Biotransformation of Flavonoids Improves Antimicrobial and Anti-Breast Cancer Activities In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Regents
2.2. Biotransformation Procedures
2.3. Extraction and Purification of the Products
2.4. Chemical Identification
2.5. Structural Characterization of the Target Compound by LC-MS
2.6. Structural Characterization of the Target Compound by NMR
2.7. Antimicrobial Biological Activity Assay
2.8. Antiproliferative and Cytotoxicity Activities Assay
2.9. Data Analysis
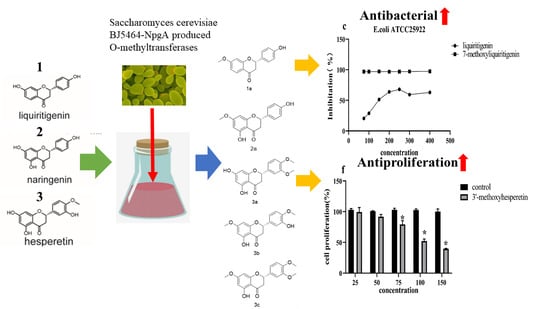
3. Results
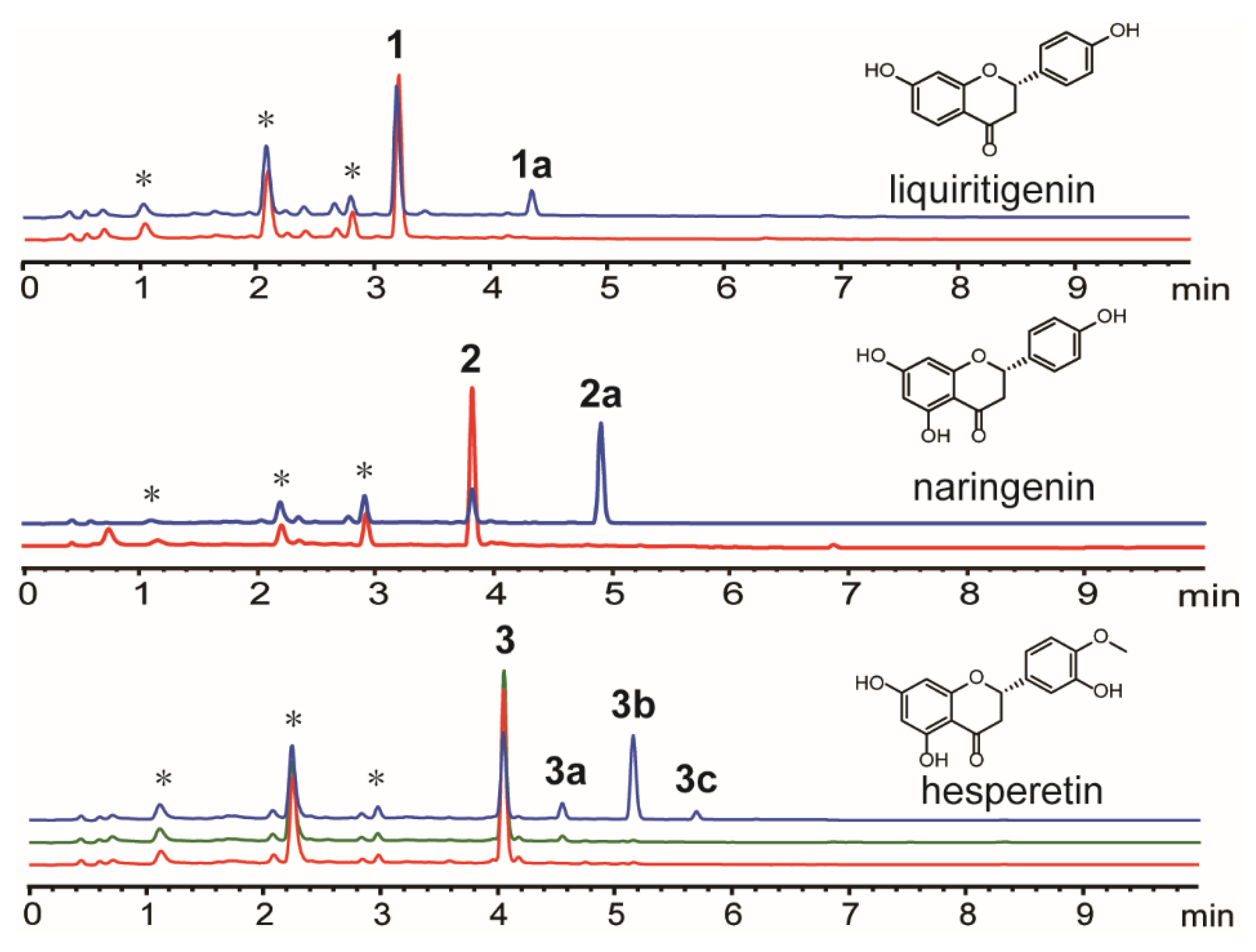
3.1. Biotransformation
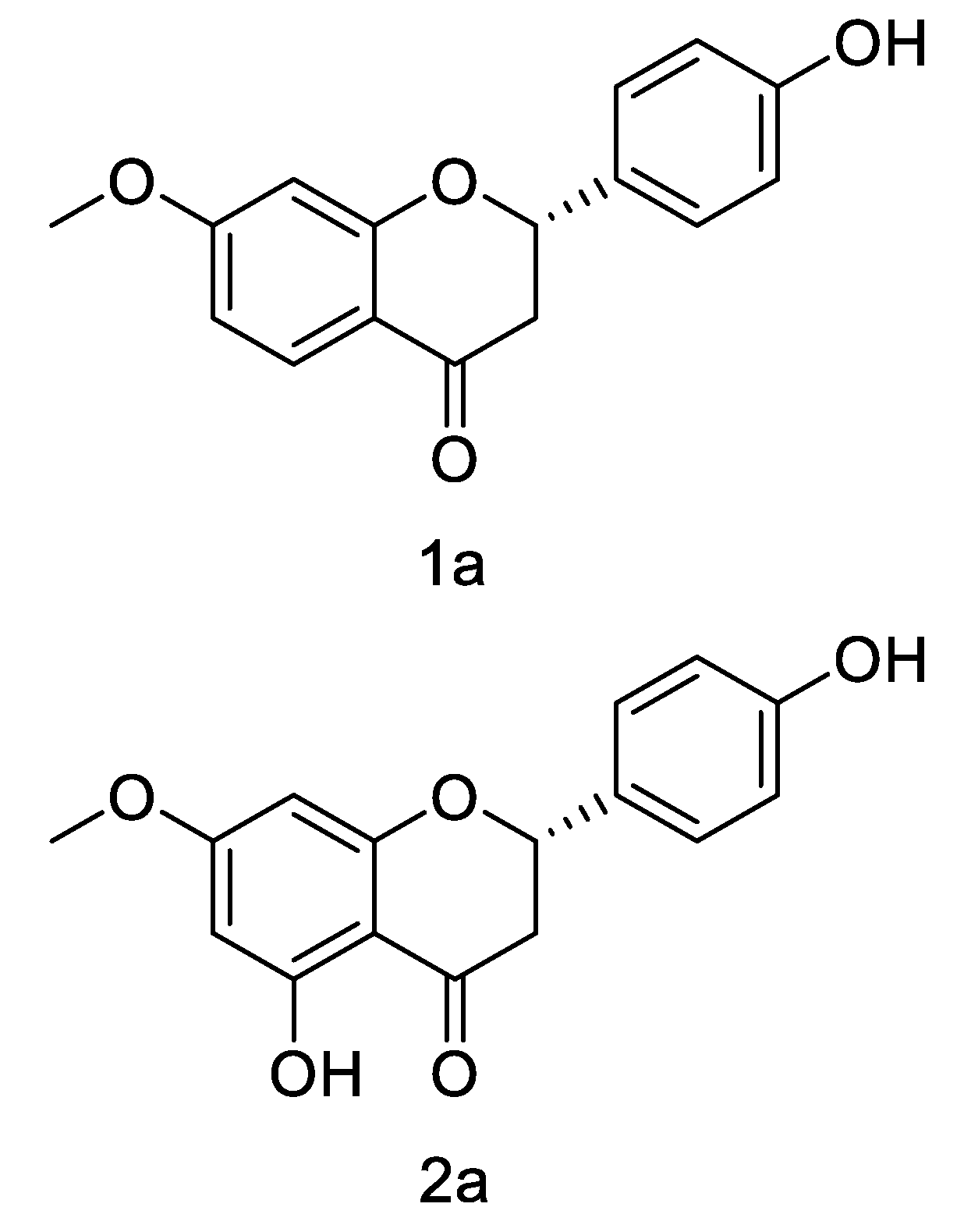
3.2. Structure Characterization
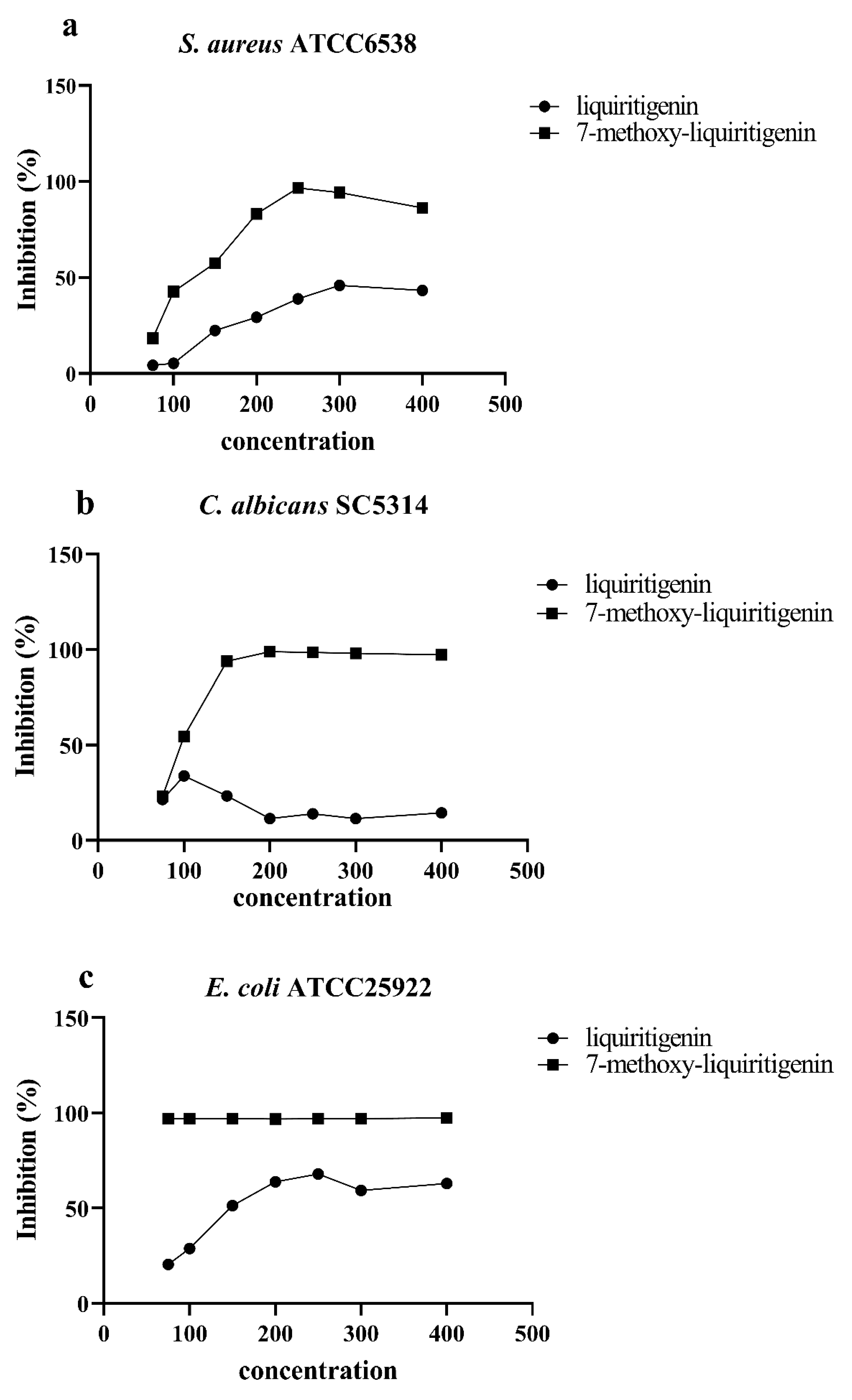
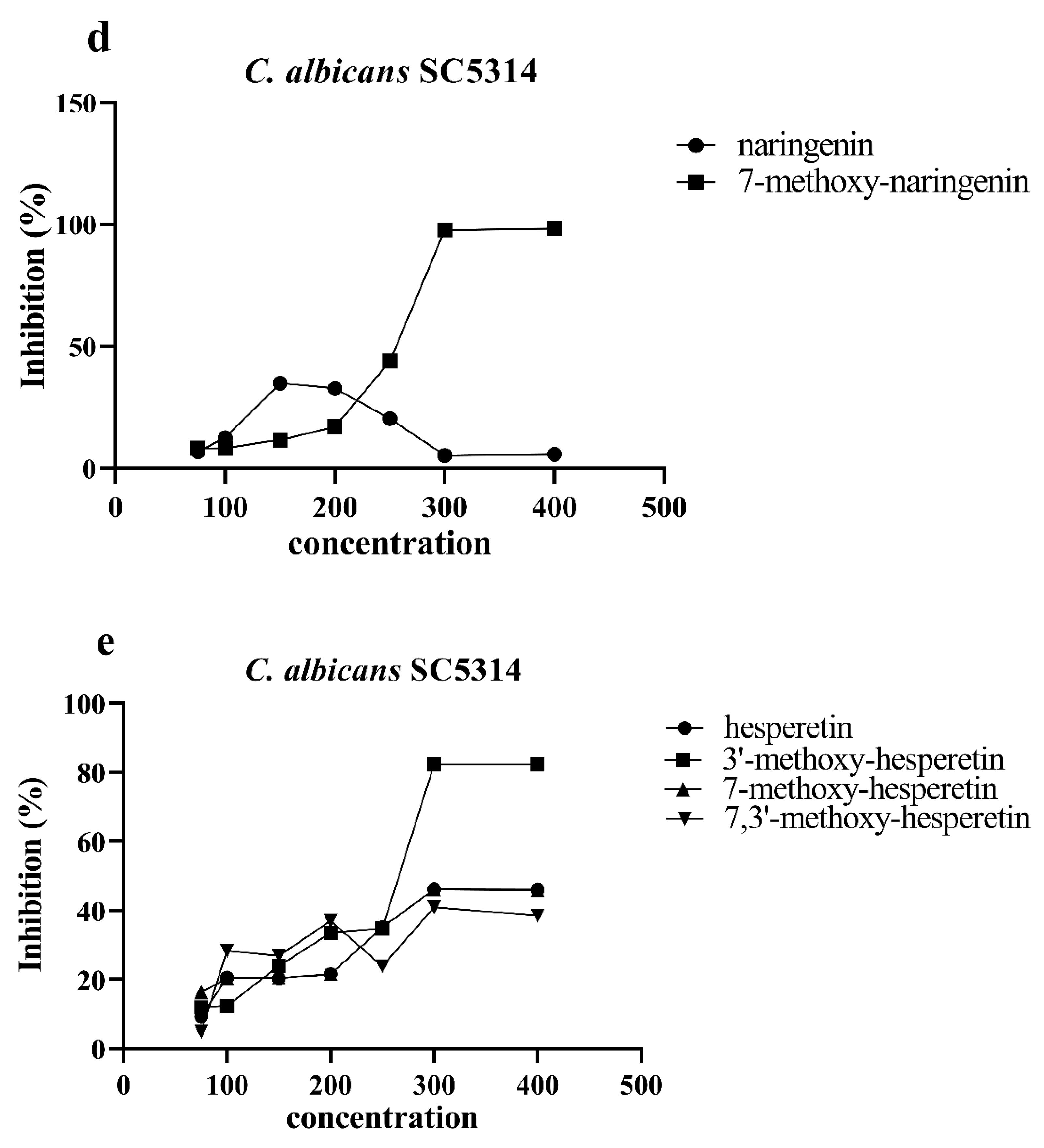
3.3. Antimicrobial Biological Activity
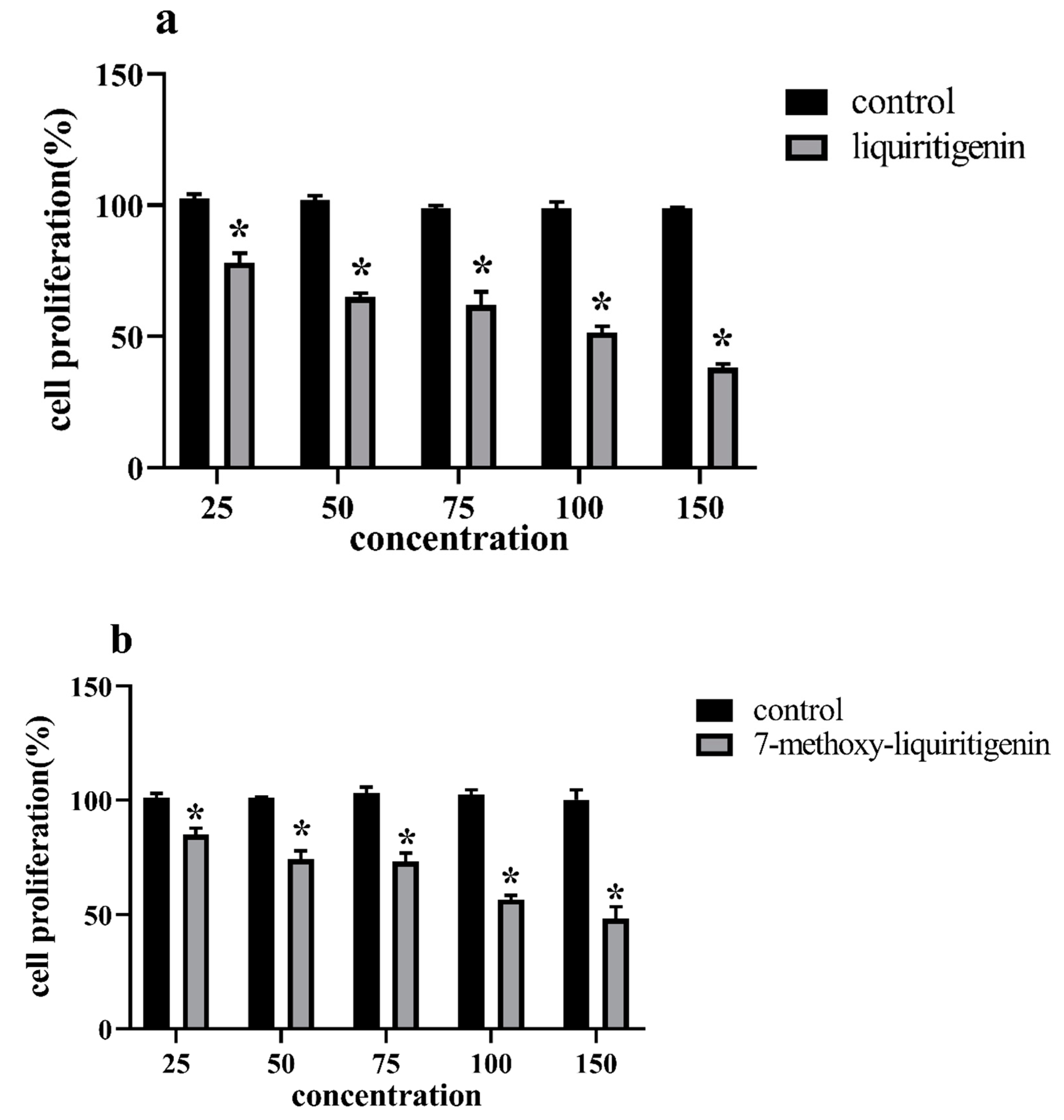
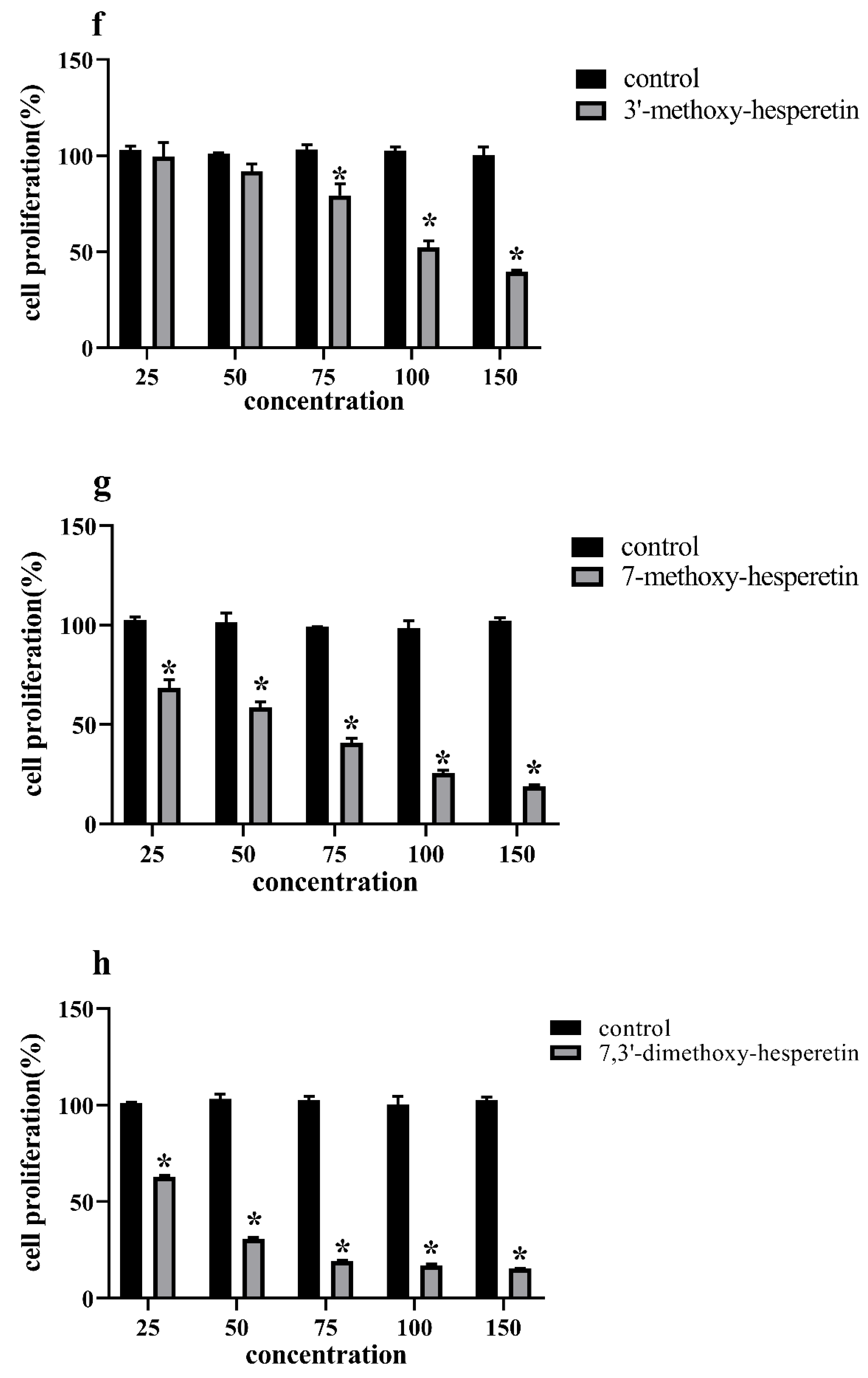
3.4. Antiproliferation Activity
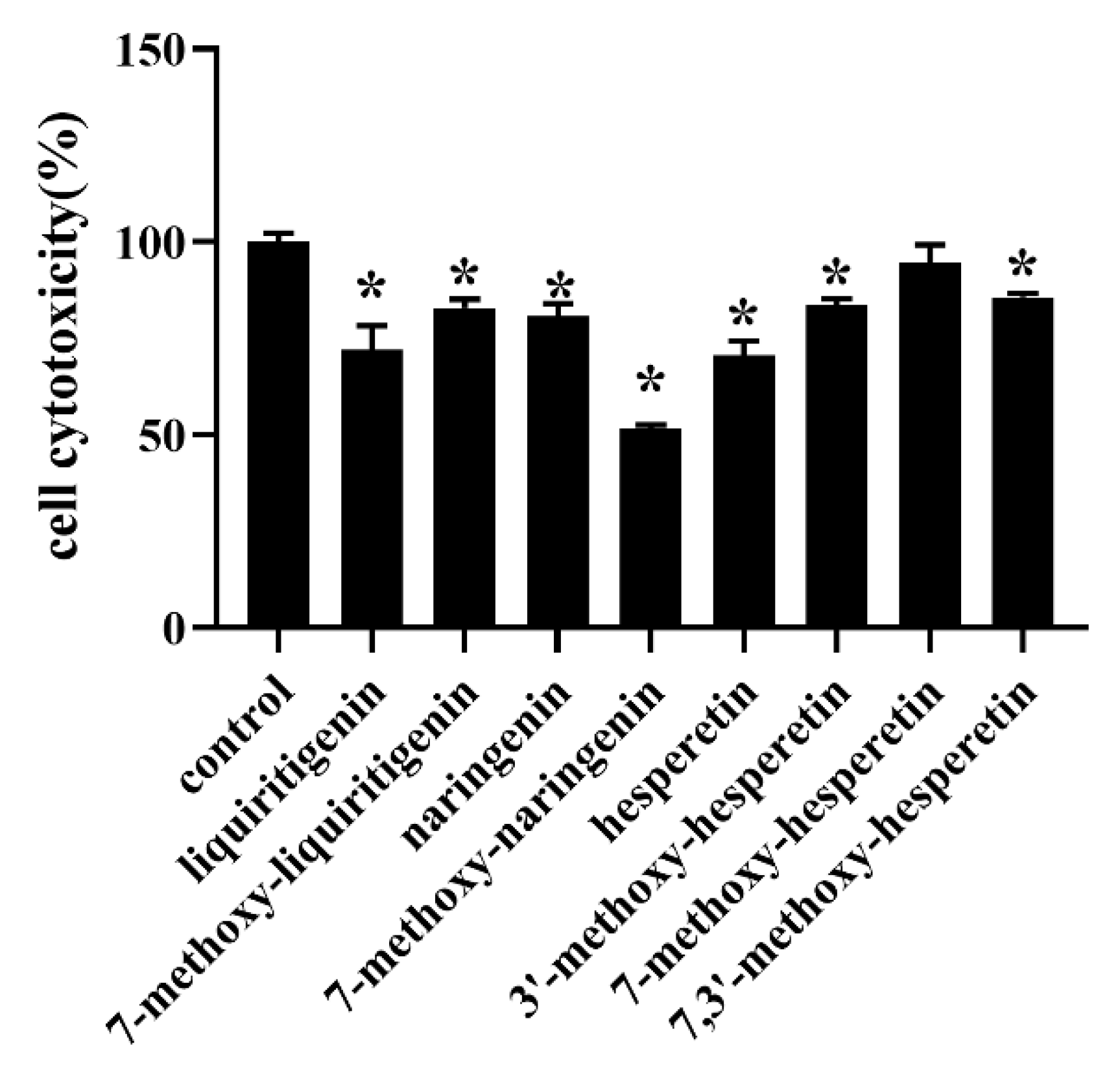
3.5. Cytotoxicity Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jang, D.; Jung, Y.S.; Kim, M.S.; Oh, S.E.; Nam, T.G.; Kim, D.O. Developing and validating a method for separating flavonoid isomers in common buckwheat sprouts using HPLC-PDA. Foods 2019, 8, 549. [Google Scholar] [CrossRef] [Green Version]
- Cannas, M.; Pulina, S.; Conte, P.; Del Caro, A.; Urgeghe, P.P.; Piga, A.; Fadda, C. Effect of substitution of rice flour with quinoa flour on the chemical-ohysical, nutritional, volatile and sensory parameters of gluten-free ladyfinger biscuits. Foods 2020, 9, 808. [Google Scholar] [CrossRef]
- Irakli, M.; Lazaridou, A.; Mylonas, I.; Biliaderis, C.G. Bioactive components and antioxidant activity distribution in pearling fractions of different greek barley cultivars. Foods 2020, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Siah, M.; Farzaei, M.H.; Ashrafi-Kooshk, M.R.; Adibi, H.; Arab, S.S.; Rashidi, M.R.; Khodarahmi, R. Inhibition of guinea pig aldehyde oxidase activity by different flavonoid compounds: An in vitro study. Bioorg. Chem. 2016, 64, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.L.; Zhang, Y.L.; Yang, X.G. Effect of lemon peel flavonoids on anti-fatigue and anti-oxidation capacities of exhaustive exercise mice. Appl. Biol. Chem. 2020, 63, 1–11. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Lin, Y.Y.; Shen, J.X.; Jiang, M.J.; Hou, Y.M. Flavonoids in Ageratum conyzoides L. exert potent antitumor effects on human cervical adenocarcinoma HeLa cells in vitro and in vivo. Biomed. Res. Int. 2020, 4, 2696350. [Google Scholar]
- Qiu, J.; Jiang, Y.; Xia, L.; Xiang, H.; Feng, H.; Pu, S.; Huang, N.; Yu, L.; Deng, X. Subinhibitory concentrations of licochalcone A decrease alpha-toxin production in both methicillin-sensitive and methicillin-resistant Staphylococcus aureus isolates. Lett. Appl. Microbiol. 2010, 50, 223–229. [Google Scholar] [CrossRef]
- Uzel, A.; Sorkun, K.; Oncag, O.; Cogulu, D.; Gencay, O.; Salih, B. Chemical compositions and antimicrobial activities of four different Anatolian propolis samples. Microbiol. Res. 2005, 160, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Vipin, C.; Saptami, K.; Fida, F.; Mujeeburahiman, M.; Rao, S.E.S.; Athmika; Arun, A.B.; Rekha, P.D. Potential synergistic activity of quercetin with antibiotics against multidrug-resistant clinical strains of Pseudomonas aeruginosa. PLoS ONE 2020, 15, e0241304. [Google Scholar] [CrossRef]
- Cheng, M.; Yuan, F.Y.; Liu, J.L.; Liu, W.; Feng, J.F.; Jin, Y.; Tu, L.X. Fabrication of Fine Puerarin Nanocrystals by Box-Behnken design to enhance intestinal absorption. Aaps Pharmscitech 2020, 21, 1–12. [Google Scholar] [CrossRef]
- Katsoura, M.H.; Polydera, A.C.; Tsironis, L.; Tselepis, A.D.; Stamatis, H. Use of ionic liquids as media for the biocatalytic preparation of flavonoid derivatives with antioxidant potency. J. Biotechnol. 2006, 123, 491–503. [Google Scholar] [CrossRef]
- Mellou, F.; Lazari, D.; Skaltsa, H.; Tselepis, A.D.; Kolisis, E.; Stamatis, H. Biocatalytic preparation of acylated derivatives of flavonoid glycosides enhances their antioxidant and antimicrobial activity. J. Biotechnol. 2005, 116, 295–304. [Google Scholar] [CrossRef]
- Lue, B.M.; Nielsen, N.S.; Jacobsen, C.; Hellgren, L.; Guo, Z.; Xu, X.B. Antioxidant properties of modified rutin esters by DPPH, reducing power, iron chelation and human low density lipoprotein assays. Food Chem. 2010, 123, 221–230. [Google Scholar] [CrossRef]
- Hoang, T.K.D.; Huynh, T.K.C.; Nguyen, T.D. Synthesis, characterization, anti-inflammatory and anti-proliferative activity against MCF-7 cells of O-alkyl and O-acyl flavonoid derivatives. Bioorg. Chem. 2015, 63, 45–52. [Google Scholar] [CrossRef]
- Araujo, K.C.F.; Costa, E.M.D.B.; Pazini, F.; Valadares, M.C.; de Oliveira, V. Bioconversion of quercetin and rutin and the cytotoxicity activities of the transformed products. Food Chem. Toxicol. 2013, 51, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Copmans, D.; Orellana-Paucar, A.M.; Steurs, G.; Zhang, Y.; Ny, A.; Fpubert, K.; Exarchou, V.; Siekierska, A.; Kim, Y.; De Borggraeve, W.; et al. Methylated flavonoids as anti-seizure agents: Naringenin 4′,7-dimethyl ether attenuates epileptic seizures. in zebrafish and mouse models. Neurochem. Int. 2018, 112, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Hua, D.; Ma, C. Microbial transformation of propenylbenzenes for natural flavour production. Trends Biotechnol. 2007, 25, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Han, F.; Lee, I.S. Microbial transformation of licochalcones. Molecules 2020, 25, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.J.; Wang, C.; Duan, L.X.; Zhang, L.W.; Liu, H.; Xu, Y.M.; Liu, Q.P.; Mao, T.L.; Zhang, W.; Chen, M.; et al. Rational reprogramming of O-methylation regioselectivity for combinatorial biosynthetic tailoring of Benzenediol Lactone Scaffolds. J. Am. Chem. Soc. 2019, 141, 4355–4364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, L.; Peng, L.; Fu, J.; Zou, L.; Zhao, G.; Zhao, J. Phytochemical, antibacterial and antioxidant activity evaluation of Rhodiola crenulata. Molecules 2020, 25, 3664. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Y.; Yao, Y.; Shi, Z.X.; Everaert, N.; Ren, G.X. Synergistic effect of bioactive anticarcinogens from soybean on anti-proliferative activity in MDA-MB-231 and MCF-7 human breast cancer vells in vitro. Molecules 2018, 23, 1557. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.N.; Cheng, J.; Zhu, X.X.; Zhang, G.H.; Yang, S.C.; Guo, X.X.; Jiang, H.F.; Ma, Y.H. De Novo biosynthesis of multiple pinocembrin derivatives in Saccharomyces cerevisiae. ACS Synth. Biol. 2020, 9, 3042–3051. [Google Scholar] [CrossRef]
- Alseekh, S.; de Souza, L.P.; Benina, M.; Fernie, A.R. The style and substance of plant flavonoid decoration; towards defining both structure and function. Phytochemistry 2020, 174, 112347. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.R.; Lyu, X.M.; Mark, R.; Chen, W.N. Antimicrobial and antioxidant activities of phenolic metabolites from flavonoid-producing yeast: Potential as natural food preservatives. Food Chem. 2019, 270, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Duranoglu, D.; Uzunoglu, D.; Mansuroglu, B.; Arasoglu, T.; Derman, S. Synthesis of hesperetin-loaded PLGA nanoparticles by two different experimental design methods and biological evaluation of optimized nanoparticles. Nanotechnology 2018, 29, 395603. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Jin, M.; Li, K.; Liu, H.; Xiao, Y.; Zhao, M.; Alseekh, S.; Li, W.; de Abreu, E.L.F.; Brotman, Y.; et al. An integrated multi-layered analysis of the metabolic networks of different tissues uncovers key genetic components of primary metabolism in maize. Plant J. 2018, 93, 1116–1128. [Google Scholar] [CrossRef] [Green Version]
- Magozwi, D.K.; Dinala, M.; Mokwana, N.; Siwe-Noundou, X.; Krause, R.W.M.; Sonopo, M.; McGaw, L.J.; Augustyn, W.A.; Tembu, V.J. Flavonoids from the genus Euphorbia: Isolation, structure, pharmacological activities and structure-activity relationships. Pharmaceuticals 2021, 14, 428. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.J.; Zhao, Y.L.; Xing, X.Y.; Ma, X.P.; Sun, X.J.; Yang, M.H.; Xiao, X.H. Antibacterial evaluation of flavonoid compounds against E-coli by microcalorimetry and chemometrics. Appl. Microbiol. Biot. 2015, 99, 6049–6058. [Google Scholar] [CrossRef]
- Liang, F.; Zhang, H.; Gao, H.; Cheng, D.; Zhang, N.; Du, J.; Yue, J.M.; Du, P.; Zhao, B.B.; Yin, L. Liquiritigenin decreases tumorigenesis by inhibiting DNMT activity and increasing BRCA1 transcriptional activity in triple-negative breast cancer. Exp. Biol. Med. 2021, 246, 459–466. [Google Scholar] [CrossRef]
- Pateliya, B.; Burade, V.; Goswami, S. Enhanced antitumor activity of doxorubicin by naringenin and metformin in breast carcinoma: An experimental study. N S Arch. Pharmacol. 2021, 394, 1949–1961. [Google Scholar] [CrossRef]
- Korga-Plewko, A.; Michalczyk, M.; Adamczuk, G.; Humeniuk, E.; Ostrowska-Lesko, M.; Jozefczyk, A.; Iwan, M.; Wojcik, M.; Dudka, J. Apigenin and hesperidin downregulate DNA repair genes in MCF-7 breast cancer cells and augment doxorubicin toxicity. Molecules 2020, 25, 4221. [Google Scholar] [CrossRef]
- Ajji, P.K.; Walder, K.; Puri, M. Combination of balsamin and flavonoids induce apoptotic effects in liver and breast cancer cells. Front. Pharmacol. 2020, 11, 574496. [Google Scholar] [CrossRef]
- Rahideh, S.T.; Shidfar, F.; Nourbakhsh, M.; Hoseini, M.; Koohdani, F.; Entezam, M.; Keramatipour, M. The individual or combinational effects of Hesperetin and Letrozole on the activity and expression of aromatase in MCF-7 cells. Cell Mol. Biol. 2016, 62, 38–43. [Google Scholar]
- Wen, L.R.; Jiang, Y.M.; Yang, J.L.; Zhao, Y.P.; Tian, M.M.; Yang, B. Structure, bioactivity, and synthesis of methylated flavonoids. Ann. N. Y. Acad. Sci. 2017, 1398, 120–129. [Google Scholar] [CrossRef]
- Katayama, K.; Masuyama, K.; Yoshioka, S.; Hasegawa, H.; Mitsuhashi, J.; Sugimoto, Y. Flavonoids inhibit breast cancer resistance protein-mediated drug resistance: Transporter specificity and structure-activity relationship. Cancer Chemother. Pharmacol. 2007, 60, 789–797. [Google Scholar] [CrossRef]
- Fan, X.; Bai, J.; Zhao, S.; Hu, M.; Sun, Y.; Wang, B.; Ji, M.; Jin, J.; Wang, X.; Hu, J.; et al. Evaluation of inhibitory effects of flavonoids on breast cancer resistance protein (BCRP): From library screening to biological evaluation to structure-activity relationship. Toxicol. Vitro 2019, 61, 104642. [Google Scholar] [CrossRef]
- Brusselmans, K.; Bono, F.; Collen, D.; Herbert, J.M.; Carmeliet, P.; Dewerchin, M. A novel role for vascular endothelial growth factor as an autocrine survival factor for embryonic stem cells during hypoxia. J. Biol. Chem. 2005, 280, 3493–3499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitagawa, S.; Nabekura, T.; Takahashi, T.; Nakamura, Y.; Sakamoto, H.; Tano, H.; Hirai, M.; Tsukahara, G. Structure-activity relationships of the inhibitory effects of flavonoids on P-glycoprotein-mediated transport in KB-C2 cells. Biol. Pharm. Bull. 2005, 28, 2274–2278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plochmann, K.; Korte, G.; Koutsilieri, E.; Richling, E.; Riederer, P.; Rethwilm, A.; Schreier, P.; Scheller, C. Structure-activity relationships of flavonoid-induced cytotoxicity on human leukemia cells. Arch. Biochem. Biophys. 2007, 460, 1–9. [Google Scholar] [CrossRef] [PubMed]


| Number | Name | Biocatalytic Enzymes | Molecular Formula | Biotransformation Yield (%) |
|---|---|---|---|---|
| 1a | 7-methoxy-liquiritigenin | Hs-OMT | C16H14O4 | 15.64 ± 1.61 |
| 2a | 7-methoxy-naringenin | Hs-OMT | C16H14O5 | 73.12 ± 3.47 |
| 3a | 3’-methoxy-hesperetin | Hs-OMT | C17H16O6 | 8.42 ± 1.33 |
| 3b | 7-methoxy- hesperetin | Lt-OMT | C17H16O6 | 3.73 ± 0.84 |
| 3b | 7-methoxy-hesperetin | Hs-OMT | C17H16O6 | 43.81 ± 0.02 |
| 3c | 7,3’-dimethoxy-hesperetin | Hs-OMT | C18H18O6 | 3.80 ± 0.84 |
| No. | 1a | 2a | ||
|---|---|---|---|---|
| δC | δH (Multi, J in Hz) | δC | δH (Multi, J in Hz) | |
| 2 | 79.26 | 5.48, brd (13.4) | 78.65 | 5.48, brd (14.8) |
| 3α | 43.14 | 3.15, m | 42.05 | 3.30, dd (17.1, 14.8) |
| 3β | 2.66, m | 2.72, dd (17.1, 2.5) | ||
| 4 | 190.42 | 197.00 | ||
| 5 | 128.03 | 7.71, d (8.6) | 163.22 | |
| 6 | 109.87 | 6.64, d (8.6) | 94.67 | 6.07, d (1.7) |
| 7 | 165.67 | 167.45 | ||
| 8 | 101.01 | 6.58, s | 93.81 | 6.10, brs |
| 9 | 163.30 | 162.90 | ||
| 10 | 114.46 | 102.62 | ||
| 1′ | 129.16 | 128.70 | ||
| 2′ | 128.32 | 7.33, d (7.3) | 115.19 | 7.32, d (8.2) |
| 3′ | 115.17 | 6.78, d (7.3) | 128.40 | 6.79, d (8.2) |
| 4′ | 157.73 | 157.79 | ||
| 5′ | 115.17 | 6.78, d (7.3) | 128.40 | 6.79, d (8.2) |
| 6′ | 128.32 | 7.32, d (7.3) | 115.19 | 7.32, d (8.2) |
| 7-OMe | 55.81 | 3.80, s | 55.92 | 3.78, s |
| No. | 3a | 3b | 3c | |||
|---|---|---|---|---|---|---|
| δC | δH (Multi, J in Hz) | δC | δH (Multi, J in Hz) | δC | δH (Multi, J in Hz) | |
| 2 | 78.48 | 5.48, dd (12.5, 2.4) | 78.41 | 5.48, d (11.4) | 78.70 | 5.53, dd (12.6, 2.1) |
| 3α | 42.10 | 3.32, m | 42.13 | 3.26, dd (17.2, 11.4) | 42.17 | 3.38, m |
| 3β | 2.71, dd (17.1, 2.4) | 2.75, d (17.2) | 2.75, dd (17.0, 2.1) | |||
| 4 | 196.22 | 196.79 | 196.86 | |||
| 5 | 163.46 | 163.18 | 163.20 | |||
| 6 | 95.85 | 5.89, brs | 94.63 | 6.08, brs | 94.71 | 6.09, brs |
| 7 | 166.72 | 167.42 | 167.46 | |||
| 8 | 95.03 | 5.91, brs | 93.82 | 6.11, brs | 93.86 | 6.13, brs |
| 9 | 162.82 | 162.73 | 162.79 | |||
| 10 | 101.73 | 102.63 | 102.61 | |||
| 1′ | 130.97 | 130.97 | 130.81 | |||
| 2′ | 110.61 | 7.13, brs | 114.08 | 6.93, brs | 110.62 | 7.14, brs |
| 3′ | 148.72 | 146.46 | 148.74 | |||
| 4′ | 149.04 | 147.92 | 149.09 | |||
| 5′ | 111.54 | 6.98, d (8.1) | 111.96 | 6.94, brs | 111.53 | 6.98, d (8.1) |
| 6′ | 119.27 | 7.01, d (8.1) | 117.71 | 6.88, d (6.4) | 119.34 | 7.02, d (8.1) |
| 7-OMe | 55.90 | 3.77, s | 55.94 | 3.79, s | ||
| 3′-OMe | 55.58 | 3.77, s | 55.58 | 3.77, s | ||
| 4′-OMe | 55.58 | 3.77, s | 55.67 | 3.77, s | 55.61 | 3.78, s |
| 5-OH | 12.14, s | 12.10, s | 12.11, s | |||
| 3′-OH | 9.10, s | |||||
| Compound | E. coli ATCC25922 | S. aureus ATCC6538 | C. albicans SC5314 | E. coli ATCC25922 | S. aureus ATCC6538 | C. albicans SC5314 |
|---|---|---|---|---|---|---|
| MIC (μM) | MIC (μM) | MIC (μM) | MBC (μM) | MBC (μM) | MBC (μM) | |
| 1 1a | 25 15 | 75 75 | 25 25 | >400 >400 | >400 >400 | >400 >400 |
| 2 2a | - - | - - | 75 75 | - - | - - | >400 >400 |
| 3 | - | - | 75 | >400 | ||
| 3a | - | - | 50 | >400 | ||
| 3b | - | - | 25 | >400 | ||
| 3c | - | - | 75 | >400 |
| Compound | IC 50 (μM) |
|---|---|
| 1 | 100.94 ± 1.83 |
| 1a | 11.23 ± 0.60 |
| 2 | 125.53 ± 2.76 |
| 2a | 93.64 ± 1.06 |
| 3 | >150 |
| 3a | 10.45 ± 0.45 |
| 3b | 30.74 ± 0.72 |
| 3c | 31.00 ± 1.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Y.; Wei, Z.; Wang, Z.; Li, G.; Yao, Y.; Dun, B. Biotransformation of Flavonoids Improves Antimicrobial and Anti-Breast Cancer Activities In Vitro. Foods 2021, 10, 2367. https://doi.org/10.3390/foods10102367
Hao Y, Wei Z, Wang Z, Li G, Yao Y, Dun B. Biotransformation of Flavonoids Improves Antimicrobial and Anti-Breast Cancer Activities In Vitro. Foods. 2021; 10(10):2367. https://doi.org/10.3390/foods10102367
Chicago/Turabian StyleHao, Yanpeng, Zuchen Wei, Zhi Wang, Guiying Li, Yang Yao, and Baoqing Dun. 2021. "Biotransformation of Flavonoids Improves Antimicrobial and Anti-Breast Cancer Activities In Vitro" Foods 10, no. 10: 2367. https://doi.org/10.3390/foods10102367
APA StyleHao, Y., Wei, Z., Wang, Z., Li, G., Yao, Y., & Dun, B. (2021). Biotransformation of Flavonoids Improves Antimicrobial and Anti-Breast Cancer Activities In Vitro. Foods, 10(10), 2367. https://doi.org/10.3390/foods10102367