Heat-Moisture Treatment Further Reduces In Vitro Digestibility and Enhances Resistant Starch Content of a High-Resistant Starch and Low-Glutelin Rice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Rice Flour Preparation
2.3. Heat Treatment
2.4. In Vitro Hydrolysis Experiments
2.5. RS Content Determination
2.6. Color and Chromaticity Analyses
2.7. Starch Extraction
2.8. Scanning Electron Microscopy
2.9. X-ray Diffraction (XRD)
2.10. Gelatinization Properties Analyses
2.11. Pasting Properties Analyses
2.12. Protein Extraction and SDS-PAGE
2.13. Preparation of Steamed Rice
2.14. Statistical Analyses
3. Results and Discussion
3.1. In Vitro Digestibility
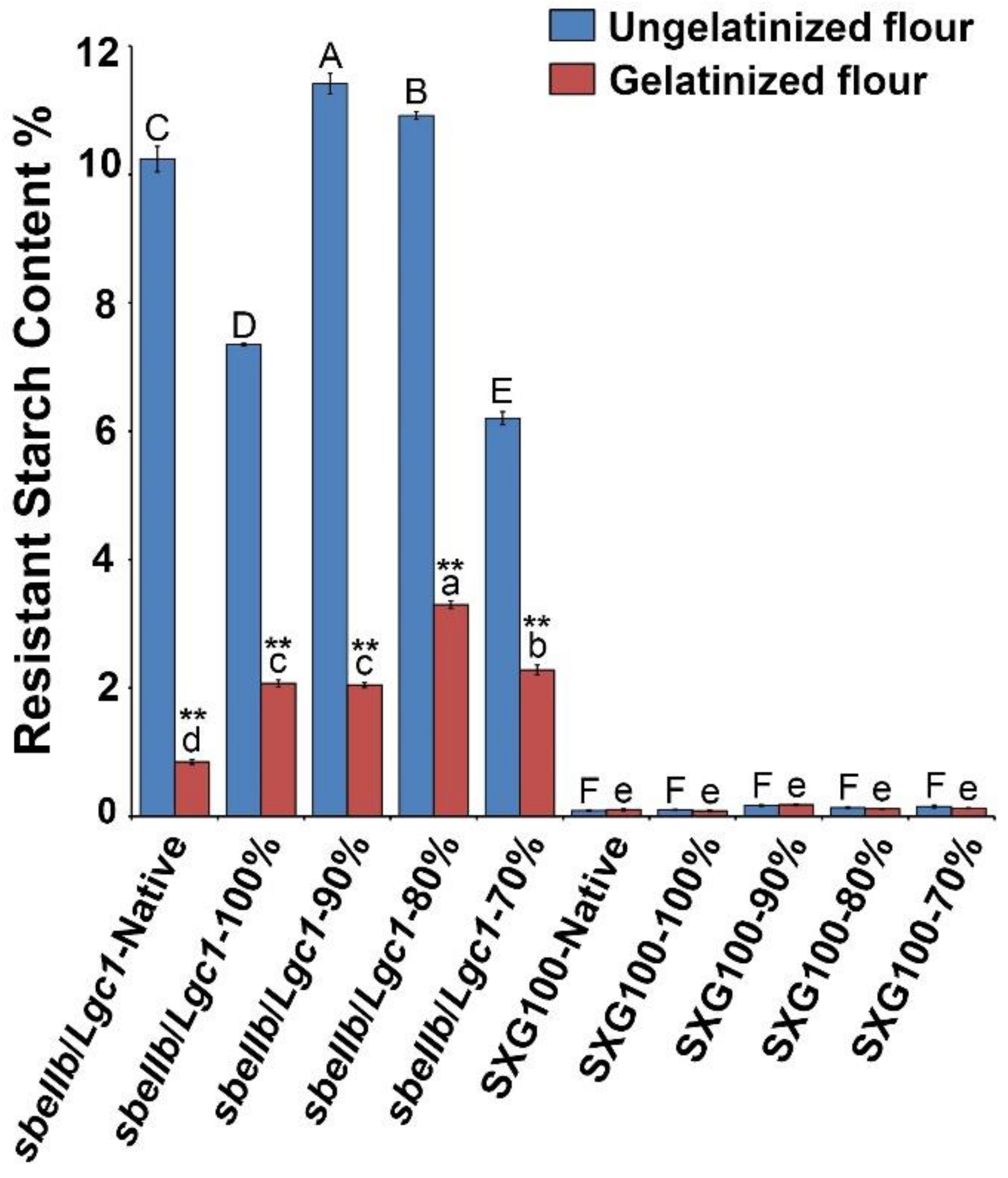
3.2. Resistant Starch Content
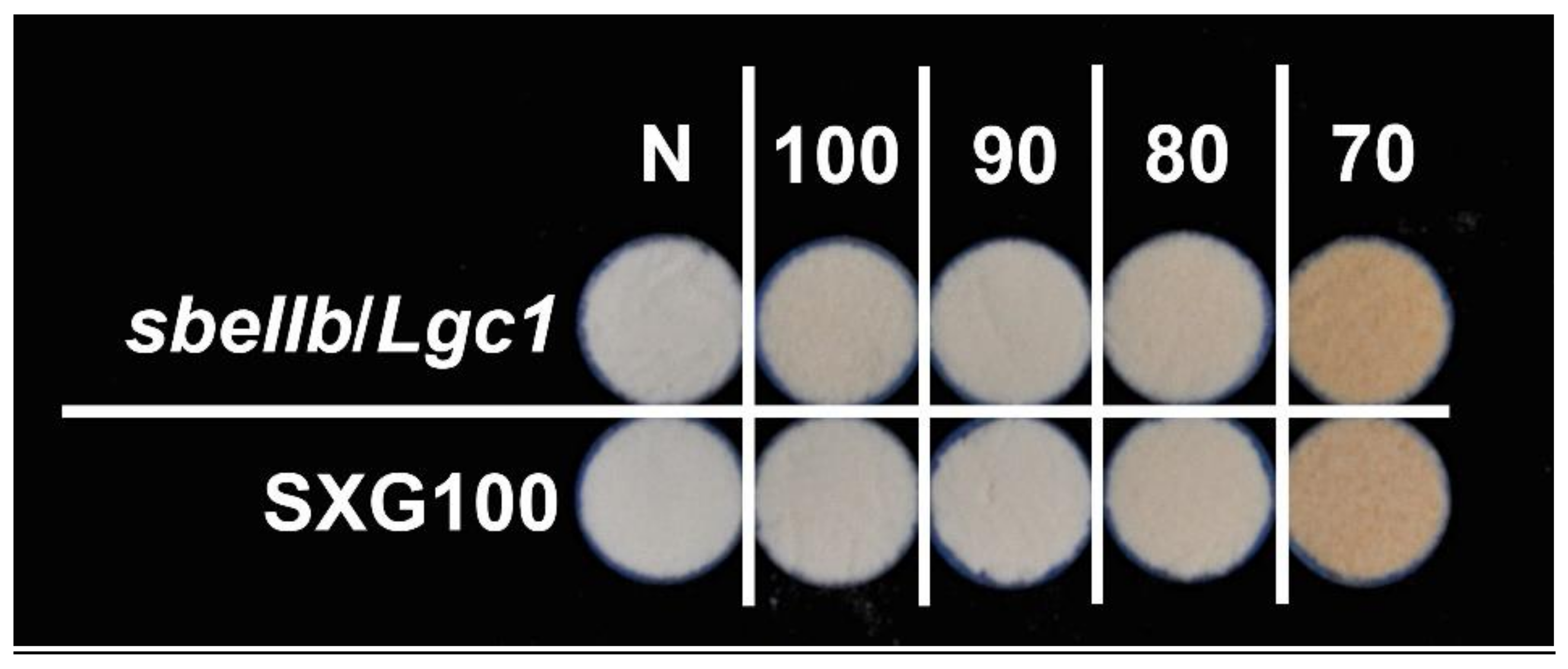
3.3. Color and Chromaticity
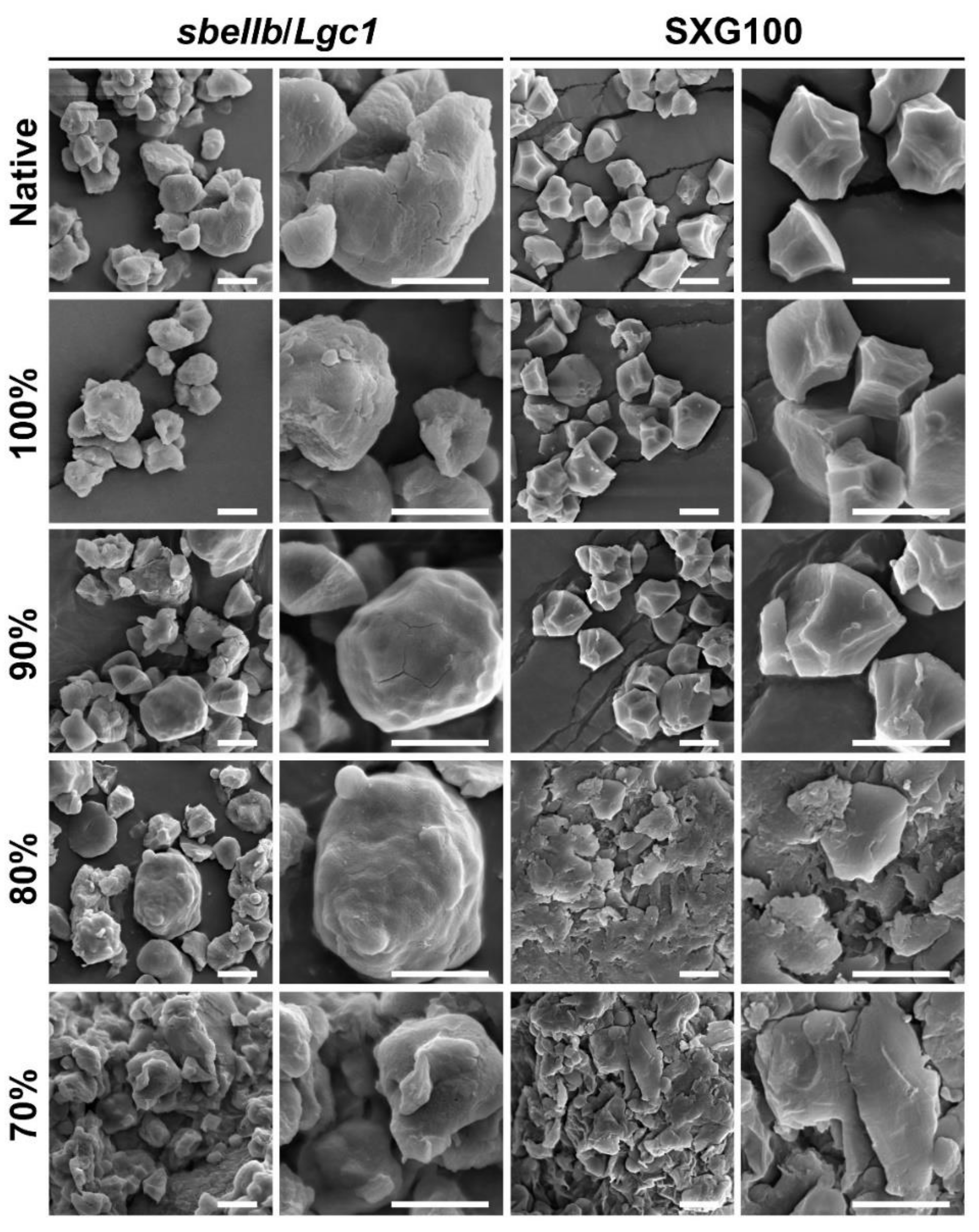
3.4. Starch Granule Morphology
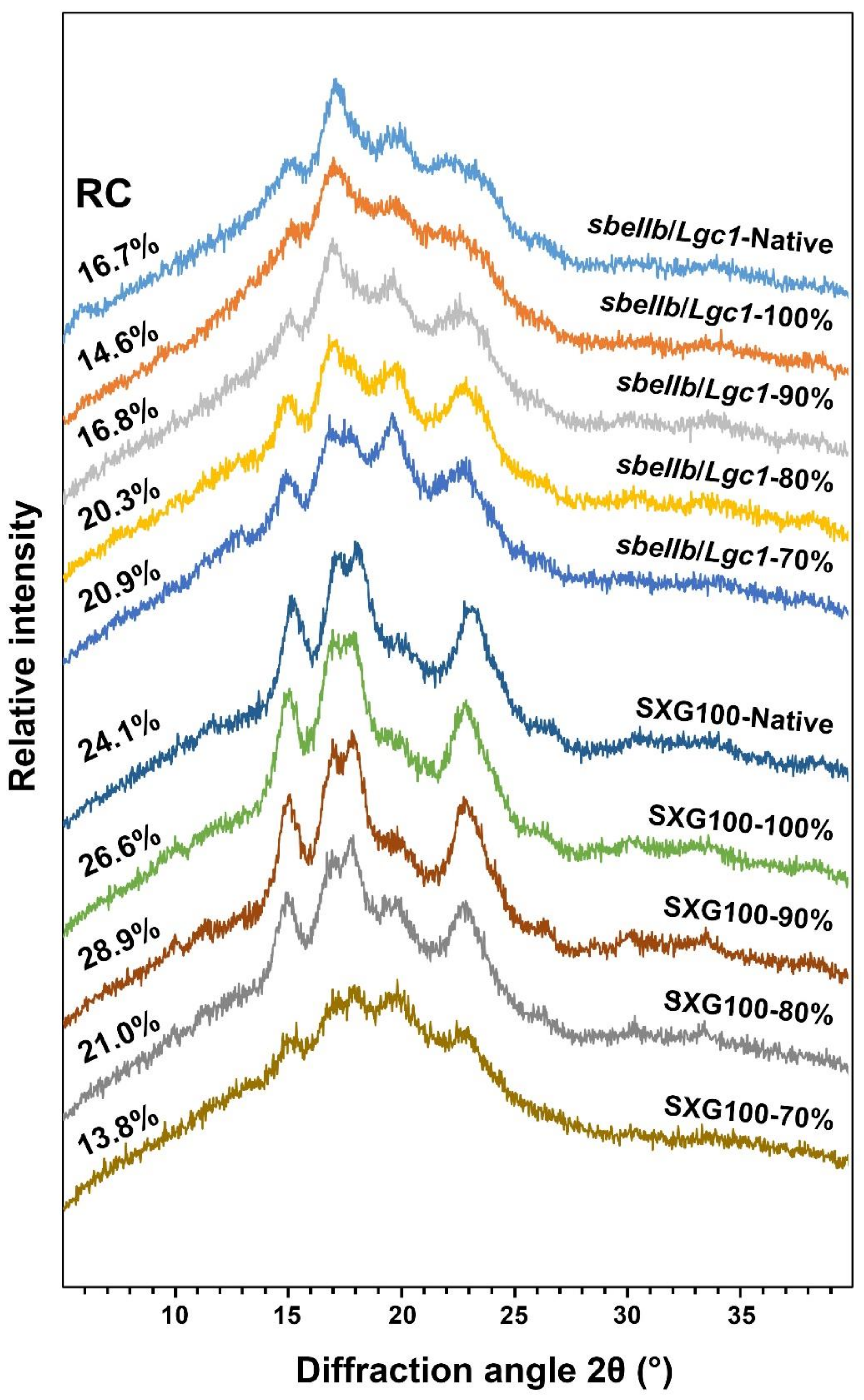
3.5. Crystalline Structure
3.6. Gelatinization Properties
3.7. Pasting Properties
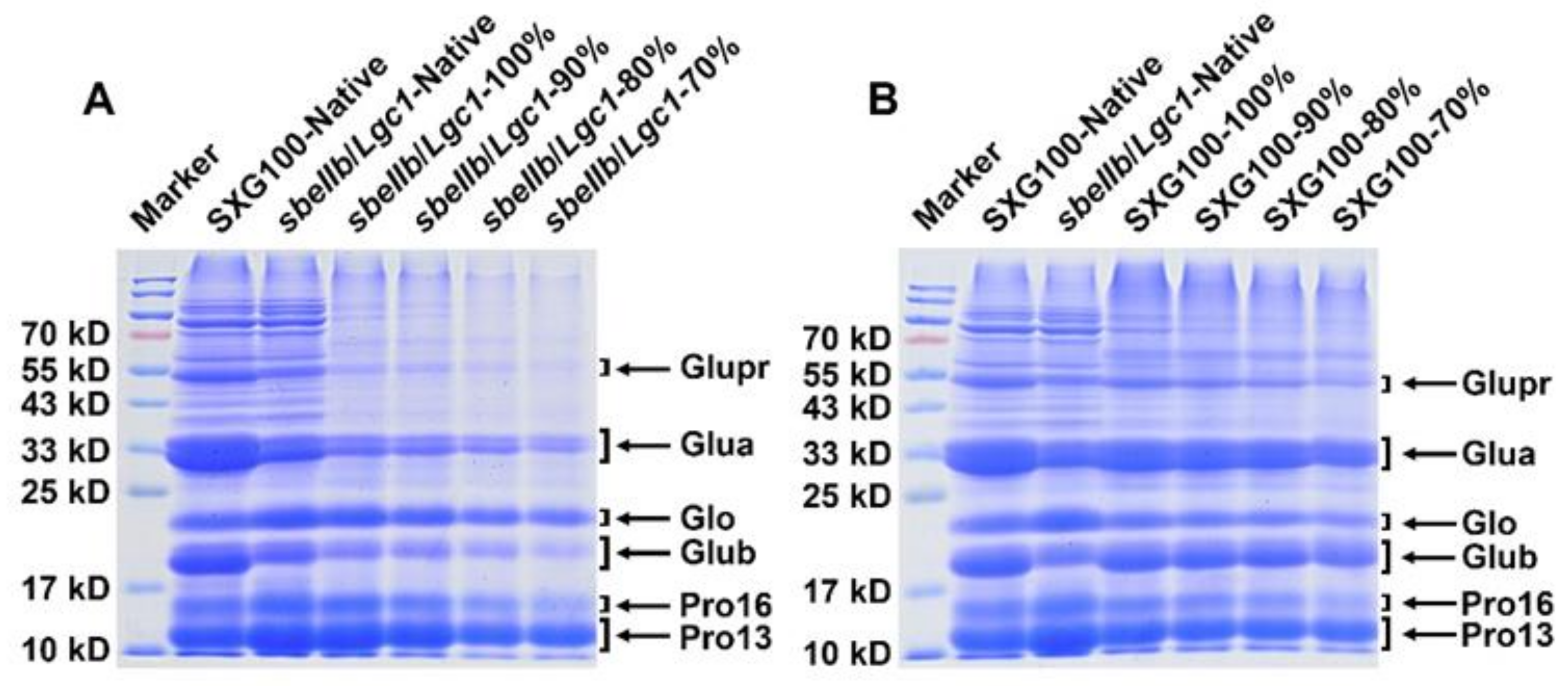
3.8. Protein Compositions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khush, G.S. What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol. Biol. 2005, 59, 15950. [Google Scholar] [CrossRef]
- Zeng, D.; Tian, Z.; Rao, Y.; Dong, G.; Yang, Y.; Huang, L.; Leng, Y.; Xu, J.; Sun, C.; Zhang, G.; et al. Rational design of high-yield and superior-quality rice. Nat. Plants 2017, 3, 17031. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Lin, Y.; Chen, H. Improving nutritional quality of rice for human health. Theor. Appl. Genet. 2020, 133, 1397–1413. [Google Scholar] [CrossRef]
- James, M.G.; Denyer, K.; Myers, A.M. Starch synthesis in the cereal endosperm. Curr. Opin. Plant Biol. 2003, 6, 215–222. [Google Scholar] [CrossRef]
- Vandeputte, G.E.; Delcour, J.A. From sucrose to starch granule to starch physical behaviour: A focus on rice starch. Carbohydr. Polym. 2004, 58, 245–266. [Google Scholar] [CrossRef]
- Zavareze, E.d.R.; Storck, C.R.; de Castro, L.A.S.; Schirmer, M.A.; Dias, A.R.G. Effect of heat-moisture treatment on rice starch of varying amylose content. Food Chem. 2010, 121, 358–365. [Google Scholar] [CrossRef]
- Yang, X.; Chi, C.; Liu, X.; Zhang, Y.; Zhang, H.; Wang, H. Understanding the structural and digestion changes of starch in heat-moisture treated polished rice grains with varying amylose content. Int. J. Biol. Macromol. 2019, 139, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.M.; Wolever, T.M. Effect of glucose, sucrose and fructose on plasma glucose and insulin responses in normal humans: Comparison with white bread. Eur. J. Clin. Nutr. 1998, 52, 924–928. [Google Scholar] [CrossRef]
- Man, J.; Yang, Y.; Huang, J.; Zhang, C.; Chen, Y.; Wang, Y.; Gu, M.; Liu, Q.; Wei, C. Effect of simultaneous inhibition of Starch Branching Enzymes I and IIb on the crystalline structure of rice starches with different amylose contents. J. Agric. Food Chem. 2013, 61, 9930–9937. [Google Scholar] [CrossRef] [PubMed]
- Raigond, P.; Ezekiel, R.; Raigond, B. Resistant starch in food: A review. J. Sci. Food Agric. 2015, 95, 1968–1978. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, L.; Liu, G.; Meng, X.; Jing, Y.; Shu, X.; Kong, X.; Sun, J.; Yu, H.; Smith, S.M.; et al. Critical roles of soluble starch synthase SSIIIa and granule-bound starch synthase Waxy in synthesizing resistant starch in rice. Proc. Natl. Acad. Sci. USA 2016, 113, 12844–12849. [Google Scholar] [CrossRef] [Green Version]
- Englyst, H.; Wiggins, H.S.; Cummings, J.H. Determination of the non-starch polysaccharides in plant foods by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst 1982, 107, 307–318. [Google Scholar] [CrossRef]
- Guo, D.; Ling, X.; Zhou, X.; Li, X.; Wang, J.; Qiu, S.; Yang, Y.; Zhang, B. Evaluation of the quality of a high-resistant starch and low-glutelin rice (Oryza sativa L.) generated through CRISPR/Cas9-mediated targeted mutagenesis. J. Agric. Food Chem. 2020, 68, 9733–9742. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiao, G.; Liu, Z.; Zhang, X.; Li, J.; Guo, X.; Du, W.; Du, J.; Francis, F.; Zhao, Y.; et al. Generation of high-amylose rice through CRISPR/Cas9-mediated targeted mutagenesis of starch branching enzymes. Front. Plant Sci. 2017, 8, 298. [Google Scholar] [CrossRef]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef] [Green Version]
- Ordonio, R.L.; Matsuoka, M. Increasing resistant starch content in rice for better consumer health. Proc. Natl. Acad. Sci. USA 2016, 113, 12616–12618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Guo, M.; Sun, S.; Zou, Y.; Yin, S.; Liu, Y.; Tang, S.; Gu, M.; Yang, Z.; Yan, C. Natural variation of OsGluA2 is involved in grain protein content regulation in rice. Nat. Commun. 2019, 10, 1949. [Google Scholar] [CrossRef] [PubMed]
- Kawakatsu, T.; Yamamoto, M.P.; Hirose, S.; Yano, M.; Takaiwa, F. Characterization of a new rice glutelin gene GluD-1 expressed in the starchy endosperm. J. Exp. Bot. 2008, 59, 4233–4245. [Google Scholar] [CrossRef] [Green Version]
- Kawakatsu, T.; Hirose, S.; Yasuda, H.; Takaiwa, F. Reducing rice seed storage protein accumulation leads to changes in nutrient quality and storage organelle formation. Plant Physiol. 2010, 154, 1842–1854. [Google Scholar] [CrossRef] [Green Version]
- Mochizuki, T.; Hara, S. Usefulness of the low protein rice on the diet therapy in patients with chronic renal failure. Nihon Jinzo Gakkai Shi 2000, 42, 24–29. [Google Scholar]
- Kusaba, M.; Miyahara, K.; Iida, S.; Fukuoka, H.; Takano, T.; Sassa, H.; Nishimura, M.; Nishio, T. Low glutelin content1: A dominant mutation that suppresses the glutelin multigene family via RNA silencing in rice. Plant Cell 2003, 15, 1455–1467. [Google Scholar] [CrossRef] [Green Version]
- Rhee, C.M.; Ahmadi, S.F.; Kovesdy, C.P.; Kalantar-Zadeh, K. Low-protein diet for conservative management of chronic kidney disease: A systematic review and meta-analysis of controlled trials. J. Cachexia Sarcopenia Muscle 2018, 9, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Yano, M.; Okuno, K.; Kawakami, J.; Satoh, H.; Omura, T. High amylose mutants of rice, Oryza sativa L. Theor. Appl. Genet. 1985, 69, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Nishi, A.; Nakamura, Y.; Tanaka, N.; Satoh, H. Biochemical and genetic analysis of the effects of amylose-extender mutation in rice endosperm. Plant Physiol. 2001, 127, 459–472. [Google Scholar] [CrossRef]
- Butardo, V.M.; Fitzgerald, M.A.; Bird, A.R.; Gidley, M.J.; Flanagan, B.M.; Larroque, O.; Resurreccion, A.P.; Laidlaw, H.K.; Jobling, S.A.; Morell, M.K.; et al. Impact of down-regulation of starch branching enzyme IIb in rice by artificial microRNA- and hairpin RNA-mediated RNA silencing. J. Exp. Bot. 2011, 62, 4927–4941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Gu, M.; Meng, X.; Cheung, S.C.; Yu, H.; Huang, J.; Sun, Y.; Shi, Y.; Liu, Q. High-amylose rice improves indices of animal health in normal and diabetic rats. Plant Biotechnol. J. 2012, 10, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Iida, S.; Amano, E.; Nishio, T. A rice (Oryza sativa L.) mutant having a low content of glutelin and a high content of prolamine. Theor. Appl. Genet. 1993, 87, 374–378. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.R. Clean label starch: Production, physicochemical characteristics, and industrial applications. Food Sci. Biotechnol. 2021, 30, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, L.; Zheng, X. Recent advances in heat-moisture modified cereal starch: Structure, functionality and its applications in starchy food systems. Food Chem. 2021, 344, 128700. [Google Scholar] [CrossRef]
- Zhang, B.; Saleh, A.S.M.; Su, C.; Gong, B.; Zhao, K.; Zhang, G.; Li, W.; Yan, W. The molecular structure, morphology, and physicochemical property and digestibility of potato starch after repeated and continuous heat-moisture treatment. J. Food Sci. 2020, 85, 4215–4224. [Google Scholar] [CrossRef]
- Silva, W.M.; Biduski, B.; Lima, K.O.; Pinto, V.Z.; Hoffmann, J.F.; Vanier, N.L.; Dias, A.R. Starch digestibility and molecular weight distribution of proteins in rice grains subjected to heat-moisture treatment. Food Chem. 2017, 219, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Chen, L.; Li, X.; Wang, J.; Xie, F. Insights into the multi-scale structure and digestibility of heat-moisture treated rice starch. Food Chem. 2018, 242, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Puncha-arnon, S.; Uttapap, D. Rice starch vs. rice flour: Differences in their properties when modified by heat-moisture treatment. Carbohydr. Polym. 2013, 91, 85–91. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. S2), S33–S50. [Google Scholar]
- Smarzyński, K.; Sarbak, P.; Kowalczewski, P.; Różańska, M.B.; Rybicka, I.; Polanowska, K.; Fedko, M.; Kmiecik, D.; Masewicz, Ł.; Nowicki, M.; et al. Low-Field NMR study of shortcake biscuits with cricket powder, and their nutritional and physical characteristics. Molecules 2021, 26, 5417. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, N.W.H.; Tao, L. Variation in crystalline type with amylose content in maize starch granules: An X-ray powder diffraction study. Carbohydr. Polym. 1998, 36, 277–284. [Google Scholar] [CrossRef]
- Kurzawska, A.; Górecka, D.; Błaszczak, W.; Szwengiel, A.; Paukszta, D.; Lewandowicz, G. The molecular and supermolecular structure of common cattail (Typha latifolia) starch. Starch-Stärke 2015, 66, 849–856. [Google Scholar] [CrossRef]
- Takemoto, Y.; Coughlan, S.J.; Okita, T.W.; Satoh, H.; Ogawa, M.; Kumamaru, T. The rice mutant esp2 greatly accumulates the glutelin precursor and deletes the protein disulfide isomerase. Plant Physiol. 2002, 128, 1212–1222. [Google Scholar] [CrossRef] [Green Version]
- Vu, T.H.; Bean, S.; Hsieh, C.F.; Shi, Y.C. Changes in protein and starch digestibility in sorghum flour during heat-moisture treatments. J. Sci. Food Agric. 2017, 97, 4770–4779. [Google Scholar] [CrossRef]
- Hasjim, J.; Ai, Y.; Jane, J.L. Novel applications of amylose-lipid complex as resistant starch type 5. In Resistant Starch: Sources, Applications and Health Benefits, 1st ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2013; Chapter 4; pp. 79–92. [Google Scholar]
- Khamthong, P.; Lumdubwong, N. Effects of heat-moisture treatment on normal and waxy rice flours and production of thermoplastic flour materials. Carbohydr. Polym. 2012, 90, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Okumura, H.; Sugawara, M.; Noro, W.; Homma, N.; Ohtsubo, K. Effects of different heat-moisture treatments on the physicochemical properties of brown rice flour. Biosci. Biotechnol. Biochem. 2017, 81, 2370–2385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piecyk, M.; Domian, K. Effects of heat-moisture treatment conditions on the physicochemical properties and digestibility of field bean starch (Vicia faba var. minor). Int. J. Biol. Macromol. 2021, 182, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Lewandowicz, G.; Fornal, J.; Walkowski, A. Effect of microwave radiation on physico-chemical properties and structure of potato and tapioca starches. Carbohydr. Polym. 1997, 34, 213–220. [Google Scholar] [CrossRef]
- Sui, Z.; Yao, T.; Ye, X.; Bao, J.; Kong, X.; Wu, Y. Physicochemical properties and starch digestibility of in-kernel heat-moisture-treated waxy, low-, and high-amylose rice starch. Starch-Stärke 2017, 69, 1600164. [Google Scholar] [CrossRef]
- Thanh-Blicharz, J.L.; Lewandowicz, J. Functionality of native starches in food systems: Cluster analysis grouping of rheological properties in different product matrices. Foods 2020, 9, 1073. [Google Scholar]
- Crosbie, G.B.; Ross, A.S. Interpretation of RVA curves. In The RVA Handbook; AACC International Press: St. Paul, MN, USA, 2019; Chapter 2; p. 22. [Google Scholar]
- Ye, J.; Hu, X.; Luo, S.; McClements, D.J.; Liang, L.; Liu, C. Effect of endogenous proteins and lipids on starch digestibility in rice flour. Food Res. Int. 2018, 106, 404–409. [Google Scholar] [CrossRef]
- Liu, K.; Zheng, J.; Chen, F. Heat-induced changes in the physicochemical properties and in vitro digestibility of rice protein fractions. J. Food Sci. Technol. 2021, 58, 1368–1377. [Google Scholar] [CrossRef]
| Sample | Glucose Release (mg/100 mg) | ||
|---|---|---|---|
| 60 Min a | 120 Min | 180 Min | |
| sbeIIb/Lgc1-Native | 31.43 ± 0.43 d | 36.92 ± 1.02 f | 40.10 ± 0.36 g |
| sbeIIb/Lgc1-100% | 31.69 ± 0.57 d | 35.79 ± 0.76 f | 41.87 ± 0.33 g |
| sbeIIb/Lgc1-90% | 22.84 ± 0.84 e | 26.49 ± 0.60 g | 29.64 ± 0.28 h |
| sbeIIb/Lgc1-80% | 19.58 ± 0.80 e | 23.74 ± 0.27 h | 26.91 ± 0.51 h |
| sbeIIb/Lgc1-70% | 35.07 ± 0.59 d | 43.10 ± 0.87 e | 48.65 ± 0.80 f |
| SXG100-Native | 45.43 ± 2.77 b | 53.47 ± 0.67 c | 61.73 ±0.74 c |
| SXG100-100% | 39.71 ± 1.38 c | 49.61 ± 0.50 d | 57.79 ± 1.59 d |
| SXG100-90% | 34.60 ± 0.63 d | 44.44 ± 0.60 e | 53.58 ± 1.53 e |
| SXG100-80% | 48.25 ± 0.68 b | 59.35 ± 1.29 b | 66.18 ± 0.99 b |
| SXG100-70% | 69.01 ± 0.81 a | 74.25 ± 0.82 a | 77.98 ± 1.59 a |
| Sample | L* | a* | b* | ΔEa | ΔEb | WI |
|---|---|---|---|---|---|---|
| sbeIIb/Lgc1-Native | 96.04 ± 1.82 b | 0.04 ± 0.04 a | 7.18 ± 0.11 a | - | 2.54 | 91.80 |
| sbeIIb/Lgc1-100% | 94.36 ± 1.17 b | 0.84 ± 0.05 d | 12.75 ± 0.16 d | 5.87 | 3.36 | 86.03 |
| sbeIIb/Lgc1-90% | 92.19 ± 0.78 c | 0.45 ± 0.03 c | 11.20 ± 0.28 c | 5.58 | 2.92 | 86.34 |
| sbeIIb/Lgc1-80% | 91.68 ± 0.63 c | 1.47 ± 0.05 e | 14.49 ± 0.30 f | 8.63 | 0.80 | 83.23 |
| sbeIIb/Lgc1-70% | 81.18 ± 1.18 d | 6.55 ± 0.17 f | 25.26 ± 0.36 h | 24.29 | 2.86 | 67.82 |
| SXG100-Native | 98.54 ± 0.66 a | −0.02 ± 0.02 a | 6.75 ± 0.04 a | - | - | 93.10 |
| SXG100-100% | 95.32 ± 1.17 b | 0.29 ± 0.04 b | 9.58 ± 0.10 b | 4.30 | - | 89.33 |
| SXG100-90% | 94.74 ± 1.82 b | 0.23 ± 0.04 b | 9.81 ± 0.33 b | 4.88 | - | 88.87 |
| SXG100-80% | 92.22 ± 0.85 c | 1.52 ± 0.07 e | 13.90 ± 0.11 e | 9.67 | - | 84.00 |
| SXG100-70% | 79.63 ± 0.53 d | 6.75 ± 0.18 g | 22.86 ± 0.37 g | 25.75 | - | 68.65 |
| Sample | To (°C) | Tp (°C) * | Tc (°C) | ΔH (J/g) |
|---|---|---|---|---|
| sbeIIb/Lgc1-Native | 72.90 ± 0.41 b | 80.87 ± 0.34 a | 93.96 ± 0.75 a | 6.82 ± 0.07 a |
| sbeIIb/Lgc1-100% | 70.80 ± 0.26 a | 79.69 ±0.07 a | 94.44 ± 0.19 a | 6.49 ± 0.14 a |
| sbeIIb/Lgc1-90% | 72.00 ± 0.39 ab | 85.65 ± 0.28 b | 109.21 ± 0.86 b | 11.65 ± 0.90 c |
| sbeIIb/Lgc1-80% | 81.14 ± 0.72 c | 92.90 ± 0.80 c | 109.85 ± 0.70 b | 9.74 ± 0.43 b |
| sbeIIb/Lgc1-70% | 88.79 ± 0.13 d | 97.59 ± 0.62 d | 109.81 ± 0.38 b | 5.47 ± 0.11 a |
| SXG100-Native | 59.74 ± 0.23 a | 68.30 ± 0.78 a | 80.81 ± 1.08 a | 7.03 ± 0.07 ab |
| SXG100-100% | 59.77 ± 0.05 a | 67.63 ± 0.17 a | 81.13 ± 0.39 a | 6.88 ± 0.17 ab |
| SXG100-90% | 61.05 ± 0.16 a | 69.54 ± 0.46 a | 82.87 ± 0.56 a | 7.68 ± 0.34 a |
| SXG100-80% | 63.49 ± 0.76 b | 79.03 ± 0.17 b | 89.05 ± 0.54 b | 2.89 ± 0.14 d |
| Sample | PV | TV | FV | BD | SB | PT |
|---|---|---|---|---|---|---|
| sbeIIb/Lgc1-Native | 817 ± 7 e | 754 ± 13 e | 1508 ± 39 e | 62 ± 14 d | 754 ± 27 e | 7.00 ± 0.00 e |
| sbeIIb/Lgc1-100% | 225 ± 2 g | 179 ± 2 g | 422 ± 6 g | 47 ± 1 d | 243 ± 4 f | 7.00 ± 0.00 e |
| sbeIIb/Lgc1-90% | 93 ± 1 h | 71 ± 1 h | 181 ± 4 h | 22 ± 1 d | 110 ± 3 g | 7.00 ± 0.00 e |
| sbeIIb/Lgc1-80% | 38 ± 6 h | 29 ± 5 h | 59 ± 9 i | 9 ± 2 d | 31 ± 5 h | 6.98 ± 0.04 e |
| sbeIIb/Lgc1-70% | 33 ± 2 h | 25 ± 2 h | 55 ± 2 i | 8 ± 1 d | 30 ± 3 h | 6.98 ± 0.04 e |
| SXG100-Native | 2768 ± 78 a | 1344 ± 12 d | 2183 ± 18 d | 1424 ± 90 a | 839 ± 8 d | 5.62 ± 0.08 a |
| SXG100-100% | 2648 ± 22 b | 2089 ± 59 a | 3869 ± 40 a | 559 ± 74 b | 1780 ± 28 a | 5.91 ± 0.03 b |
| SXG100-90% | 2306 ± 64 c | 2001 ± 37 b | 3630 ± 7 b | 305 ± 35 c | 1629 ± 31 b | 6.13 ± 0.07 c |
| SXG100-80% | 1982 ± 7 d | 1904 ± 11 c | 3248 ± 6 c | 78 ± 8 d | 1344 ± 8 c | 6.55 ± 0.04 d |
| SXG100-70% | 496 ± 2 f | 417 ± 2 f | 672 ± 5 f | 80 ± 4 d | 255 ± 7 f | 7.00 ± 0.00 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Guo, D.; Li, X.; Tang, Z.; Ling, X.; Zhou, T.; Zhang, B. Heat-Moisture Treatment Further Reduces In Vitro Digestibility and Enhances Resistant Starch Content of a High-Resistant Starch and Low-Glutelin Rice. Foods 2021, 10, 2562. https://doi.org/10.3390/foods10112562
Li Z, Guo D, Li X, Tang Z, Ling X, Zhou T, Zhang B. Heat-Moisture Treatment Further Reduces In Vitro Digestibility and Enhances Resistant Starch Content of a High-Resistant Starch and Low-Glutelin Rice. Foods. 2021; 10(11):2562. https://doi.org/10.3390/foods10112562
Chicago/Turabian StyleLi, Zhiyuan, Dongshu Guo, Xiao Li, Zhaocheng Tang, Xitie Ling, Tiantian Zhou, and Baolong Zhang. 2021. "Heat-Moisture Treatment Further Reduces In Vitro Digestibility and Enhances Resistant Starch Content of a High-Resistant Starch and Low-Glutelin Rice" Foods 10, no. 11: 2562. https://doi.org/10.3390/foods10112562
APA StyleLi, Z., Guo, D., Li, X., Tang, Z., Ling, X., Zhou, T., & Zhang, B. (2021). Heat-Moisture Treatment Further Reduces In Vitro Digestibility and Enhances Resistant Starch Content of a High-Resistant Starch and Low-Glutelin Rice. Foods, 10(11), 2562. https://doi.org/10.3390/foods10112562





