ± 0.04 ab | 1.23 ± 0.03 b | 1.12 ± 0.05 ab |
| 10.15 | 1075 | cis-4,5-Epoxy-(E)-2-decenal | 0.83 ± 0.02 a | 1.02 ± 0.02 b | 0.99 ± 0.00 b | 1.23 ± 0.11 c | 1.06 ± 0.04 b |
| 11.3 | 1104 | Nonanal | 0.13 ± 0.02 a | 0.15 ± 0.04 a | 0.12 ± 0.02 a | 0.58 ± 0.02 b | 0.57 ± 0.03 b |
| 13.55 | 1156 | 2,6-Nonadienal, (E,Z)- | 0.14 ± 0.00 a | 0.14 ± 0.00 a | 0.16 ± 0.01 a | 0.27 ± 0.01 c | 0.22 ± 0.01 b |
| 13.73 | 1169 | Lilac aldehyde D | 0.08 ± 0.01 a | 0.09 ± 0.01 a | 0.12 ± 0.05 a | 0.12 ± 0.01 a | 0.13 ± 0.00 a |
| 13.91 | 1162 | 2-Nonenal, (E)- | 0.14 ± 0.03 a | 0.17 ± 0.01 a | 0.29 ± 0.01 c | 0.15 ± 0.01 b | 0.22 ± 0.01 b |
| 16.31 | 1206 | Decanal | 0.24 ± 0.09 a | 0.20 ± 0.01 a | 0.26 ± 0.02 ab | 0.37 ± 0.02 b | 0.23 ± 0.01 a |
| 16.95 | 1220 | β-Cyclocitral | 1.37 ± 0.10 b | 1.02 ± 0.01 a | 0.91 ± 0.01 a | 1.04 ± 0.07 a | 0.98 ± 0.06 a |
| 19.49 | 1263 | 2-Decenal, (E)- | 0.20 ± 0.09 ab | 0.14 ± 0.01 a | 0.26 ± 0.02 b | 0.26 ± 0.00 b | 0.15 ± 0.01 a |
| 19.78 | 1276 | Citral | 0.45 ± 0.03 b | 0.34 ± 0.02 a | 0.33 ± 0.02 a | 0.54 ± 0.01 c | 0.46 ± 0.02 b |
| 21.03 | 1296 | (E,Z,Z)-2,4,7-Tridecatrienal | 0.23 ± 0.06 ab | 0.29 ± 0.02 bc | 0.28 ± 0.03 abc | 0.35 ± 0.01 c | 0.22 ± 0.01 a |
| 21.28 | 1317 | 2,4-Decadienal, (E,E)- | 0.34 ± 0.04 a | 0.30 ± 0.01 a | 0.36 ± 0.03 ab | 0.45 ± 0.01 b | 0.30 ± 0.02 a |
| 24.92 | 1367 | 2-Undecenal, E- | 0.30 ± 0.05 b | 0.11 ± 0.01 a | 0.20 ± 0.01 ab | 0.18 ± 0.01 ab | 0.14 ± 0.01 a |
| 27.17 | 1409 | Dodecanal | 0.20 ± 0.01 b | 0.08 ± 0.00 a | 0.06 ± 0.00 a | 0.07 ± 0.01 a | 0.06 ± 0.00 a |
| Hydrocarbons | |||||||
| 10.78 | 1089 | 1-Undecyne | 0.39 ± 0.00 a | 0.45 ± 0.02 a | 0.66 ± 0.08 c | 0.58 ± 0.01 b | 0.80 ± 0.01 d |
| 15.06 | 1182 | Naphthalene | 0.07 ± 0.00 a | 0.12 ± 0.01 bc | 0.13 ± 0.01 c | 0.15 ± 0.01 d | 0.11 ± 0.00 b |
| 16.03 | 1200 | Dodecane | 0.05 ± 0.01 a | 0.06 ± 0.00 a | 0.06 ± 0.00 a | 0.09 ± 0.00 c | 0.08 ± 0.01 b |
| 21.72 | 1300 | Tetradecane | 0.26 ± 0.03 c | 0.21 ± 0.01 b | 0.20 ± 0.01 b | 0.15 ± 0.02 a | 0.12 ± 0.00 a |
| 22.3 | 1321 | 1,5,5-Trimethyl-6-methylene-cyclohexene | 0.41 ± 0.04 b | 0.16 ± 0.02 a | 0.16 ± 0.01 a | 0.16 ± 0.01 a | 0.12 ± 0.01 a |
| 26.8 | 1400 | Hexadecane | 0.42 ± 0.08 c | 0.28 ± 0.00 b | 0.26 ± 0.02 b | 0.17 ± 0.02 ab | 0.13 ± 0.03 a |
| 29.34 | 1461 | cis-β-Farnesene | 0.03 ± 0.00 b | 0.03 ± 0.00 b | 0.04 ± 0.00 c | 0.05 ± 0.00 c | 0.01 ± 0.00 a |
| 36.61 | 1600 | Heptadecane, 2,6,10,15-tetramethyl- | 0.16 ± 0.02 b | 0.09 ± 0.00 a | 0.12 ± 0.01 ab | 0.11 ± 0.00 ab | 0.10 ± 0.02 a |
| Esters | |||||||
| 15.44 | 1192 | Methyl salicylate | 1.35 ± 0.25 a | 1.85 ± 0.03 b | 1.70 ± 0.16 b | 2.13 ± 0.06 bc | 2.45 ± 0.15 c |
| 20.03 | 1280 | Geranyl formate | 1.54 ± 0.30 b | 0.76 ± 0.01 a | 0.75 ± 0.01 a | 0.75 ± 0.02 a | 0.58 ± 0.01 a |
| 25.03 | 1373 | Hexanoic acid, 3-hexenyl ester, (Z)- | 0.26 ± 0.06 b | 0.21 ± 0.02 b | 0.23 ± 0.01 b | 0.25 ± 0.04 b | 0.13 ± 0.01 a |
| 25.5 | 1390 | 2-isopropenyl-5-methyl-4-hexenyl acetate | ND | 0.05 ± 0.01 a | 0.10 ± 0.01 b | 0.14 ± 0.02 c | 0.09 ± 0.00 b |
| 32.74 | 1532 | Dihydroactinidiolide | 1.27 ± 0.12 c | 0.47 ± 0.08 b | 0.41 ± 0.01 b | 0.26 ± 0.04 a | 0.27 ± 0.01 a |
| 35.67 | 1571 | E-8-Methyl-9-tetradecen-1-ol acetate | 0.03 ± 0.00 a | 0.05 ± 0.00 b | 0.06 ± 0.00 b | 0.06 ± 0.01 b | 0.05 ± 0.00 b |
| 40.63 | 1778 | benzyl ester | 0.08 ± 0.01 a | 0.06 ± 0.00 a | 0.08 ± 0.01 a | 0.07 ± 0.00 a | 0.06 ± 0.00 a |
| Ketone | |||||||
| 9.81 | 1073 | 3,5-Octadien-2-one, (E,E)- | 0.91 ± 0.01 ab | 0.94 ± 0.02 ab | 0.90 ± 0.05 a | 1.10 ± 0.15 ab | 1.12 ± 0.01 b |
| 12.03 | 1124 | Isophorone | 0.12 ± 0.00 a | 0.14 ± 0.00 a | 0.13 ± 0.01 a | 0.15 ± 0.02 a | 0.13 ± 0.01 a |
| 23.47 | 1328 | 2,6,6-Trimethyl-2-cyclohexene-1,4-dione | 0.62 ± 0.09 b | 0.29 ± 0.02 ba | 0.36 ± 0.03 ab | 0.24 ± 0.01 a | 0.24 ± 0.01 a |
| 27.58 | 1426 | α-Ionone | 0.24 ± 0.05 a | 0.23 ± 0.00 a | 0.21 ± 0.01 a | 0.23 ± 0.00 a | 0.18 ± 0.00 a |
| 29.02 | 1456 | Geranyl acetone | 1.62 ± 0.05 a | 1.63 ± 0.02 a | 1.70 ± 0.03 a | 1.73 ± 0.02 a | 1.42 ± 0.01 a |
| 30.71 | 1473 | β-Ionone epoxide | 1.44 ± 0.07 b | 0.77 ± 0.05 a | 0.82 ± 0.05 a | 0.61 ± 0.03 a | 0.55 ± 0.02 a |
| 41.7 | 1844 | Phytone | 0.16 ± 0.03 a | 0.32 ± 0.02 b | 0.19 ± 0.01 ab | 0.15 ± 0.01 a | 0.22 ± 0.01 b |
| Sample | DPPH IC50 (µg/mL) | α-amylase IC50 (mg/mL) | α-glucosidase IC50 (µg/mL) | Glucose Uptake/% |
|---|---|---|---|---|
| WT4 | 27.49 ± 0.28 a | 17.97 ± 0.10 a | 35.54 ± 0.71 a | 54.29 ± 1.57 c |
| WT6 | 28.63 ± 0.21 b | 18.10 ± 0.04 a | 36.55 ± 0.86 ab | 57.43 ± 1.43 d |
| WT8 | 29.48 ± 0.18 c | 18.28 ± 1.59 a | 37.38 ± 0.31 b | 50.81 ± 1.44 b |
| WT10 | 30.32 ± 0.32 d | 18.37 ± 0.04 a | 37.92 ± 0.11 b | 46.36 ± 1.20 a |
| WT12 | 32.29 ± 0.65 e | 19.89 ± 0.08 b | 39.67 ± 1.36 c | 47.60 ± 0.97 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntezimana, B.; Li, Y.; He, C.; Yu, X.; Zhou, J.; Chen, Y.; Yu, Z.; Ni, D. Different Withering Times Affect Sensory Qualities, Chemical Components, and Nutritional Characteristics of Black Tea. Foods 2021, 10, 2627. https://doi.org/10.3390/foods10112627
Ntezimana B, Li Y, He C, Yu X, Zhou J, Chen Y, Yu Z, Ni D. Different Withering Times Affect Sensory Qualities, Chemical Components, and Nutritional Characteristics of Black Tea. Foods. 2021; 10(11):2627. https://doi.org/10.3390/foods10112627
Chicago/Turabian StyleNtezimana, Bernard, Yuchuan Li, Chang He, Xinlei Yu, Jingtao Zhou, Yuqiong Chen, Zhi Yu, and Dejiang Ni. 2021. "Different Withering Times Affect Sensory Qualities, Chemical Components, and Nutritional Characteristics of Black Tea" Foods 10, no. 11: 2627. https://doi.org/10.3390/foods10112627
APA StyleNtezimana, B., Li, Y., He, C., Yu, X., Zhou, J., Chen, Y., Yu, Z., & Ni, D. (2021). Different Withering Times Affect Sensory Qualities, Chemical Components, and Nutritional Characteristics of Black Tea. Foods, 10(11), 2627. https://doi.org/10.3390/foods10112627







