Storage Proteins Are Driving Pediatric Hazelnut Allergy in a Lipid Transfer Protein-Rich Area
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Oral Hazelnut Challenges
2.3. Skin Prick Test
2.4. Determination of Specific IgE to Hazelnut
2.5. Statistical Analyses
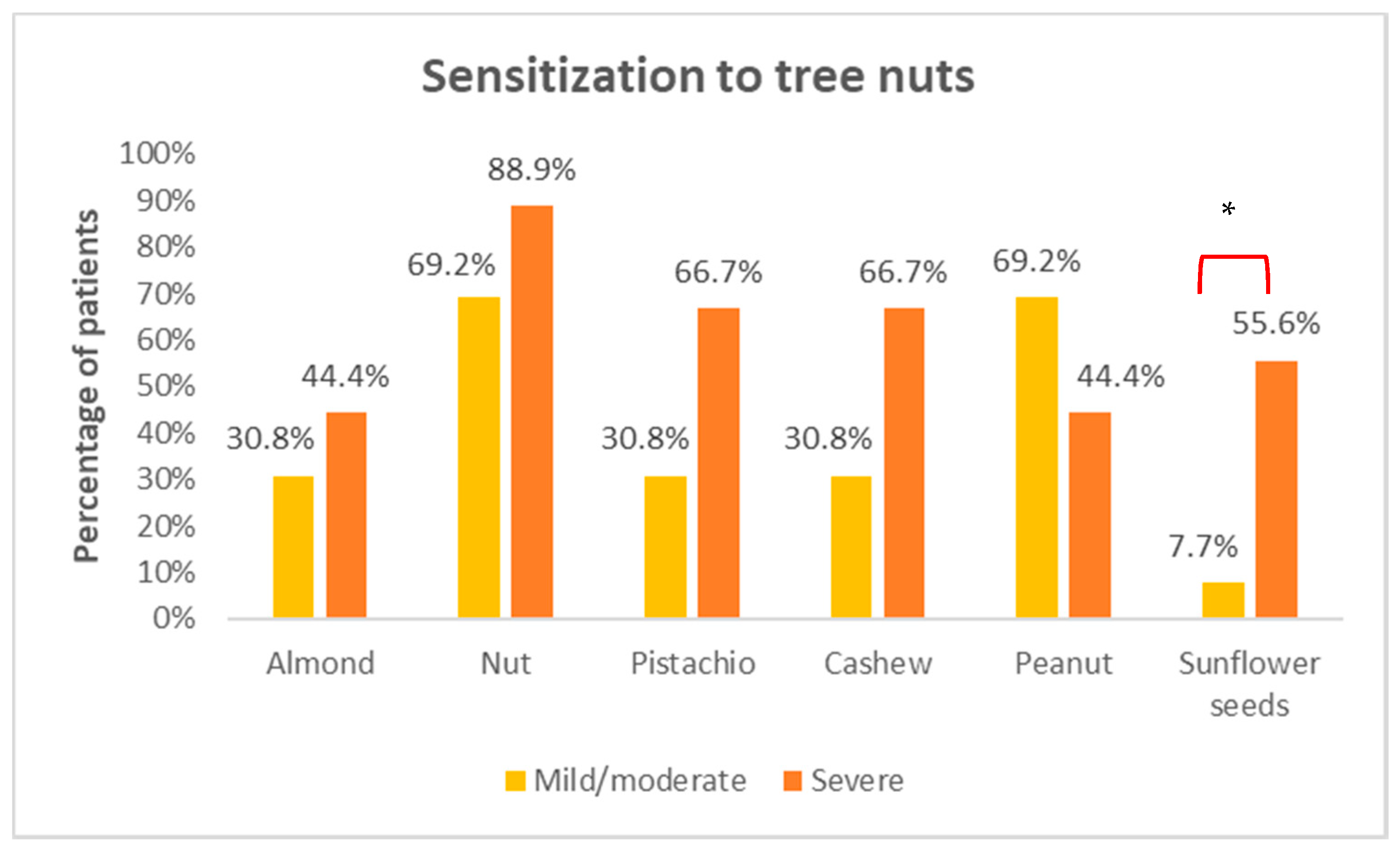
3. Results
3.1. Patients Characteristics
3.2. Food Challenge Results
3.3. Skin Prick Tests
3.4. Sensitization to Hazelnut Allergens
4. Discussion
4.1. Demographic and Clinical Characteristics
4.2. Allergen Dose
4.3. SPT and Specific IgE to Hazelnut
4.4. Component-Resolved Diagnosis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McWilliam, V.; Koplin, J.; Lodge, C.; Tang, M.; Dharmage, S.; Allen, K. The Prevalence of Tree Nut Allergy: A Systematic Review. Curr. Allergy Asthma Rep. 2015, 15, 54. [Google Scholar] [CrossRef]
- McWilliam, V.L.; Perrett, K.P.; Dang, T.; Peters, R.L. Prevalence and Natural History of Tree Nut Allergy. Ann. Allergy. Asthma. Immunol. 2020, 124, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, D.M.; Conover-Walker, M.K.; Matsui, E.C.; Wood, R.A. The Natural History of Tree Nut Allergy. J. Allergy Clin. Immunol. 2005, 116, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Sampson, H.A. Food Allergy: A Review and Update on Epidemiology, Pathogenesis, Diagnosis, Prevention, and Management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wainstein, B.K.; Studdert, J.; Ziegler, M.; Ziegler, J.B. Prediction of Anaphylaxis during Peanut Food Challenge: Usefulness of the Peanut Skin Prick Test (SPT) and Specific IgE Level: Prediction of Anaphylaxis Risk in Peanut Allergy. Pediatr. Allergy Immunol. 2010, 21, 603–611. [Google Scholar] [CrossRef]
- Zhu, J.; Pouillot, R.; Kwegyir-Afful, E.K.; Luccioli, S.; Gendel, S.M. A Retrospective Analysis of Allergic Reaction Severities and Minimal Eliciting Doses for Peanut, Milk, Egg, and Soy Oral Food Challenges. Food Chem. Toxicol. 2015, 80, 92–100. [Google Scholar] [CrossRef]
- Rolinck-Werninghaus, C.; Niggemann, B.; Grabenhenrich, L.; Wahn, U.; Beyer, K. Outcome of Oral Food Challenges in Children in Relation to Symptom-Eliciting Allergen Dose and Allergen-Specific IgE. Allergy 2012, 67, 951–957. [Google Scholar] [CrossRef]
- Santos, A.F.; Du Toit, G.; O’Rourke, C.; Becares, N.; Couto-Francisco, N.; Radulovic, S.; Khaleva, E.; Basting, M.; Harris, K.M.; Larson, D.; et al. Biomarkers of Severity and Threshold of Allergic Reactions during Oral Peanut Challenges. J. Allergy Clin. Immunol. 2020, 146, 344–355. [Google Scholar] [CrossRef]
- Pettersson, M.E.; Koppelman, G.H.; Flokstra-de Blok, B.M.J.; Kollen, B.J.; Dubois, A.E.J. Prediction of the Severity of Allergic Reactions to Foods. Allergy 2018, 73, 1532–1540. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Wang, J.; Leung, N.; Wang, L.X.; Lisann, L.; Sicherer, S.H.; Scurlock, A.M.; Pesek, R.; Perry, T.T.; Jones, S.M.; et al. Correlations between Basophil Activation, Allergen-Specific IgE with Outcome and Severity of Oral Food Challenges. Ann. Allergy. Asthma. Immunol. 2015, 114, 319–326. [Google Scholar] [CrossRef]
- Clark, A.T.; Ewan, P.W. Interpretation of Tests for Nut Allergy in One Thousand Patients, in Relation to Allergy or Tolerance: Interpretation of Tests for Nut Allergy. Clin. Exp. Allergy 2003, 33, 1041–1045. [Google Scholar] [CrossRef]
- Reier-Nilsen, T.; Michelsen, M.M.; Lødrup Carlsen, K.C.; Carlsen, K.-H.; Mowinckel, P.; Nygaard, U.C.; Namork, E.; Borres, M.P.; Håland, G. Predicting Reactivity Threshold in Children with Anaphylaxis to Peanut. Clin. Exp. Allergy 2018, 48, 415–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumchen, K.; Beder, A.; Beschorner, J.; Ahrens, F.; Gruebl, A.; Hamelmann, E.; Hansen, G.; Heinzmann, A.; Nemat, K.; Niggemann, B.; et al. Modified Oral Food Challenge Used with Sensitization Biomarkers Provides More Real-Life Clinical Thresholds for Peanut Allergy. J. Allergy Clin. Immunol. 2014, 134, 390–398.e4. [Google Scholar] [CrossRef]
- Ta, V. Use of Specific IgE and Skin Prick Test to Determine Clinical Reaction Severity. Br. J. Med. Med. Res. 2011, 1, 410–429. [Google Scholar] [CrossRef] [PubMed]
- Summers, C.W.; Pumphrey, R.S.; Woods, C.N.; McDowell, G.; Pemberton, P.W.; Arkwright, P.D. Factors Predicting Anaphylaxis to Peanuts and Tree Nuts in Patients Referred to a Specialist Center. J. Allergy Clin. Immunol. 2008, 121, 632–638.e2. [Google Scholar] [CrossRef] [PubMed]
- Calamelli, E.; Trozzo, A.; Di Blasi, E.; Serra, L.; Bottau, P. Hazelnut Allergy. Medicina 2021, 57, 67. [Google Scholar] [CrossRef] [PubMed]
- Datema, M.R.; van Ree, R.; Asero, R.; Barreales, L.; Belohlavkova, S.; de Blay, F.; Clausen, M.; Dubakiene, R.; Fernández-Perez, C.; Fritsche, P.; et al. Component-Resolved Diagnosis and beyond: Multivariable Regression Models to Predict Severity of Hazelnut Allergy. Allergy 2018, 73, 549–559. [Google Scholar] [CrossRef]
- Giovannini, M.; Comberiati, P.; Piazza, M.; Chiesa, E.; Piacentini, G.L.; Boner, A.; Zanoni, G.; Peroni, D.G. Retrospective Definition of Reaction Risk in Italian Children with Peanut, Hazelnut and Walnut Allergy through Component-Resolved Diagnosis. Allergol. Immunopathol. (Madr.) 2019, 47, 73–78. [Google Scholar] [CrossRef]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; duToit, G.; Eigenmann, P.; et al. EAACI Food Allergy and Anaphylaxis Guidelines: Diagnosis and Management of Food Allergy. Allergy 2014, 69, 1008–1025. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; Gerth van Wijk, R.; Bindslev-Jensen, C.; Sicherer, S.; Teuber, S.S.; Burks, A.W.; Dubois, A.E.J.; Beyer, K.; Eigenmann, P.A.; Spergel, J.M.; et al. Standardizing Double-Blind, Placebo-Controlled Oral Food Challenges: American Academy of Allergy, Asthma & Immunology–European Academy of Allergy and Clinical Immunology PRACTALL Consensus Report. J. Allergy Clin. Immunol. 2012, 130, 1260–1274. [Google Scholar] [CrossRef]
- Ballmer-Weber, B.K.; Beyer, K. Food Challenges. J. Allergy Clin. Immunol. 2018, 141, 69–71.e2. [Google Scholar] [CrossRef] [Green Version]
- Ansotegui, I.J.; Melioli, G.; Canonica, G.W.; Caraballo, L.; Villa, E.; Ebisawa, M.; Passalacqua, G.; Savi, E.; Ebo, D.; Gómez, R.M.; et al. IgE Allergy Diagnostics and Other Relevant Tests in Allergy, a World Allergy Organization Position Paper. World Allergy Organ. J. 2020, 13, 100080. [Google Scholar] [CrossRef] [PubMed]
- Cetinkaya, P.G.; Buyuktiryaki, B.; Soyer, O.; Sahiner, U.M.; Sekerel, B.E. Factors Predicting Anaphylaxis in Children with Tree Nut Allergies. Allergy Asthma Proc. 2019, 40, 180–186. [Google Scholar] [CrossRef]
- Turner, P.J.; Baumert, J.L.; Beyer, K.; Boyle, R.J.; Chan, C.-H.; Clark, A.T.; Crevel, R.W.R.; DunnGalvin, A.; Fernández-Rivas, M.; Gowland, M.H.; et al. Can We Identify Patients at Risk of Life-Threatening Allergic Reactions to Food? Allergy 2016, 71, 1241–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballmer-Weber, B.K.; Fernandez-Rivas, M.; Beyer, K.; Defernez, M.; Sperrin, M.; Mackie, A.R.; Salt, L.J.; Hourihane, J.O.; Asero, R.; Belohlavkova, S.; et al. How Much Is Too Much? Threshold Dose Distributions for 5 Food Allergens. J. Allergy Clin. Immunol. 2015, 135, 964–971. [Google Scholar] [CrossRef]
- Villalta, D.; Scala, E.; Mistrello, G.; Amato, S.; Asero, R. Evidence of Cross-Reactivity between Different Seed Storage Proteins from Hazelnut (Corylus Avellana) and Walnut (Juglans Regia) Using Recombinant Allergen Proteins. Int. Arch. Allergy Immunol. 2019, 178, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Datema, M.R.; Zuidmeer-Jongejan, L.; Asero, R.; Barreales, L.; Belohlavkova, S.; de Blay, F.; Bures, P.; Clausen, M.; Dubakiene, R.; Gislason, D.; et al. Hazelnut Allergy across Europe Dissected Molecularly: A EuroPrevall Outpatient Clinic Survey. J. Allergy Clin. Immunol. 2015, 136, 382–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Bravo, L.; Laiseca-García, J.; Pineda, F.; Rosado, A. Anaphylaxis to Sunflower Seed with Tolerance to Other Nuts. The Role of Lipophilic Allergens. J. Investig. Allergol. Clin. Immunol. 2021, 32. [Google Scholar] [CrossRef]
- Hansen, K.S.; Ballmer-Weber, B.K.; Sastre, J.; Lidholm, J.; Andersson, K.; Oberhofer, H.; Lluch-Bernal, M.; Östling, J.; Mattsson, L.; Schocker, F.; et al. Component-Resolved in Vitro Diagnosis of Hazelnut Allergy in Europe. J. Allergy Clin. Immunol. 2009, 123, 1134–1141.e3. [Google Scholar] [CrossRef]
- Asero, R.; Piantanida, M.; Pinter, E.; Pravettoni, V. The Clinical Relevance of Lipid Transfer Protein. Clin. Exp. Allergy 2018, 48, 6–12. [Google Scholar] [CrossRef] [Green Version]
- Ojeda, P.; Ibáñez, M.; Olaguibel, J.; Sastre, J.; Chivato, T. Alergólogica 2015: A National Survey on Allergic Diseases in the Spanish Pediatric Population. J. Investig. Allergol. Clin. Immunol. 2018, 28, 321–329. [Google Scholar] [CrossRef]
- Haroun-Díaz, E.; Azofra, J.; González-Mancebo, E.; de las Heras, M.; Pastor-Vargas, C.; Esteban, V.; Villalba, M.; Díaz-Perales, A.; Cuesta-Herranz, J. Nut Allergy in Two Different Areas of Spain: Differences in Clinical and Molecular Pattern. Nutrients 2017, 9, 909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goikoetxea, M.J.; D’Amelio, C.M.; Martínez-Aranguren, R.; Gamboa, P.; Garcia, B.E.; Gómez, F.; Fernández, J.; Bartra, J.; Parra, A.; Alvarado, M.I.; et al. Is Microarray Analysis Really Useful and Sufficient to Diagnose Nut Allergy in the Mediterranean Area? J. Investig. Allergol. Clin. Immunol. 2016, 26, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Blanc, F.; Bernard, H.; Ah-Leung, S.; Przybylski-Nicaise, L.; Skov, P.S.; Purohit, A.; Blay, F.; Ballmer-Weber, B.; Fritsche, P.; Rivas, M.F.; et al. Further Studies on the Biological Activity of Hazelnut Allergens. Clin. Transl. Allergy 2015, 5, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kattan, J.D.; Sicherer, S.H.; Sampson, H.A. Clinical Reactivity to Hazelnut May Be Better Identified by Component Testing than Traditional Testing Methods. J. Allergy Clin. Immunol. Pract. 2014, 2, 633–634.e1. [Google Scholar] [CrossRef] [Green Version]
- Buyuktiryaki, B.; Cavkaytar, O.; Sahiner, U.M.; Yilmaz, E.A.; Yavuz, S.T.; Soyer, O.; Sekerel, B.E.; Tuncer, A.; Sackesen, C. Cor a 14, Hazelnut-Specific IgE, and SPT as a Reliable Tool in Hazelnut Allergy Diagnosis in Eastern Mediterranean Children. J. Allergy Clin. Immunol. Pract. 2016, 4, 265–272.e3. [Google Scholar] [CrossRef]
- Carraro, S.; Berardi, M.; Bozzetto, S.; Baraldi, E.; Zanconato, S. COR a 14-Specific IgE Predicts Symptomatic Hazelnut Allergy in Children. Pediatr. Allergy Immunol. 2016, 27, 322–324. [Google Scholar] [CrossRef]
- Verweij, M.; Hagendorens, M.; Trashin, S.; Cucu, T.; Meulenaer, B.D.; Devreese, B.; Bridts, C. Age-Dependent Sensitization to the 7S-Vicilin-Like Protein Cor a 11 From Hazelnut (Corylus Avellana) in a Birch- Endemic Region. J. Investig. Allergol. Clin. Immunol. 2012, 22, 7. [Google Scholar]
| General (n = 22) | Mild/Moderate (n = 13) | Severe (n = 9) | p | |
|---|---|---|---|---|
| Gender | 0.264 | |||
| Male | 18 (81.8%) | 12 (92.3%) | 6 (66. 7%) | |
| Female | 4 (18.2%) | 1 (7.7%) | 3 (33.3%) | |
| Age | 0.357 | |||
| mean ± SD | 8.1 ± 2.7 | 8.5 ± 2.6 | 7.4 ± 2.7 | |
| (min, max) | (4–14) | (5–14) | (4–12) | |
| Pollen-induced rhinoconjunctivitis | 0.380 | |||
| No | 14 (63.6%) | 7 (53.8%) | 7 (77.8%) | |
| Yes | 8 (36.4%) | 6 (46.2%) | 2 (22.2%) | |
| Bronchial asthma | 1 | |||
| No | 16 (72.7%) | 9 (69.2%) | 7 (77.8%) | |
| Yes | 6 (27.3%) | 4 (30.8%) | 2 (22.2%) | |
| Atopic dermatitis | 0.074 | |||
| No | 8 (36.4%) | 7 (53.8%) | 1 (11.1%) | |
| Yes | 14 (63.6%) | 6 (46.2%) | 8 (88.9%) | |
| Food allergy | 0.609 | |||
| No | 5 (22.3%) | 2 (15.4%) | 3 (33.3%) | |
| Yes | 17 (77.3%) | 11 (84.6%) | 6 (66.7%) |
| Skin Tests | Mild/Moderate (n = 13) | Severe (n = 9) | p |
|---|---|---|---|
| Hazelnut SPT | 0.077 | ||
| mean ± SD | 5.4 ± 2.3 | 7.1 ± 1.9 | |
| (min–max) | (0–8) | (5–10) | |
| Hazelnut SPPT | 0.490 | ||
| mean ± SD | 5.8 ± 3.5 | 6.7 ± 1.9 | |
| (min–max) | (0–13) | (4–9) |
| ImmunoCAP® Median [IQR] | ALEX® Median [IQR] | |||||
|---|---|---|---|---|---|---|
| Mild/Mod (n = 13) | Severe (n = 9) | p | Mild/Mod (n = 13) | Severe (n = 9) | p | |
| sIgE hazelnut | 1.6 [5.1] | 14 [37] | 0.042 | NA | NA | |
| rCor a 1 | 0 [0.1] | 0 [0] | 0.683 | 0 [0] | 0 [0.1] | 0.691 |
| rCor a 8 | 0 [1.5] | 0.1 [4.1] | 0.331 | 0 [4.7] | 0.1 [20.5] | 0.498 |
| nCor a 9 | 0.5 [2.1] | 0.9 [11] | 0.216 | 0.4 [1.4] | 2.1 [9] | 0.225 |
| nCor a 11 | NA | NA | 0.8 [5.8] | 7 [13.2] | 0.049 | |
| rCor a 14 | 0.3 [0.9] | 3.5 [14.9] | 0.027 | 9.3 [21.6] | 10.5 [36.6] | 0.548 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valbuena, T.; Reche, M.; Marco, G.; Toboso, I.; Ringauf, A.; Thuissard-Vasallo, I.J.; Lozano-Ojalvo, D.; Martínez-Blanco, M.; Molina, E. Storage Proteins Are Driving Pediatric Hazelnut Allergy in a Lipid Transfer Protein-Rich Area. Foods 2021, 10, 2463. https://doi.org/10.3390/foods10102463
Valbuena T, Reche M, Marco G, Toboso I, Ringauf A, Thuissard-Vasallo IJ, Lozano-Ojalvo D, Martínez-Blanco M, Molina E. Storage Proteins Are Driving Pediatric Hazelnut Allergy in a Lipid Transfer Protein-Rich Area. Foods. 2021; 10(10):2463. https://doi.org/10.3390/foods10102463
Chicago/Turabian StyleValbuena, Teresa, Marta Reche, Guadalupe Marco, Inmaculada Toboso, Anna Ringauf, Israel J. Thuissard-Vasallo, Daniel Lozano-Ojalvo, Mónica Martínez-Blanco, and Elena Molina. 2021. "Storage Proteins Are Driving Pediatric Hazelnut Allergy in a Lipid Transfer Protein-Rich Area" Foods 10, no. 10: 2463. https://doi.org/10.3390/foods10102463






