A Year Following the Onset of the COVID-19 Pandemic: Existing Challenges and Ways the Food Industry Has Been Impacted
Abstract
:1. Introduction
2. COVID-19: Consequences for Food Safety and Food Security
2.1. COVID-19 Pandemic: Food Security vs. Dietary Behavior
2.2. The Transmission of SARS-CoV-2 through Oral/Alimentary Routes
2.3. Animal Origin Products: The Risks of Consumption
3. Live Animals: The Risks of SARS-Cov-2 Transmission to Humans
3.1. Animal Species Susceptible to Infection with SARS-CoV-2
3.2. Humans and Animals with Close Contact: Possible Routes of Transmission and Precautionary Measures for SARS-CoV-2
4. Food Industry: Steps to Prevent SARS-CoV-2 Contamination (Human, Surfaces, and Food)
4.1. Food Inspection Services (Government Agencies): Global Trading (Importing and Exporting)
4.2. Personal Hygiene of Food Workers
4.3. Cleaning the Environment and Facilities
4.4. Coronaviruses (SARS-CoV): Minimum Conditions to Environment Survival and Methods for Inactivation
4.5. Processing Facilities: Food Industry Challenges to Prevent and Control COVID-19
5. From Farm-to-Fork: Protocols to Identify and/or Prevent SARS-Cov-2 on (or in) Foods
5.1. Detection Tools for SARS-CoV-2 in Workers and Facilities
5.2. Coronavirus Disease (COVID-19): Protocol to Employee Illness Working in the Food Industry
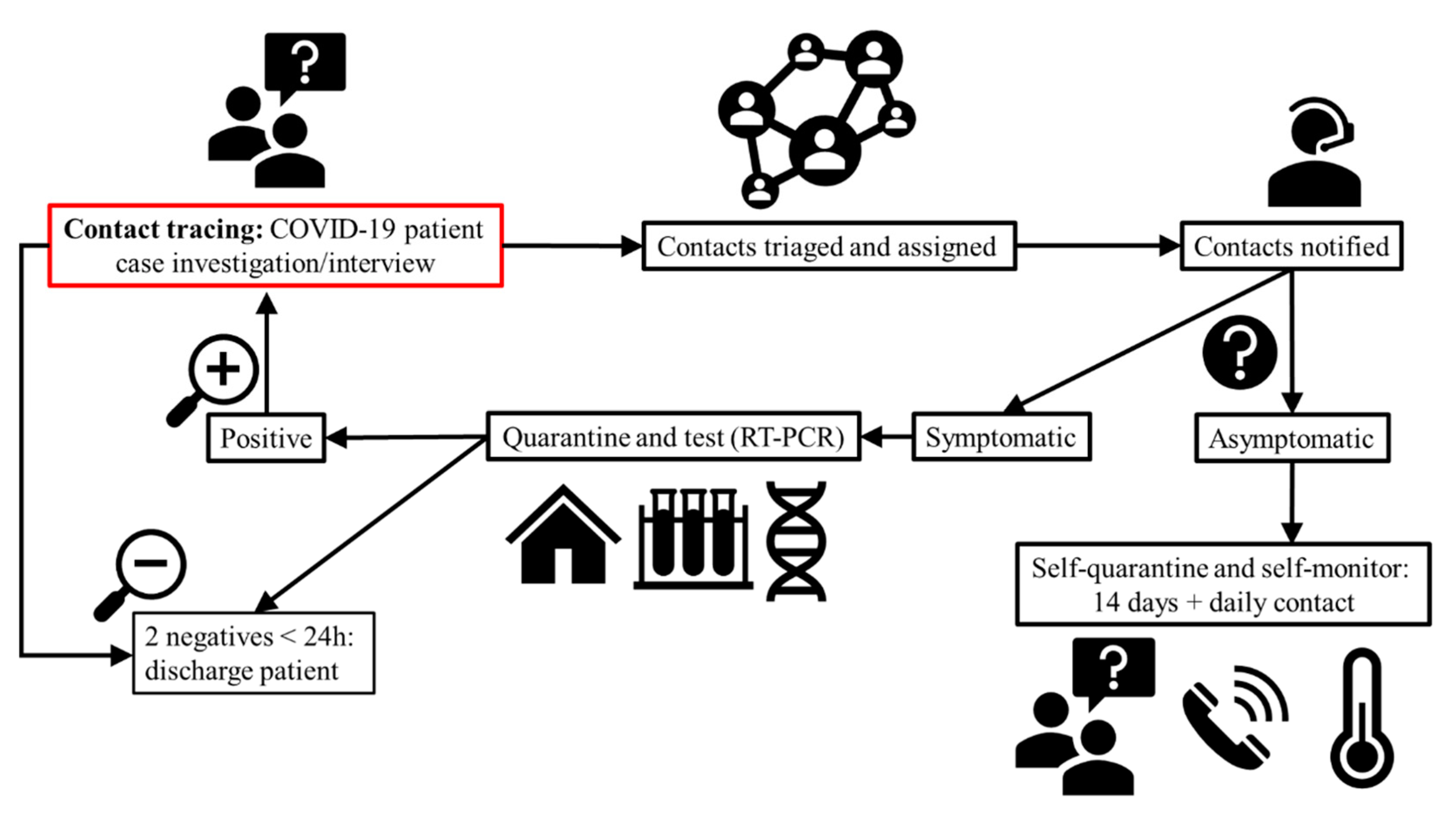
5.3. Exposure to COVID-19: Contact Tracing
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galanakis, C.M. The Food Systems in the Era of the Coronavirus (COVID-19) Pandemic Crisis. Foods 2020, 9, 523. [Google Scholar] [CrossRef]
- WHO. WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 13 January 2021).
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA J. Am. Med. Assoc. 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, Q.; Zhang, Z. Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Curr. Biol. 2020, 30, 1346–1351. [Google Scholar] [CrossRef]
- Hossain, M.G.; Javed, A.; Akter, S.; Saha, S. SARS-CoV-2 host diversity: An update of natural infections and experimental evidence. J. Microbiol. Immunol. Infect. 2020, 54, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Rizou, M.; Galanakis, I.M.; Aldawoud, T.M.S.; Galanakis, C.M. Safety of foods, food supply chain and environment within the COVID-19 pandemic. Trends Food Sci. Technol. 2020, 102, 293–299. [Google Scholar] [CrossRef]
- Yuan, J.; Lu, Y.; Cao, X.; Cui, H. Regulating wildlife conservation and food safety to prevent human exposure to novel virus. Ecosyst. Heal. Sustain. 2020, 6, 1741325. [Google Scholar] [CrossRef] [Green Version]
- WHO. Origin of SARS-CoV-2. Available online: https://www.who.int/publications-detail/origin-of-sars-cov-2 (accessed on 6 July 2020).
- Brief, F.P. Food Security. FAO’s Agric. Dev. Econ. Div. 2006, 43, 1–4. [Google Scholar]
- Xie, X.; Huang, L.; Li, J.J.; Zhu, H. Generational Differences in Perceptions of Food Health/Risk and Attitudes toward Organic Food and Game Meat: The Case of the COVID-19 Crisis in China. Int. J. Environ. Res. Public Health 2020, 17, 3148. [Google Scholar] [CrossRef]
- AIFS What is Food Safety? Australian Institute of Food Safety. Available online: https://www.foodsafety.com.au/blog/what-is-food-safety (accessed on 6 July 2020).
- FAO. Food Safety: Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 6 July 2020).
- Desai, A.N.; Aronoff, D.M. Food Safety and COVID-19. JAMA J. Am. Med. Assoc. 2020, 323, 1982. [Google Scholar] [CrossRef]
- Sidor, A.; Rzymski, P. Dietary Choices and Habits during COVID-19 Lockdown: Experience from Poland. Nutrients 2020, 12, 1657. [Google Scholar] [CrossRef]
- EFSA. Coronavirus: No Evidence That Food is a Source or Transmission Route. Available online: https://www.efsa.europa.eu/en/news/coronavirus-no-evidence-food-source-or-transmission-route (accessed on 6 July 2020).
- FAO/WHO. Food Safety in The Time of COVID-19. Available online: http://www.fao.org/policy-support/tools-and-publications/resources-details/en/c/1271409/ (accessed on 17 July 2021).
- Niles, M.T.; Bertmann, F.; Belarmino, E.H.; Wentworth, T.; Biehl, E.; Neff, R. The Early Food Insecurity Impacts of COVID-19. Nutrients 2020, 12, 2096. [Google Scholar] [CrossRef] [PubMed]
- Dyal, J.W.; Grant, M.P.; Broadwater, K.; Bjork, A.; Waltenburg, M.A.; Gibbins, J.D.; Hale, C.; Silver, M.; Fischer, M.; Steinberg, J.; et al. COVID-19 Among Workers in Meat and Poultry Processing Facilities—19 States, April 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Laborde, D.; Martin, W.; Swinnen, J.; Vos, R. COVID-19 risks to global food security. Science 2020, 369, 500–502. [Google Scholar] [CrossRef]
- Middleton, J.; Reintjes, R.; Lopes, H. Meat plants—A new front line in the covid-19 pandemic. BMJ 2020, 370, m2716. [Google Scholar] [CrossRef] [PubMed]
- Bakalis, S.; Valdramidis, V.P.; Argyropoulos, D.; Ahrne, L.; Chen, J.; Cullen, P.J.; Cummins, E.; Datta, A.K.; Emmanouilidis, C.; Foster, T.; et al. Perspectives from CO+RE: How COVID-19 changed our food systems and food security paradigms. Curr. Res. Food Sci. 2020, 3, 166. [Google Scholar] [CrossRef]
- Batlle-Bayer, L.; Aldaco, R.; Bala, A.; Puig, R.; Laso, J.; Margallo, M.; Vázquez-Rowe, I.; Antó, J.M.; Fullana-i-Palmer, P. Environmental and nutritional impacts of dietary changes in Spain during the COVID-19 lockdown. Sci. Total Environ. 2020, 748, 141410. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Molina-Montes, E.; Verardo, V.; Artacho, R.; García-Villanova, B.; Guerra-Hernández, E.J.; Ruíz-López, M.D. Changes in Dietary Behaviours during the COVID-19 Outbreak Confinement in the Spanish COVIDiet Study. Nutrients 2020, 12, 1730. [Google Scholar] [CrossRef]
- Ruiz-Roso, M.B.; de Carvalho Padilha, P.; Mantilla-Escalante, D.C.; Ulloa, N.; Brun, P.; Acevedo-Correa, D.; Peres, W.A.F.; Martorell, M.; Aires, M.T.; de Oliveira Cardoso, L.; et al. Covid-19 Confinement and Changes of Adolescent’s Dietary Trends in Italy, Spain, Chile, Colombia and Brazil. Nutrients 2020, 12, 1807. [Google Scholar] [CrossRef]
- Scarmozzino, F.; Visioli, F. Covid-19 and the Subsequent Lockdown Modified Dietary Habits of Almost Half the Population in an Italian Sample. Foods 2020, 9, 675. [Google Scholar] [CrossRef]
- Elleby, C.; Domínguez, I.P.; Adenauer, M.; Genovese, G. Impacts of the COVID-19 Pandemic on the Global Agricultural Markets. Environ. Resour. Econ. 2020, 76, 1067–1079. [Google Scholar] [CrossRef]
- Wolfson, J.A.; Leung, C.W. Food Insecurity and COVID-19: Disparities in Early Effects for US Adults. Nutrients 2020, 12, 1648. [Google Scholar] [CrossRef]
- Ayittey, F.K.; Ayittey, M.K.; Chiwero, N.B.; Kamasah, J.S.; Dzuvor, C. Economic impacts of Wuhan 2019-nCoV on China and the world. J. Med. Virol. 2020, 92, 473. [Google Scholar] [CrossRef] [Green Version]
- Commission, E. A Farm to Fork Strategy for a fair, Healthy and Environmentally-Friendly Food Dystem. Available online: https://ec.europa.eu/food/horizontal-topics/farm-fork-strategy_ro (accessed on 23 July 2021).
- WHO. COVID-19 and Food Safety: Guidance for Competent Authorities Responsible for National Food Safety Control Systems. Available online: https://www.who.int/publications/i/item/covid-19-and-food-safety-guidance-for-competent-authorities-responsible-for-national-food-safety-control-systems (accessed on 6 July 2020).
- WHO. Estimating the Burden of Foodborne Diseases. Available online: https://www.who.int/activities/estimating-the-burden-of-foodborne-diseases#:~:text=Eachyearworldwide%2Cunsafefood,under5yearsofage (accessed on 6 July 2020).
- Murdoch, J.; Marsden, T.; Banks, J. Quality, Nature, and Embeddedness: Some Theoretical Considerations in the Context of the Food Sector. Econ. Geogr. 2000, 76, 107–125. [Google Scholar] [CrossRef]
- Pussemier, L.; Larondelle, Y.; Van Peteghem, C.; Huyghebaert, A. Chemical safety of conventionally and organically produced foodstuffs: A tentative comparison under Belgian conditions. Food Control. 2006, 17, 14–21. [Google Scholar] [CrossRef]
- CDC. Food and Coronavirus Disease 2019 (COVID-19). Available online: https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/food-and-COVID-19.html (accessed on 14 January 2021).
- Huang, Z.; Liu, Y.; Qi, G.; Brand, D.; Zheng, S.G. Role of Vitamin A in the Immune System. J. Clin. Med. 2018, 7, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, A.; Edgar, J.D.; Neville, C.E.; Gilchrist, S.E.; McKinley, M.C.; Patterson, C.C.; Young, I.S.; Woodside, J. V Effect of fruit and vegetable consumption on immune function in older people: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 96, 1429–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wang, Y.; Ye, D.; Liu, Q. Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. Int. J. Antimicrob. Agents 2020, 55, 105948. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Bikle, D.; Hewison, M.; Lazaretti-Castro, M.; Formenti, A.M.; Gupta, A.; Madhavan, M.V.; Nair, N.; Babalyan, V.; Hutchings, N.; et al. Mechanisms in Endocrinology: Vitamin D and COVID-19. Eur. J. Endocrinol. 2020, 183, R133–R147. [Google Scholar] [CrossRef]
- Djekic, I.; Nikolić, A.; Uzunović, M.; Marijke, A.; Liu, A.; Han, J.; Brnčić, M.; Knežević, N.; Papademas, P.; Lemoniati, K.; et al. Covid-19 pandemic effects on food safety-Multi-country survey study. Food Control 2021, 122, 107800. [Google Scholar] [CrossRef]
- International Monetary Fund World Economic Outlook Update, June 2020. A Crisis Like No Other, An Uncertain Recovery. Available online: https://www.imf.org/en/Publications/WEO/Issues/2020/06/24/WEOUpdateJune2020 (accessed on 6 July 2020).
- Jalava, K. First respiratory transmitted food borne outbreak? Int. J. Hyg. Environ. Health 2020, 226, 113490. [Google Scholar] [CrossRef] [PubMed]
- Nijman, V. An overview of international wildlife trade from Southeast Asia. Biodivers. Conserv. 2010, 19, 1101–1114. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, J.J.; Xie, X.; Cai, X.; Huang, J.; Tian, X.; Zhu, H. Game consumption and the 2019 novel coronavirus. Lancet Infect. Dis. 2020, 20, 275–276. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.H.; Sridhar, S.; Zhang, R.R.; Chu, H.; Fung, A.Y.F.; Chan, G.; Chan, J.F.W.; To, K.K.W.; Hung, I.F.N.; Cheng, V.C.C.; et al. Factors affecting stability and infectivity of SARS-CoV-2. J. Hosp. Infect. 2020, 106, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.; Smith, D.; Ghai, R.R.; Wallace, R.M.; Torchetti, M.K.; Loiacono, C.; Murrell, L.S.; Carpenter, A.; Moroff, S.; Rooney, J.A.; et al. First Reported Cases of SARS-CoV-2 Infection in Companion Animals—New York, March–April 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 710–713. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Disinfection of Environments in Healthcare and Non-Healthcare Settings Potentially Contaminated with SARS-CoV-2. Available online: https://www.ecdc.europa.eu/en/publications-data/disinfection-environments-covid-19 (accessed on 17 July 2021).
- WHO. COVID-19 and Food Safety: Guidance for Food Businesses. Available online: https://www.who.int/publications/i/item/covid-19-and-food-safety-guidance-for-food-businesses (accessed on 17 July 2021).
- WHO. Q&A: How Is COVID-19 Transmitted? Available online: https://www.who.int/news-room/q-a-detail/q-a-how-is-covid-19-transmitted (accessed on 17 July 2020).
- WHO. Transmission of SARS-CoV-2: Implications for Infection Prevention Precautions. Available online: https://www.who.int/publications/i/item/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations (accessed on 16 July 2020).
- Fears, A.C.; Klimstra, W.B.; Duprex, P.; Hartman, A.; Weaver, S.C.; Plante, K.S.; Mirchandani, D.; Plante, J.A.; Aguilar, P.V.; Fernández, D.; et al. Persistence of Severe Acute Respiratory Syndrome Coronavirus 2 in Aerosol Suspensions. Emerg. Infect. Dis. 2020, 26, 2168. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- WHO. Questions Relating to Food Businesses. Available online: https://www.who.int/news-room/q-a-detail/questions-relating-to-food-businesses (accessed on 16 July 2020).
- OIE. Questions and Answers on COVID-19. Available online: https://www.oie.int/en/scientific-expertise/specific-information-and-recommendations/questions-and-answers-on-2019novel-coronavirus/ (accessed on 21 July 2020).
- Sit, T.H.C.; Brackman, C.J.; Ip, S.M.; Tam, K.W.S.; Law, P.Y.T.; To, E.M.W.; Yu, V.Y.T.; Sims, L.D.; Tsang, D.N.C.; Chu, D.K.W.; et al. Infection of dogs with SARS-CoV-2. Nature 2020, 586, 776–778. [Google Scholar] [CrossRef]
- OIE. Infection with SARS-Cov-2 in Animals. Available online: https://www.oie.int/fileadmin/Home/MM/EN_Factsheet_SARS-CoV-2.pdf (accessed on 17 July 2021).
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [Green Version]
- WHO. Questions Relating to Food Safety Authorities. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/questions-relating-to-food-safety-authorities (accessed on 7 July 2020).
- OIE. Events in Animals. OIE—World Organisation for Animal Health. Available online: https://www.oie.int/en/scientific-expertise/specific-information-and-recommendations/questions-and-answers-on-2019novel-coronavirus/events-in-animals/ (accessed on 21 July 2020).
- CDC. What to Do If Your Pet Tests Positive for the Virus that Causes COVID-19. National Center for Immunization and Respiratory Diseases (NCIRD). Available online: https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/positive-pet.html (accessed on 17 September 2020).
- Durand-Moreau, Q.; Adisesh, A.; Mackenzie, G.; Bowley, J.; Straube, S.; Chan, X.H.; Zelyas, N.; Greenhalgh, T. What Explains the High Rate of SARS-CoV-2 Transmission in Meat and Poultry Facilities? Available online: https://www.cebm.net/covid-19/what-explains-the-high-rate-of-sars-cov-2-transmission-in-meat-and-poultry-facilities-2/ (accessed on 6 July 2020).
- MAGyP. Lineamientos de Buenas Prácticas en Distintas Producciones Agropecuarias. Available online: https://www.argentina.gob.ar/agricultura/covid-19 (accessed on 7 January 2021).
- Senasa/MAGyP. Lineamientos de Buenas Prácticas para la Producción Agropecuaria Para el COVID-19 Plantas Frigoríficas. Available online: https://magyp.gob.ar/covid-19/COVID-19_PLANTASFRIGORIFICAS_x.pdf (accessed on 7 January 2021).
- ME/MS/MAPA. Orientações Gerais para Frigoríficos em Razão da Pandemia da Covid-19. Available online: https://www.gov.br/agricultura/pt-br/campanhas/mapacontracoronavirus/documentos/manual-orientacoes-gerais-para-frigorificos-em-razao-da-pandemia-da-covid-19/view (accessed on 23 July 2021).
- ME. Portaria Conjunta no 19, de 18 de Junho de 2020. Available online: http://www.in.gov.br/en/web/dou/-/portaria-conjunta-n-19-de-18-de-junho-de-2020-262407973 (accessed on 30 July 2020).
- NDDB. Covid-19-Updates. Available online: https://www.nddb.coop/covid-19-updates (accessed on 5 January 2021).
- USDA. Coronavirus Disease (COVID-19). Available online: https://www.usda.gov/coronavirus (accessed on 5 January 2021).
- Lai, M.Y.Y.; Cheng, P.K.C.; Lim, W.W.L. Survival of severe acute respiratory syndrome coronavirus. Clin. Infect. Dis. an Off. Publ. Infect. Dis. Soc. Am. 2005, 41, e67–e71. [Google Scholar] [CrossRef] [Green Version]
- EPA. Is Drinking Tap Water Safe? Available online: https://www.epa.gov/coronavirus/drinking-tap-water-safe (accessed on 6 July 2020).
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A.; Hui, K.P.Y.; Yen, H.-L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef]
- WHO. Considerations for Quarantine of Contacts of COVID-19 Cases: Interim guidance. Available online: https://apps.who.int/iris/handle/10665/333901 (accessed on 23 July 2021).
- Goldman, E. Exaggerated risk of transmission of COVID-19 by fomites. Lancet Infect. Dis. 2020, 20, 892–893. [Google Scholar] [CrossRef]
- Duan, S.M.; Zhao, X.S.; Wen, R.F.; Huang, J.J.; Pi, G.H.; Zhang, S.X.; Han, J.; Bi, S.L.; Ruan, L.; Dong, X.P. Stability of SARS Coronavirus in Human Specimens and Environment and Its Sensitivity to Heating and UV Irradiation. Biomed. Environ. Sci. 2003, 16, 246–255. [Google Scholar] [PubMed]
- Rabenau, H.F.; Cinatl, J.; Morgenstern, B.; Bauer, G.; Preiser, W.; Doerr, H.W. Stability and inactivation of SARS coronavirus. Med. Microbiol. Immunol. 2005, 194, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BfR. Can the New Type of Coronavirus Be Transmitted via Food and Objects? Available online: https://www.bfr.bund.de/en/can_the_new_type_of_coronavirus_be_transmitted_via_food_and_objects_-244090.html. (accessed on 23 July 2021).
- Riddell, S.; Goldie, S.; Hill, A.; Eagles, D.; Drew, T.W. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol. J. 2020, 17, 145. [Google Scholar] [CrossRef] [PubMed]
- CISA. Advisory Memorandum on Ensuring Essential Critical Infrastructure Workers Ability To Work During the Covid-19 Response; U.S. Department of Homeland Security: Washington, DC, USA, 2020. [Google Scholar]
- Liu, F.; Rhim, H.; Park, K.; Xu, J.; Lo, C.K.Y. HACCP certification in food industry: Trade-offs in product safety and firm performance. Int. J. Prod. Econ. 2021, 231, 107838. [Google Scholar] [CrossRef]
- ISO. ISO Management System Standards. Available online: https://www.iso.org/management-system-standards.html%0Ahttps://www.iso.org/management-system-standards-list.html (accessed on 11 January 2021).
- ISO. ISO 22000—Food Safety Management. Available online: http://www.iso.org/iso/home/standards/management-standards/iso22000.htm (accessed on 11 January 2021).
- FDA. HACCP Principles & Application Guidelines. National Advisory Committee on Microbiological Criteria for Foods (NACMCF). Available online: http://www.fda.gov/Food/GuidanceRegulation/HACCP/ucm2006801.htm#execsum (accessed on 6 July 2020).
- Rai, P.; Kumar, B.K.; Deekshit, V.K.; Karunasagar, I.; Karunasagar, I. Detection technologies and recent developments in the diagnosis of COVID-19 infection. Appl. Microbiol. Biotechnol. 2021, 105, 1–15. [Google Scholar] [CrossRef]
- Pascarella, G.; Strumia, A.; Piliego, C.; Bruno, F.; Del Buono, R.; Costa, F.; Scarlata, S.; Agrò, F.E. COVID-19 diagnosis and management: A comprehensive review. J. Intern. Med. 2020, 288, 192–206. [Google Scholar] [CrossRef]
- Udugama, B.; Kadhiresan, P.; Kozlowski, H.N.; Malekjahani, A.; Osborne, M.; Li, V.Y.C.; Chen, H.; Mubareka, S.; Gubbay, J.B.; Chan, W.C.W. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano 2020, 14, 3822–3835. [Google Scholar] [CrossRef] [Green Version]
- Yüce, M.; Filiztekin, E.; Özkaya, K.G. COVID-19 diagnosis —A review of current methods. Biosens. Bioelectron. 2021, 172, 112752. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Chen, Y.; Qin, Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J. Med. Virol. 2020, 92, 568–576. [Google Scholar] [CrossRef] [Green Version]
- Chia, P.Y.; Coleman, K.K.; Tan, Y.K.; Ong, S.W.X.; Gum, M.; Lau, S.K.; Lim, X.F.; Lim, A.S.; Sutjipto, S.; Lee, P.H.; et al. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat. Commun. 2020, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, W.; Truchado, P.; Cuevas-Ferrando, E.; Simón, P.; Allende, A.; Sánchez, G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020, 181, 115942. [Google Scholar] [CrossRef] [PubMed]
- WHO. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19. Available online: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf (accessed on 23 July 2021).
- WHO. International Health Regulations (2005), 3rd. ed.; World Health Organization: Geneva, Switzerland, 2016; 91p. [Google Scholar]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J.; El-harakeh, A.; Bognanni, A.; Lotfi, T.; Loeb, M.; et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef]
- Jones, N.R.; Qureshi, Z.U.; Temple, R.J.; Larwood, J.P.J.; Greenhalgh, T.; Bourouiba, L. Two metres or one: What is the evidence for physical distancing in covid-19? BMJ 2020, 370, m3223. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, Z.; Jones, N.; Temple, R.; Larwood, J.P.J.; Greenhalgh, T.; Bourouiba, L. What is the Evidence to Support the 2-Metre Social Distancing Rule to Reduce COVID-19 Transmission? Available online: https://www.cebm.net/covid-19/what-is-the-evidence-to-support-the-2-metre-social-distancing-rule-to-reduce-covid-19-transmission/ (accessed on 5 January 2021).
- CDC. Contact Tracing for COVID-19. National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. Available online: https://www.cdc.gov/coronavirus/2019-ncov/php/contact-tracing/contact-tracing-plan/contact-tracing.html (accessed on 27 August 2020).
- CDC. Public Health Guidance for Community-Related Exposure. Available online: https://www.cdc.gov/coronavirus/2019-ncov/php/public-health-recommendations.html (accessed on 27 August 2020).
- WHO. Contact Tracing in the Context of COVID-19—Interim Guidance. Available online: https://www.who.int/publications/i/item/contact-tracing-in-the-context-of-covid-19 (accessed on 6 July 2020).
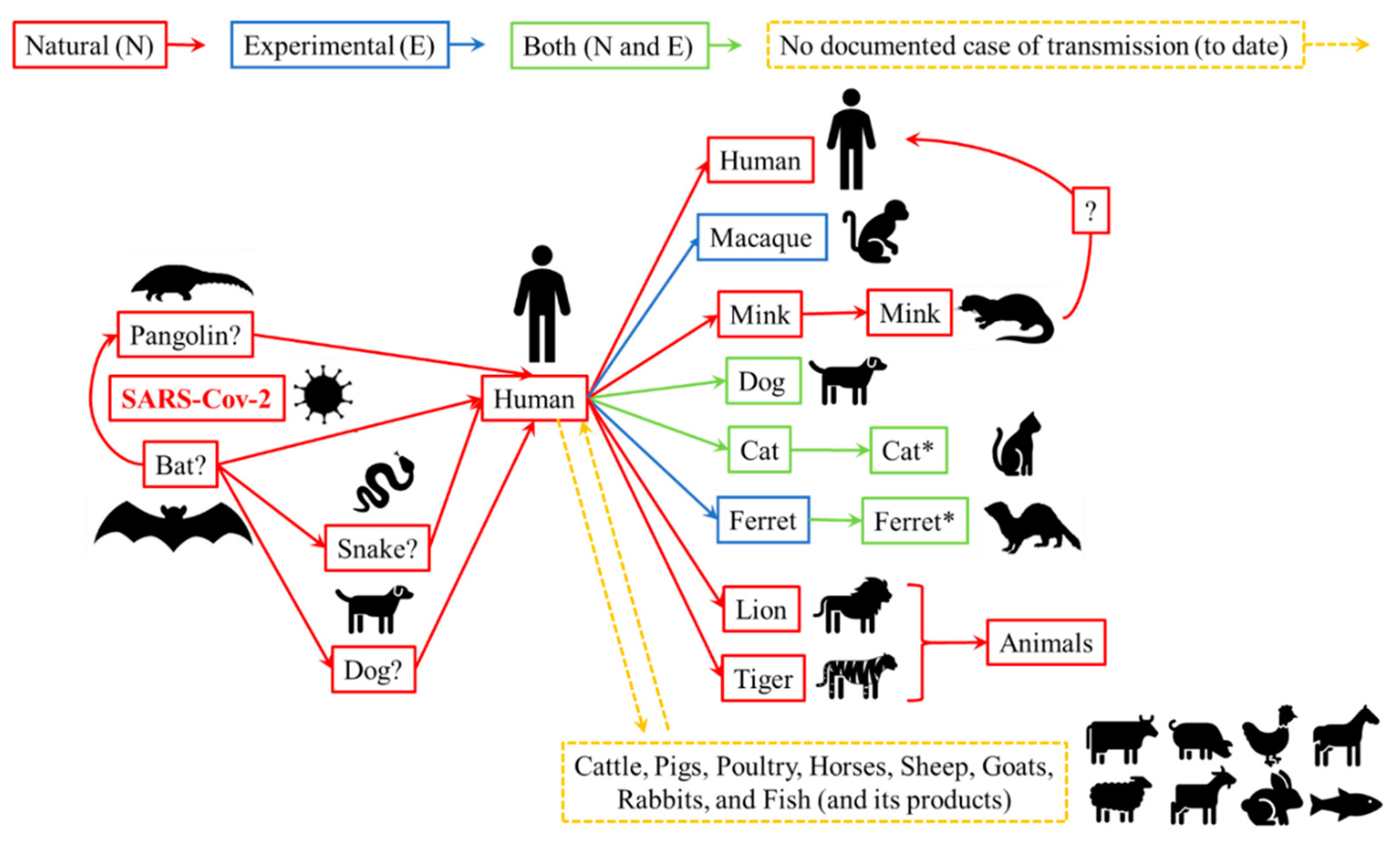
| Species * | Infection (N or/and E) | Susceptibility (None/Low/High) | Clinical Signs | Transmission |
|---|---|---|---|---|
| Pigs | E | None | None | None |
| Poultry | E | None | None | None |
| Dogs | N and E | Low | Possible | Possible (suggested) |
| Cats | N and E | High | Possible ** | Possible *** |
| Tigers and lions | N | High | Possible | Possible (between animals) |
| Ferrets | E | High | None or very mild | Possible *** |
| Minks | N | High | Possible | Possible ***; Mink to humans (suggested) |
| Fruit bats | E | High | No | Possible *** |
| Hamsters | E | High | Possible ** | Possible *** |
| Macaques | E | High | Possible | Possible |
| Places | Disinfectant | Concentration | Time | Ref. |
|---|---|---|---|---|
| Hands | Ethanol | 60% | NS | [13] |
| Hands & Surfaces | Ethanol | 70% | NS | [47,64,65,70,71] * |
| Surfaces | Alcohol-based disinfectants (ethanol, propan-2-ol, propan-1-ol) | 70–80% | within 1 min | [53] |
| Ethanol | 62–71% | within 1 min | [56] | |
| 60–85% | NS | [16] ** | ||
| Sodium hypochlorite (NaClO) | 0.05% | NS | [16,47] | |
| 1% | NS | [68,70] + | ||
| Povidone-iodine | 7.5% | NS | [70] | |
| Chloroxylenol | 0.05% | NS | [70] | |
| Chlorhexidine | 0.05% | NS | [70] | |
| Chlorhexidine digluconate | 0.02% | NS | [56] ++ | |
| Hydrogen peroxide | 0.5% | within 1 min | [56] | |
| Benzalkonium chloride | 0.05–0.2% | NS | [56] ++ | |
| 0.1% | NS | [70] | ||
| Cleaning equipmentused | Household soap or detergent | 0.1% | within 1 min | [47] *+ |
| Sodium hypochlorite (NaClO) | [47,56] ** | |||
| Textiles | Virucidal disinfectant | NS | [47] | |
| Hot-water cycle (90 °C) and regular laundry detergent | [47,71] | |||
| Lower temperature cycle and bleach | [47] |
| Source | Survival Time (up to) | Ref. |
|---|---|---|
| Dishes (dishwasher) | wash cycle * | [75] |
| Paper/Tissue paper | 3 h | [68,70] |
| 28 days (20 ℃) *+ | [76] | |
| Copper surfaces | 4 h ** | [52] |
| Environmental + | 3 h ** | [52,56] |
| 16 h | [51] | |
| Stool specimen (20 °C) | 1 day ++ | [68] |
| 2 days | [45] | |
| Cardboard | 24 h ** | [52] |
| Wood | 2 days | [70] |
| Cloth | 1 day ++ | [68] |
| 2 days | [70] | |
| Stainless steel | 3 days ** | [52] |
| 7 days | [70] | |
| 9 days ++ | [56] | |
| 28 days (20 ℃) *+ | [76] | |
| Plastic | 2 days ++ | [68] |
| 3 days ** | [52] | |
| 7 days | [70] | |
| 9 days ++ | [56] | |
| Glass | 4 days | [45,70] |
| 9 days ++ | [56] | |
| 28 days (20 ℃) *+ | [76] | |
| Mask | ||
| Inner layer | 7 days | [70] |
| Outer layer | More than 14 days | |
| Ambient | ||
| 70 °C | 5 min | [16,70] |
| 37 °C | from 1 to 2 days | [45,70] |
| from 20 to 25 °C | from 3 to 5 days | [45] |
| 4 °C | >14 days (highly stable) | [45,48,70] |
| –20 °C | 2 years | [1,16,48,75] |
| Sewage water | ||
| 20 °C | 2 days ++ | [56] |
| 4 °C | 14 days ++ | |
| Respiratory specimen (throat and nasal) | ||
| 20 °C | 5 days ++ | [68] |
| 4 °C | 21 days ++ | |
| Category | Challenges According to Practices and Sectors | Suggestions |
|---|---|---|
| Structural | Breaks, entering and exiting the facility (physical distancing) |
|
| Production line (Physical distancing) |
| |
| Worker’s health |
| |
| Operational | Production line (physical distancing) |
|
| Face covering recommendations |
| |
| Cleaning and disinfection routine |
| |
| Processing rate for animals andcarcasses |
| |
| Sociocultural | Employees that live in crowded, multigenerational settings |
|
| Employee transportation (to and from work) |
|
| Sample Collect and/or Technique | Assays | Laboratory Method |
|---|---|---|
| Swab (NAAT) | RNA | RT-qPCR RT-LAMP NGS CRISPR ddPCR CBNAAT |
| Blood (Serological) | Ag/Ab | ELISA POC Lateral Flow CLIA |
| Image | Patient Chest | CT X-ray Ultrasound |
| Varied specimens | Virus particles | Electron microscopy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Ramella, M.; Lorenzo, J.M.; Bohrer, B.M.; Pateiro, M.; Cantalapiedra, J.J.; Franco, D. A Year Following the Onset of the COVID-19 Pandemic: Existing Challenges and Ways the Food Industry Has Been Impacted. Foods 2021, 10, 2389. https://doi.org/10.3390/foods10102389
Vargas-Ramella M, Lorenzo JM, Bohrer BM, Pateiro M, Cantalapiedra JJ, Franco D. A Year Following the Onset of the COVID-19 Pandemic: Existing Challenges and Ways the Food Industry Has Been Impacted. Foods. 2021; 10(10):2389. https://doi.org/10.3390/foods10102389
Chicago/Turabian StyleVargas-Ramella, Márcio, José M. Lorenzo, Benjamin M. Bohrer, Mirian Pateiro, Jesús J. Cantalapiedra, and Daniel Franco. 2021. "A Year Following the Onset of the COVID-19 Pandemic: Existing Challenges and Ways the Food Industry Has Been Impacted" Foods 10, no. 10: 2389. https://doi.org/10.3390/foods10102389








