Lactobacillus rhamnosus Probio-M9 Improves the Quality of Life in Stressed Adults by Gut Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Questionnaire Survey
2.4. DNA Extraction and Metagenomic Sequencing
2.5. Bioinformatics Analysis
2.6. Fecal Metabolomics
2.7. Statistical Analysis
2.8. Data and Code Availability
3. Results
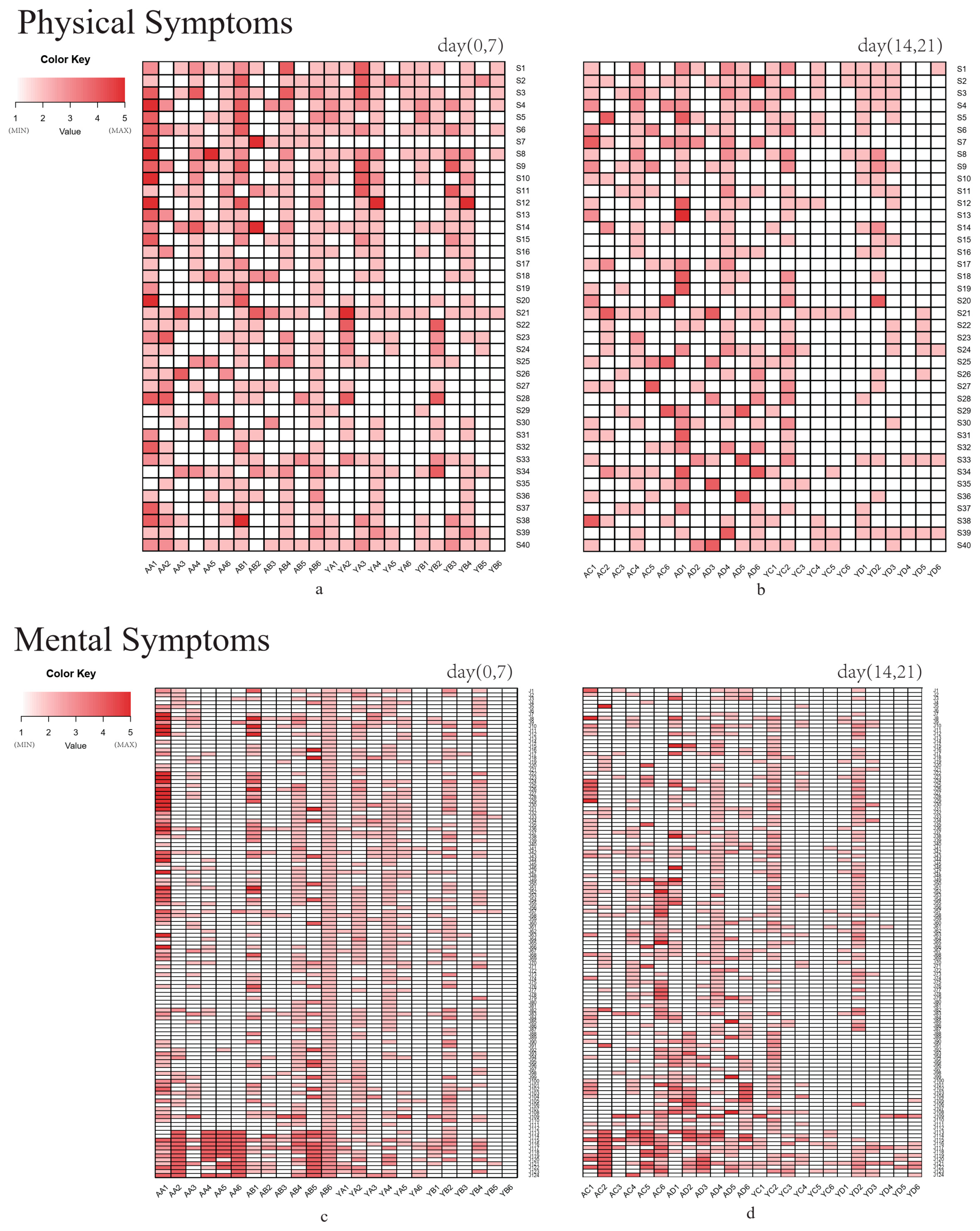
3.1. Quality of Life Questionnaire Analysis
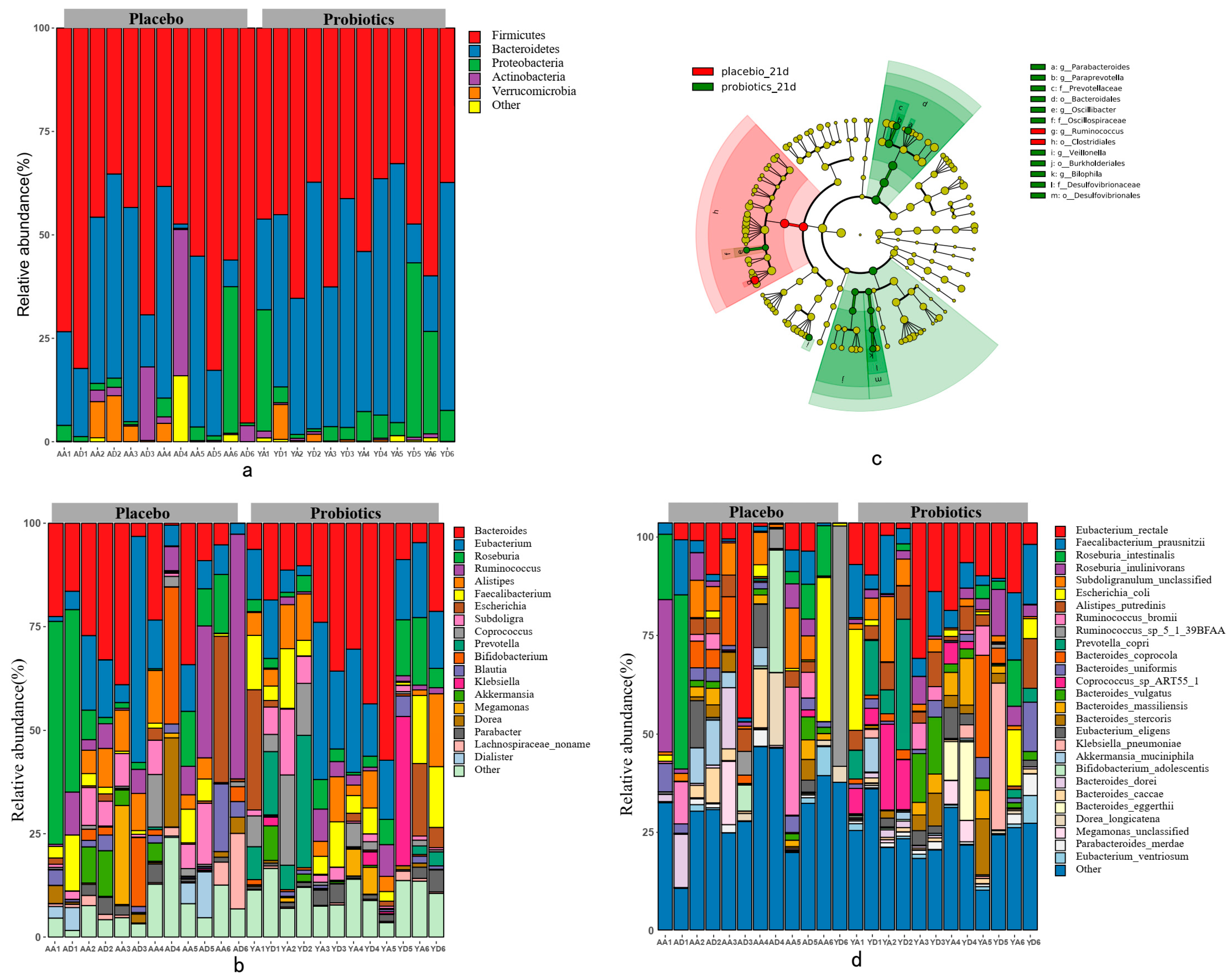
3.2. The Effect of the Probiotic Intervention on the Gut Microbiota Composition
3.3. Genomic Characteristics of the Gut Microbiome
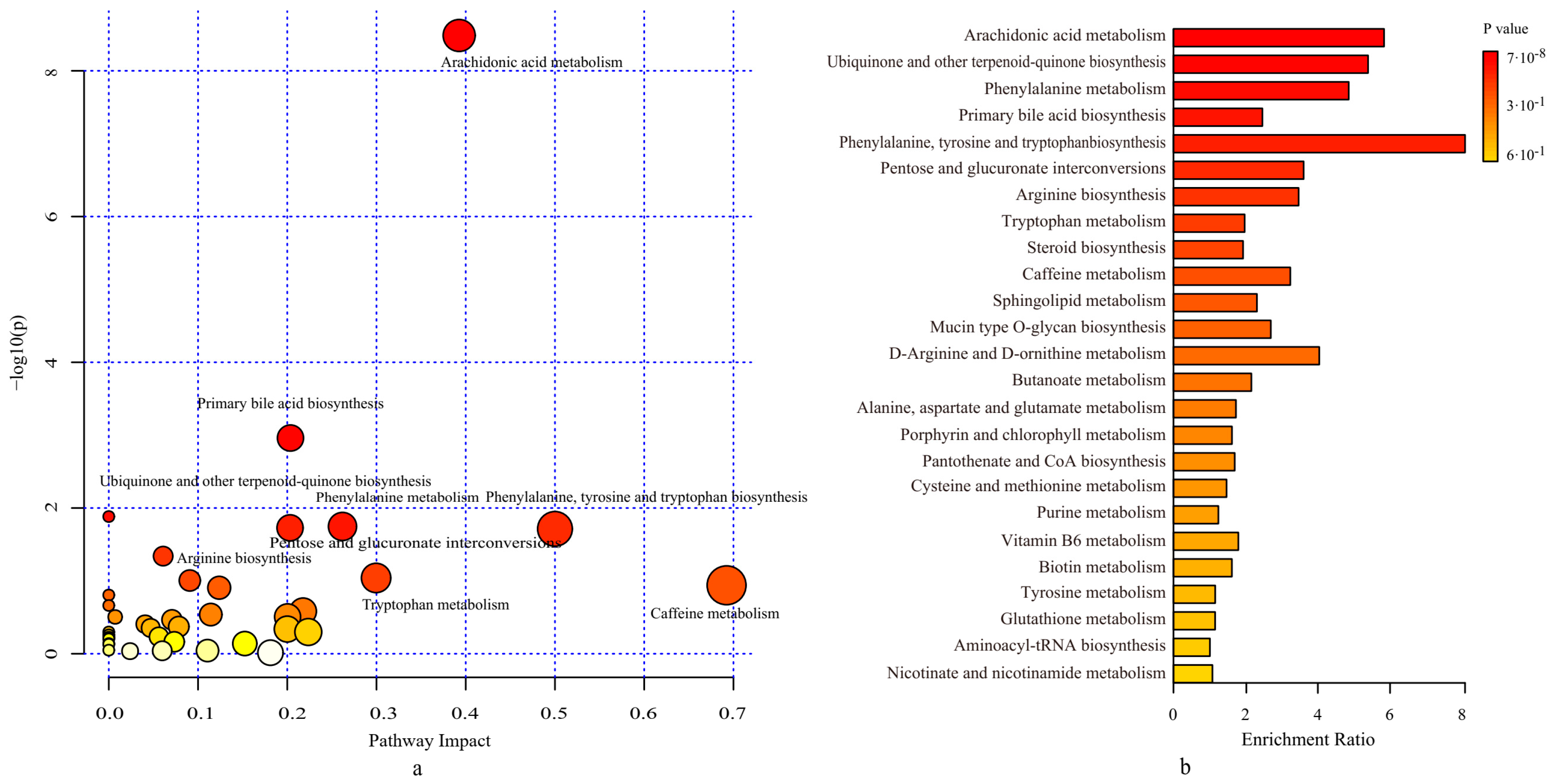
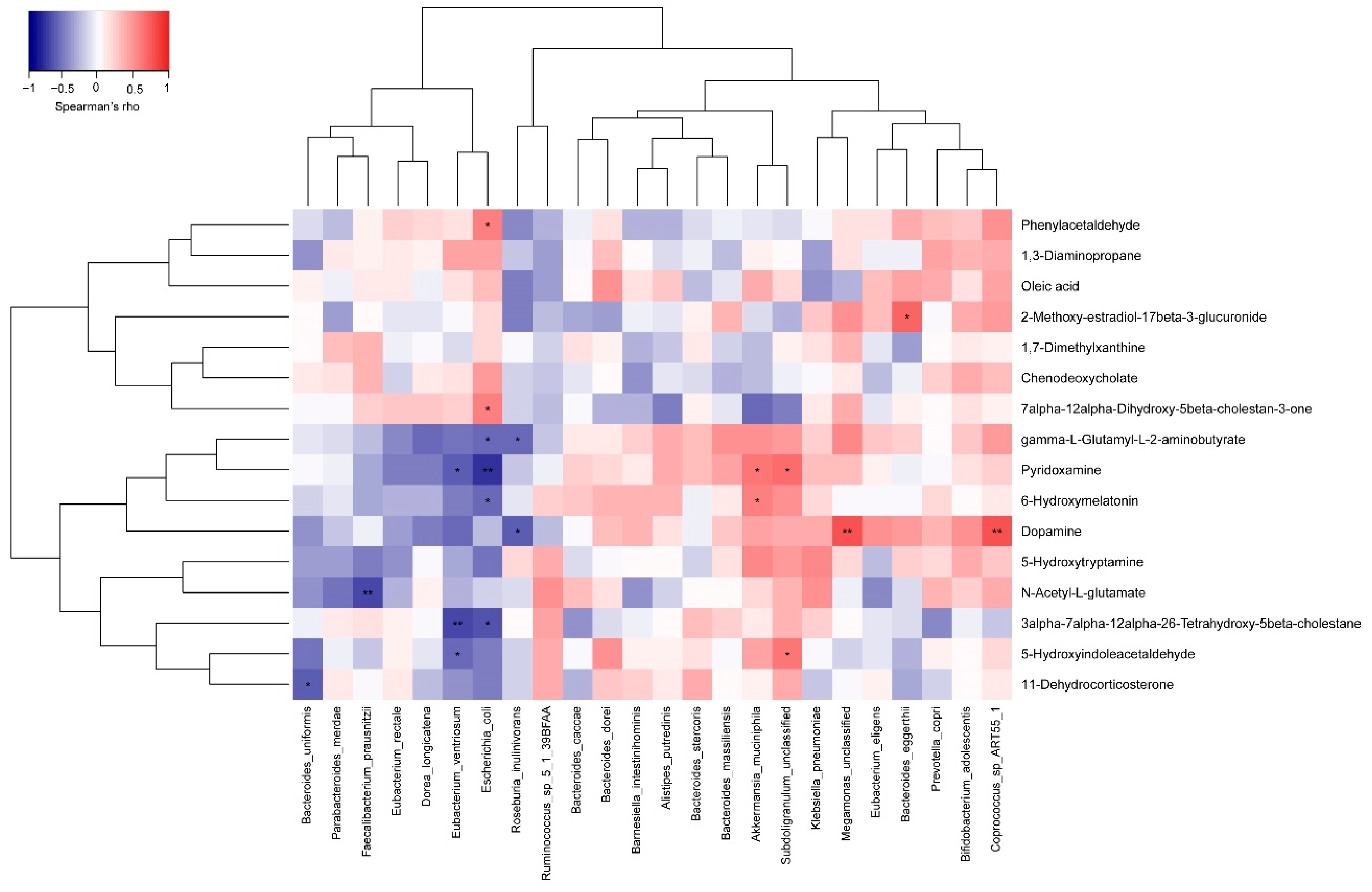
3.4. Probiotics Regulated the Metabolic Pathways of the Gut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelly, P. Postgraduate Students Likely to Experience Stress and Depression, According to GSU Survey. Available online: http://trinitynews.ie/2018/05/postgraduate-students-likely-to-experience-stress-and-depression-according-to-gsu-survey/ (accessed on 31 May 2018).
- Carlijn, K.; John, V.; Remco, P. The Influence of the Social Environment Context in Stress and Coping in Sport. Front Psychol. 2016, 7, 875. [Google Scholar]
- Bedewy, D.; Gabriel, A. Examining Perceptions of Academic Stress and Its Sources among University Students: The Perception of Academic Stress Scale. Health Psychol. Open 2015, 2. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The Neuroactive Potential of the Human Gut Microbiota in Quality of Life and Depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced Anxiety-Like Behavior and Central Neurochemical Change in Germ-Free Mice. Neurogastroenterol. Motil. 2011, 23, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Vojinovic, D.; Radjabzadeh, D.; Kurilshikov, A.; Amin, N.; Wijmenga, C.; Franke, L.; Ikram, M.A.; Uitterlinden, A.G.; Zhernakova, A.; Fu, J. Relationship between Gut Microbiota and Circulating Metabolites in Population-Based Cohorts. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, V.B. The Role of the Microbiome in Human Health and Disease: An Introduction for Clinicians. BMJ 2017, 356, j831. [Google Scholar] [CrossRef]
- Sarkar, A.; Harty, S.; Lehto, S.M.; Moeller, A.H.; Dinan, T.G.; Dunbar, R.I.M.; Cryan, J.F.; Burnet, P.W.J. The Microbiome in Psychology and Cognitive Neuroscience. Trends Cogn. Sci. 2018, 22, 611–636. [Google Scholar] [CrossRef] [PubMed]
- Backhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [Green Version]
- Cryan, J.F.; Dinan, T.G. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Rios-Covian, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilan, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [Green Version]
- Strandwitz, P. Neurotransmitter Modulation by the Gut Microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Chen, M.; Duo, L.; Wang, J.; Guo, S.; Sun, H.; Menghe, B.; Zhang, H. Characterization of Potentially Probiotic Lactic Acid Bacteria and Bifidobacteria Isolated from Human Colostrum. J. Dairy Sci. 2020, 103, 4013–4025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, J.; Jin, H.; Lv, R.; Shi, H.; De, G.; Yang, B.; Sun, Z.; Zhang, H. Probiotics Maintain the Intestinal Microbiome Homeostasis of the Sailors during a Long Sea Voyage. Gut Microbes 2020, 11, 930–943. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharp, J.W. Expanding the Definition of Quality of Life for Prostate Cancer. Cancer 1993, 71, 1078–1082. [Google Scholar] [CrossRef]
- Lipman, R.S.; Covi, L.; Shapiro, A.K. The Hopkins Symptom Checklist (HSCL): Factors Derived from the HSCL-90. J. Affect. Disord. 1979, 1, 9–24. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Zung, W.W. A Self-Rating Depression Scale. Arch. Gen. Psychiatry 1965, 12, 63–70. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [Green Version]
- Catherine Lozupone, R.K. UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, J.; Othman, S.; Li, C.; Iurie, C.; Li, S.; Guillaume, B.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards More Transparent and Integrative Metabolomics Analysis. Nucleic Acids Res. 2018, 46, 486–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P. A Core Gut Microbiome in Obese and Lean Twins. Nature 2009, 457, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, Tryptophan Metabolism and the Brain-Gut-Microbiome Axis-Sciencedirect. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.C.; Kien, C.L.; Bouthillier, L.; Levy, E. Short-Chain Fatty Acids: Ready for Prime Time? Nutr. Clin. Pract. 2006, 21, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Ji, W.; He, T.; Becker, S.; Xi, M. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar]
- Stilling, R.M.; van de Wouw, M.; Clarcke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The Neuropharmacology of Butyrate: The Bread and Butter of the Microbiota-Gut-Brain Axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Karl, J.P.; Hatch, A.M.; Arcidiacono, S.M.; Pearce, S.C.; Soares, J.W. Effects of Psychological, Environmental and Physical Stressors on the Gut Microbiota. Front. Microbiol. 2018, 9, 2013. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Lu, W.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; Chen, W. Probiotics Modulate the Gut Microbiota Composition and Immune Responses in Patients with Atopic Dermatitis: A Pilot Study. Eur. J. Nutr. 2020, 59, 2119–2130. [Google Scholar] [CrossRef]
- Wei, X.; Tao, J.; Xiao, S.; Jiang, S.; Shang, E.; Zhu, Z.; Qian, D.; Duan, J. Xiexin Tang Improves the Symptom of Type 2 Diabetic Rats by Modulation of the Gut Microbiota. Sci. Rep. 2018, 8, 3685. [Google Scholar] [CrossRef]
- Weiss, G.A.; Chassard, C.; Hennet, T. Selective Proliferation of Intestinal Barnesiella under Fucosyllactose Supplementation in Mice. Br. J. Nutr. 2014, 111, 1602–1610. [Google Scholar] [CrossRef] [Green Version]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia muciniphila Gen. Nov., Sp. Nov., a Human Intestinal Mucin-Degrading Bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, N.-R.; Lee, J.-C.; Lee, H.-Y.; Kim, M.-S.; Whon, T.W.; Lee, M.-S.; Bae, J.-W. An Increase in the Akkermansia Spp. Population Induced by Metformin Treatment Improves Glucose Homeostasis in Diet-Induced Obese Mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef] [Green Version]
- Naito, Y.; Uchiyama, K.; Takagi, T. A Next-Generation Beneficial Microbe: Akkermansia muciniphila. J. Clin. Biochem. Nutr. 2018, 63, 33–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, K. Strategies to Promote Abundance of Akkermansia muciniphila, an Emerging Probiotics in the Gut, Evidence from Dietary Intervention Studies. J. Funct. Foods 2017, 33, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G. Transferring the Blues: Depression-Associated Gut Microbiota Induces Neurobehavioural Changes in the Rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.A.; Diaz-Arteche, C.; Eliby, D.; Schwartz, O.S.; Simmons, J.G.; Cowan, C.S. The Gut Microbiota in Anxiety and Depression–A Systematic Review. Clin. Psychol. Rev. 2020, 83, 101943. [Google Scholar] [CrossRef]
- Jameson, K.G.; Hsiao, E.Y. Linking the Gut Microbiota to a Brain Neurotransmitter. Trends Neurosci. 2018, 41, 413–414. [Google Scholar] [CrossRef]
- Chittoor-Vinod, V.G.; Villalobos-Cantor, S.; Roshak, H.; Shea, K.; Martin, I. Dietary Amino Acids Impact LRRK2-induced Neurodegeneration in Parkinson’s Disease Models. J. Neurosci. 2020, 40, 6234–6249. [Google Scholar] [CrossRef]
- Chi, L.; Mahbub, R.; Gao, B.; Bian, X.; Tu, P.; Ru, H.; Lu, K. Nicotine Alters the Gut Microbiome and Metabolites of Gut-Brain Interactions in a Sex-Specific Manner. Chem. Res. Toxicol. 2017, 30, 2110–2119. [Google Scholar] [CrossRef]
- Kaelberer, M.M.; Rupprecht, L.E.; Liu, W.W.; Weng, P.; Bohórquez, D. Neuropod Cells: Emerging Biology of the Gut-Brain Sensory Transduction. Annu. Rev. Neurosci. 2020, 43, 337–353. [Google Scholar] [CrossRef] [Green Version]
- Gao, K.; Pi, Y.; Mu, C.-L.; Farzi, A.; Liu, Z. Increasing Carbohydrate Availability in the Hindgut Promotes Hypothalamic Neurotransmitter Synthesis: Aromatic Amino Acids Linking the Microbiota-Brain Axis. J. Neurochem. 2019, 149, 641–659. [Google Scholar] [CrossRef]
- Allott, K.; Mcgorry, P.D.; Yuen, H.P.; Firth, J.; Proffitt, T.M.; Berger, G.; Maruff, P.; O’Regan, M.K.; Papas, A.; Stephens, T.C.B. The Vitamins in Psychosis Study: A Randomized, Double-Blind, Placebo-Controlled Trial of the Effects of Vitamin B12, B6 and Folic Acid on Symptoms and Neurocognition in First-Episode Psychosis. Biol. Psychiatry 2019, 86, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Koole, J.L.; Bours, M.J.L.; Geijsen, A.J.M.R.; Gigic, B.; Ulvik, A.; E Kok, D.; Brezina, S.; Ose, J.; Baierl, A.; Böhm, J.; et al. Circulating B-Vitamin Biomarkers and B-Vitamin Supplement Use in Relation to Quality of Life in Patients with Colorectal Cancer: Results from the Focus Consortium. Am. J. Clin. Nutr. 2021, 113, 1468–1481. [Google Scholar] [CrossRef]
- Qin, B.; Xun, P.; Jacobs, D.R.; Zhu, N.; Daviglus, M.L.; Reis, J.P.; Steffen, L.M.; Linda, V.H.; Stephen, S.; He, K. Intake of Niacin, Folate, Vitamin B-6, and Vitamin B-12 through Young Adulthood and Cognitive Function in Midlife: The Coronary Artery Risk Development in Young Adults (Cardia) Study. Am. J. Clin. Nutr. 2017, 106, 1032–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina-Rodriguez, E.M.; Madorma, D.; O’Connor, G.; Mason, B.L.; Beurel, E. Identification of a Signaling Mechanism by Which the Microbiome Regulates Th17 Cell-Mediated Depressive-Like Behaviors in Mice. Am. J. Psychiatry 2020, 177, 974–990. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, J.; Li, J.; Kim, G.; Stewart, A.; Urban, J.F.; Huang, Y.; Chen, S.; Wu, L.G.; Chesler, A. Interleukin-33 Promotes Serotonin Release from Enterochromaffin Cells for Intestinal Homeostasis. Immunity 2020, 54, 151–163. [Google Scholar] [CrossRef]
- Israelyan, N.; Margolis, K.G. Serotonin as a Link Between the Gut-Brain-Microbiome Axis in Autism Spectrum Disorders. Pharmacol. Res. 2018, 132, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.; Belkaid, Y. Gut Microbiota: The Link to Your Second Brain. Cell 2015, 161, 193–194. [Google Scholar] [CrossRef] [Green Version]
- Burnet, P.; Cowen, P.J. Psychobiotics Highlight the Pathways to Happiness. Biol. Psychiatry 2013, 74, 708–709. [Google Scholar] [CrossRef]
- Bambury, A.; Sandhu, K.; Cryan, J.F.; Dinan, T.G. Finding the Needle in the Haystack: Systematic Identification of Psychobiotics. Br. J. Pharmacol. 2018, 175, 4430–4438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makris, A.P.; Karianaki, M.; Tsamis, K.I.; Paschou, S.A. The Role of the Gut-Brain Axis in Depression: Endocrine, Neural, and Immune Pathways. Hormones 2021, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]




| Group | Subject | Sex | Age | Weight (kg) | Height (cm) | Body Mass Index (kg/m2) |
|---|---|---|---|---|---|---|
| Probiotic | A1 | male | 25 | 48 | 152 | 20.8 |
| A2 | male | 26 | 55 | 155 | 22.9 | |
| A3 | male | 24 | 49 | 155 | 20.4 | |
| A4 | female | 24 | 51 | 166 | 18.5 | |
| A5 | female | 26 | 61 | 176 | 19.7 | |
| A6 | female | 24 | 81 | 179 | 25.3 | |
| Placebo | Y1 | male | 23 | 87 | 162 | 33.2 |
| Y2 | male | 26 | 55 | 158 | 22.0 | |
| Y3 | male | 24 | 50 | 162 | 19.1 | |
| Y4 | female | 40 | 65 | 1.7 | 22.5 | |
| Y5 | female | 25 | 71 | 185 | 20.7 | |
| Y6 | female | 25 | 71 | 177 | 22.7 |
| Gradient Time (min) | Flow Rate (mL/min) | Mobile Phase A (%) | Mobile Phase B (%) | Curve |
|---|---|---|---|---|
| 0 | 0.32 | 95.0 | 5.0 | 6 |
| 2.50 | 0.32 | 84.0 | 16.0 | 6 |
| 12.00 | 0.32 | 45.0 | 55.0 | 6 |
| 15.00 | 0.32 | 35.0 | 65.0 | 6 |
| 18.00 | 0.32 | 28.0 | 72.0 | 6 |
| 20.00 | 0.32 | 15.0 | 85.0 | 6 |
| 25.00 | 0.32 | 2.0 | 98.0 | 6 |
| 28.00 | 0.32 | 95.0 | 5.0 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Yu, Z.; Zhang, W.; Sun, T. Lactobacillus rhamnosus Probio-M9 Improves the Quality of Life in Stressed Adults by Gut Microbiota. Foods 2021, 10, 2384. https://doi.org/10.3390/foods10102384
Zheng Y, Yu Z, Zhang W, Sun T. Lactobacillus rhamnosus Probio-M9 Improves the Quality of Life in Stressed Adults by Gut Microbiota. Foods. 2021; 10(10):2384. https://doi.org/10.3390/foods10102384
Chicago/Turabian StyleZheng, Yan, Zhongjie Yu, Wenyi Zhang, and Tiansong Sun. 2021. "Lactobacillus rhamnosus Probio-M9 Improves the Quality of Life in Stressed Adults by Gut Microbiota" Foods 10, no. 10: 2384. https://doi.org/10.3390/foods10102384
APA StyleZheng, Y., Yu, Z., Zhang, W., & Sun, T. (2021). Lactobacillus rhamnosus Probio-M9 Improves the Quality of Life in Stressed Adults by Gut Microbiota. Foods, 10(10), 2384. https://doi.org/10.3390/foods10102384






