Tracing the Geographical Origin of Thai Hom Mali Rice in Three Contiguous Provinces of Thailand Using Stable Isotopic and Elemental Markers Combined with Multivariate Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation of THMR
2.2. Chemicals and Reference Materials
2.3. ICP-MS
2.4. Stable Oxygen (O) Isotope by EA-IRMS in Pyrolyzer Mode
2.5. Multivariate Analysis
3. Results and Discussion
3.1. Stable Isotope and Selected Elemental Analyses in THMR
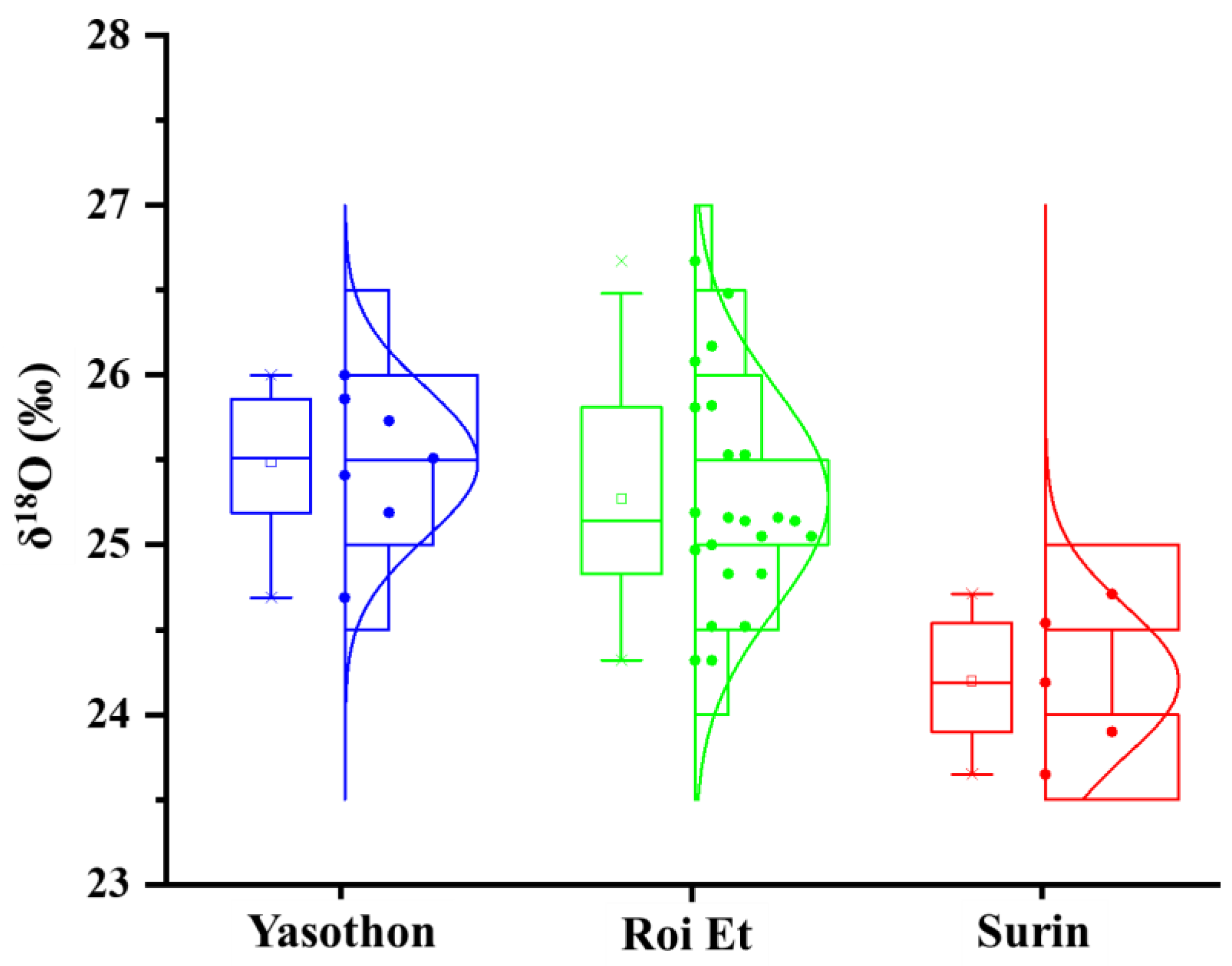
3.1.1. δ18O Analysis
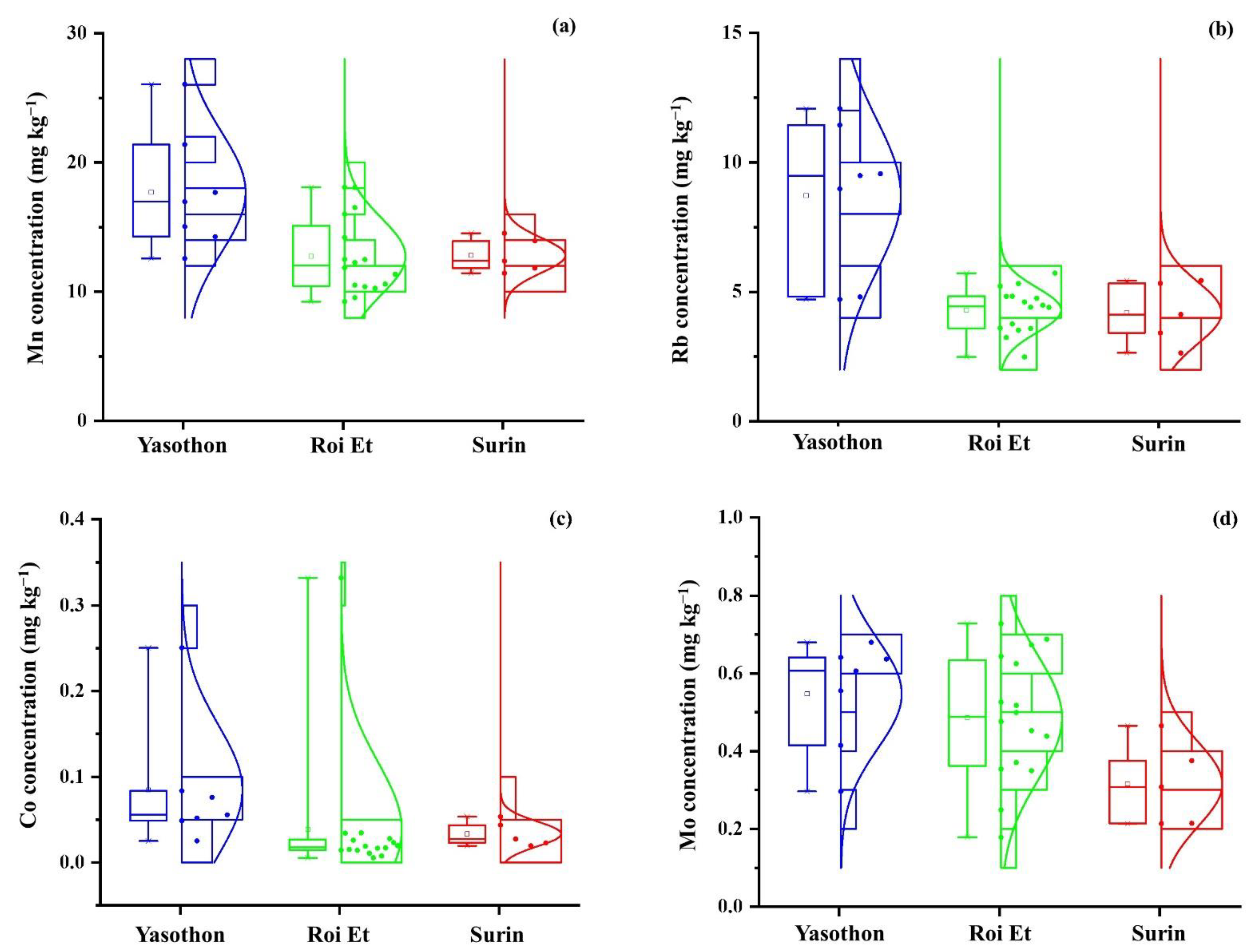
3.1.2. Elemental Analysis
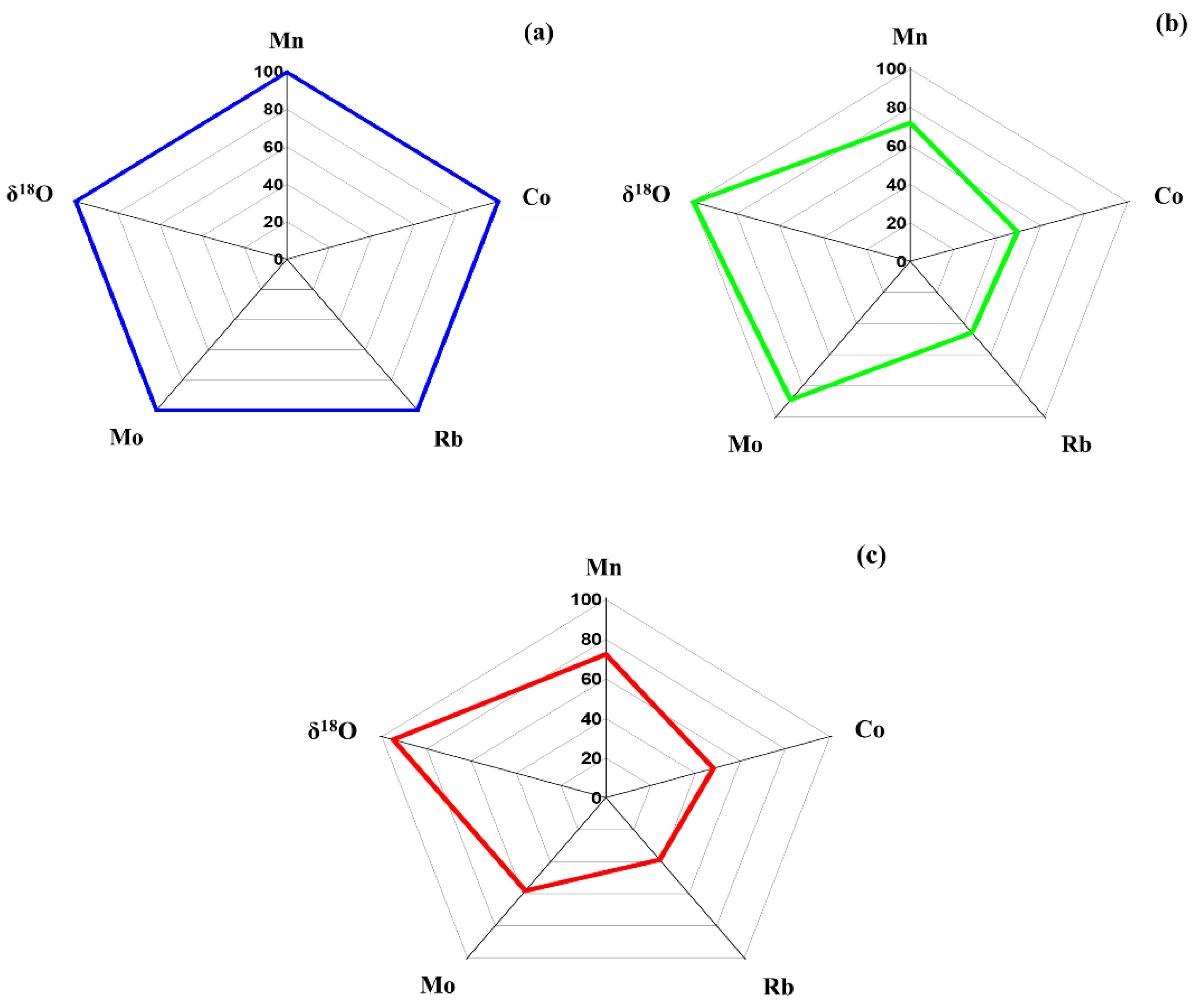
3.2. Radar Plot Analysis
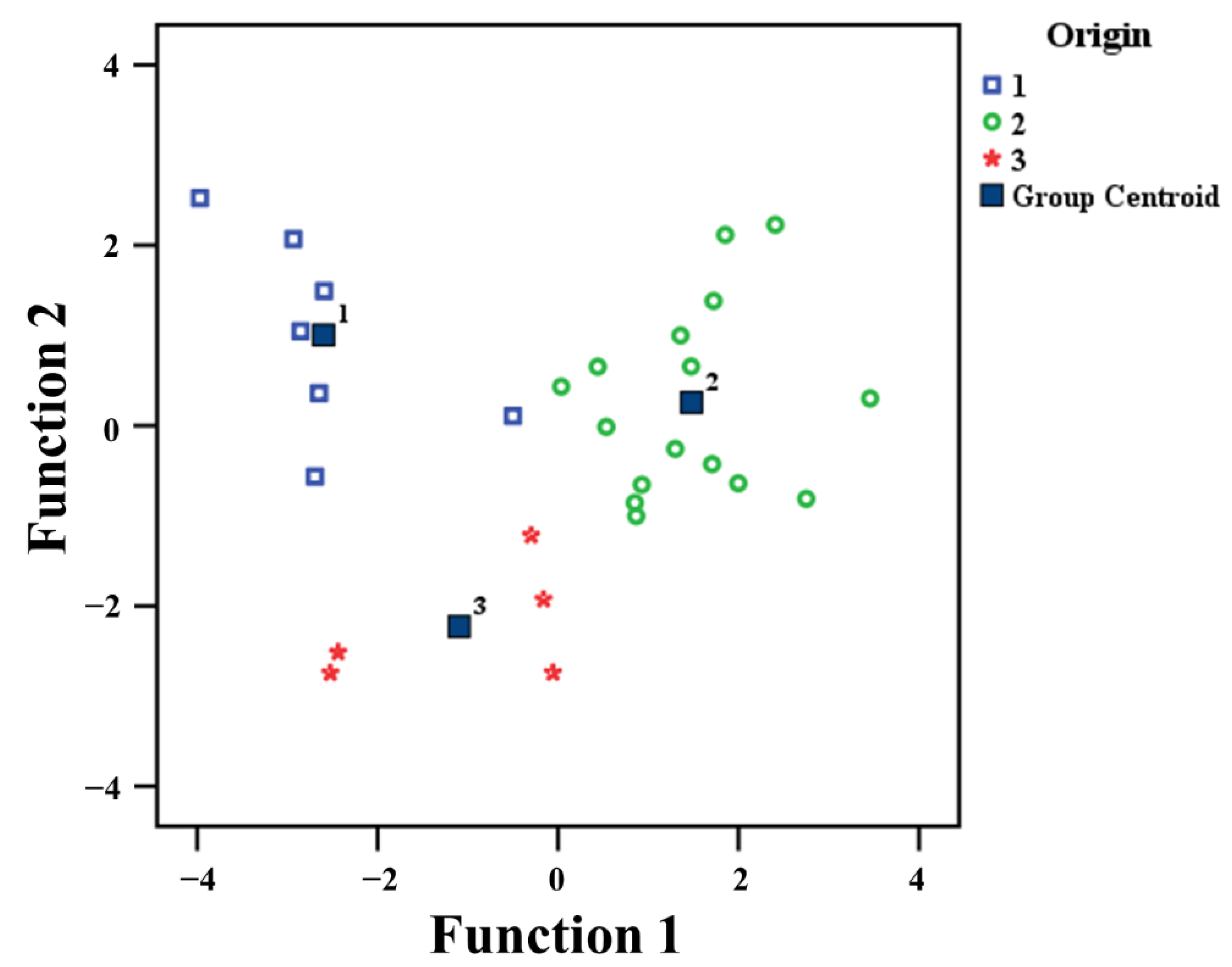
3.3. LDA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, S.; Xia, L.; Xiong, N.; Liu, Z.; Sun, H. Determination of the geographic origin of rice by element fingerprints and correlation analyses with the soil of origin. Anal. Methods 2013, 5, 6177–6185. [Google Scholar] [CrossRef]
- Kukusamude, C.; Kongsri, S. Elemental and isotopic profiling of Thai jasmine rice (Khao Dawk Mali 105) for discrimination of geographical origins in Thung Kula Rong Hai area. Thailand. Food Control 2018, 91, 357–364. [Google Scholar] [CrossRef]
- Kukusamude, C.; Sricharoen, P.; Limchoowong, N.; Kongsri, S. Heavy metals and probabilistic risk assessment via rice consumption in Thailand. Food Chem. 2021, 334, 127402. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, T.; Zhang, W.; Zhao, Y.; Yang, S.; Chen, A. Tracing the geographical origin of rice by stable isotopic analyses combined with chemometrics. Food Chem. 2020, 313, 126093. [Google Scholar] [CrossRef] [PubMed]
- Cheajesadagul, P.; Arnaudguilhem, C.; Shiowatana, J.; Siripinyanond, A.; Szpunar, J. Discrimination of geographical origin of rice based on multi-element fingerprinting by high resolution inductively coupled plasma mass spectrometry. Food Chem. 2013, 141, 3504–3509. [Google Scholar] [CrossRef]
- Cotecna. World Rice Production & Trade in Brief. 2020. Available online: https://www.cotecna.com/en/media/articles/world-rice-trade-in-brief (accessed on 15 April 2021).
- EU. Commission Implementing Regulation (EU) No 120/2013 of 11 February 2013 entering a name in the register of protected designations of origin and protected geographical indications ((Khao Hom Mali Thung Kula Rong-Hai) (PGI)). Off. J. Eur. Union 2013, 56, 3–8. [Google Scholar]
- Asean-gidatabase. Khao HomMali Thung Kula Rong-Hai. 2007. Available online: http://www.asean-gidatabase.org/gidatabase/sites/default/files/gidocs/THGI0000049100020-en.pdf (accessed on 15 April 2021).
- DIP (Department of Intellectual Property). One Province One Geographical Indication, GI Thailand. 2013. Available online: https://ipthailand.go.th/images/gibook/GI_Thailand_1.pdf (accessed on 15 April 2021).
- Pérez-Rodríguez, M.; Dirchwolf, P.M.; Rodríguez-Negrín, Z.; Pellerano, R.G. Assessing mineral profiles for rice flour fraud detection by principal component analysis based data fusion. Food Chem. 2021, 339, 128125. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.N.; Monteiro, L.R.; Freire, B.M.; Franco, D.F.; de Souza, R.O.; dos Reis Ferreira, C.E.S.; da Silva, J.J.C.; Batista, B.L. Mineral profile exploratory analysis for rice grains traceability. Food Chem. 2019, 300, 125145. [Google Scholar] [CrossRef]
- Kelly, S.; Baxter, M.; Chapman, S.; Rhodes, C.; Dennis, J.; Brereton, P. The application of isotopic and elemental analysis to determine the geographical origin of premium long grain rice. Euro. Food Res. Technol. 2002, 214, 72–78. [Google Scholar] [CrossRef]
- Suzuki, Y.; Chikaraishi, Y.; Ogawa, N.O.; Ohkouchi, N.; Korenaga, T. Geographical origin of polished rice based on multiple element and stable isotope analyses. Food Chem. 2008, 109, 470–475. [Google Scholar] [CrossRef]
- Maione, C.; Barbosa, R.M. Recent applications of multivariate data analysis methods in the authentication of rice and the most analyzed parameters: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1868–1879. [Google Scholar] [CrossRef]
- Kato, L.S.; De Nadai Fernandes, E.A.; Bacchi, M.A.; Sarriés, G.A. Elemental composition of Brazilian rice grains from different cultivars and origins. J. Radioanal. Nucl. Chem. 2018, 318, 745–751. [Google Scholar] [CrossRef]
- Salim, N.A.A.; Mostapa, R.; Othman, Z.; Daud, N.M.; Harun, A.R.; Mohamed, F. Geographical identification of Oryza sativa “MR 220CL” from Peninsular Malaysia using elemental and isotopic profiling. Food Control 2020, 110, 106967. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, Y.; Zhang, W.; Yang, S.; Ye, Z.; Zhang, G. Variation of the light stable isotopes in the superior and inferior grains of rice (Oryza sativa L.) with different geographical origins. Food Chem. 2016, 209, 95–98. [Google Scholar] [CrossRef]
- Chung, I.M.; Kim, J.K.; Prabakaran, M.; Yang, J.H.; Kim, S.H. Authenticity of rice (Oryza sativa L.) geographical origin based on analysis of C, N, O and S stable isotope ratios: A preliminary case report in Korea, China and Philippine. J. Sci. Food Agric. 2016, 96, 2433–2439. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Park, S.K.; Lee, K.J.; An, M.J.; Lee, J.H.; Oh, Y.T.; Kim, S.H. Authenticity testing of environment-friendly Korean rice (Oryza sativa L.) using carbon and nitrogen stable isotope ratio analysis. Food Chem. 2017, 234, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Korenaga, T.; Musashi, M.; Nakashita, R.; Suzuki, Y. Statistical analysis of rice samples for compositions of multiple light elements (H, C, N, and O) and their stable isotopes. Anal. Sci. 2010, 26, 873–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, I.M.; Kim, J.K.; Lee, J.K.; Kim, S.H. Discrimination of geographical origin of rice (Oryza sativa L.) by multielement analysis using inductively coupled plasma atomic emission spectroscopy and multivariate analysis. J. Cereal Sci. 2015, 65, 252–259. [Google Scholar] [CrossRef]
- Gonzálvez, A.; Armenta, S.; de la Guardia, M. Geographical traceability of “Arròs de Valencia” rice grain based on mineral element composition. Food Chem. 2011, 126, 1254–1260. [Google Scholar] [CrossRef]
- Kokot, S.; Phuong, T.D. Elemental content of Vietnamese rice. Part 2. Multivariate data analysis. Analyst 1999, 124, 561–569. [Google Scholar] [CrossRef]
- Li, G.; Nunes, L.; Wang, Y.; Williams, P.N.; Zheng, M.; Zhang, Q.; Zhu, Y. Profiling the ionome of rice and its use in discriminating geographical origins at the regional scale, China. J. Environ. Sci. 2012, 25, 144–154. [Google Scholar] [CrossRef]
- Yasui, A.; Shindoh, K. Analytical Chemistry for Environmental and Human Health. Determination of the geographic origin of brown-rice with trace-element composition. Bunseki Kagaku 2000, 49, 405–410. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, R.M.; de Paula, E.S.; Paulelli, A.C.; Moore, A.F. Souza, J.M.O.; Batista, B.L.; Campiglia, A.D.; Barbosa, F., Jr. Recognition of organic rice samples based on trace elements and support vector machines. J. Food Compos. Anal. 2016, 45, 95–100. [Google Scholar] [CrossRef]
- Lagad, R.A.; Singh, S.K.; Rai, V.K. Rare earth elements and 87Sr/86Sr isotopic characterization of Indian Basmati rice as potential tool for its geographical authenticity. Food Chem. 2017, 217, 254–265. [Google Scholar] [CrossRef]
- Du, M.; Fang, Y.; Shen, F.; Mao, B.; Zou, Y.; Li, P.; Pei, F.; Hu, Q. Multiangle discrimination of geographical origin of rice based on analysis of mineral elements and characteristic volatile components. Int. J. Food Sci. Technol. 2018, 53, 2088–2096. [Google Scholar] [CrossRef]
- Qian, L.; Zuo, F.; Liu, H.; Zhang, C.; Chi, X.; Zhang, D. Determination of Geographical Origin of Wuchang Rice with the Geographical Indicator by Multielement Analysis. J. Food Qual. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Promchan, J.; Günther, D.; Siripinyanond, A.; Shiowatana, J. Elemental imaging and classifying rice grains by using laser ablation inductively coupled plasma mass spectrometry and linear discriminant analysis. J. Cereal Sci. 2016, 71, 198–203. [Google Scholar] [CrossRef]
- Ariyama, K.; Shinozaki, M.; Kawasaki, A. Determination of the Geographic Origin of Rice by Chemometrics with Strontium and Lead Isotope Ratios and Multielement Concentrations. J. Agric. Food Chem. 2012, 60, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.; Heaton, K.; Hoogewerff, J. Tracing the geographical origin of food: The application of multi-element and multi-isotope analysis. Trends Food Sci. Technol. 2005, 16, 555–567. [Google Scholar] [CrossRef]
- Chung, I.M.; Kim, J.K.; Lee, K.J.; Park, S.K.; Lee, J.H.; Son, N.Y.; Jin, Y.I.; Kim, S.H. Geographic authentication of Asian rice (Oryza sativa L.) using multi-elemental and stable isotopic data combined with multivariate analysis. Food Chem. 2018, 240, 840–849. [Google Scholar] [CrossRef]
- Li, C.; Dong, H.; Luo, D.; Xian, Y.; Fu, X. Recent Developments in Application of Stable Isotope and Multi-element Analysis on Geographical Origin Traceability of Cereal Grains. Food Anal. Methods 2016, 9, 1512–1519. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, W.; Zhang, Y.; Chen, T.; Shao, S.; Zhou, L.; Yuan, Y.; Xie, T.; Rogers, K.M. Assuring food safety and traceability of polished rice from different production regions in China and Southeast Asia using chemometric models. Food Control 2019, 99, 1–10. [Google Scholar] [CrossRef]
- Magnusson, B.; Ornemark, U. (Eds.) Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; Eurachem: London, UK, 2014; pp. 1–70. [Google Scholar]
- Melini, V.; Melini, F. Asian grain-based food products and the European scheme for food protected designations of origin: A critical analysis. Trends Food Sci. Technol. 2019, 91, 83–94. [Google Scholar] [CrossRef]
- Ni, K.; Wang, J.; Zhang, Q.; Yi, X.; Ma, L.; Shi, Y.; Ruan, J. Multi-element composition and isotopic signatures for the geographical origin discrimination of green tea in China: A case study of Xihu Longjing. J. Food Compos. Anal. 2018, 67, 104–109. [Google Scholar] [CrossRef]
| Stable Isotope (‰)/Element (mg kg−1) | Province of Thai Hom Mali Rice Origin 1 | |||||
|---|---|---|---|---|---|---|
| YP (n =7) | RP (n = 16) | SP (n = 5) | ||||
| Average | RSD 2 | Average | RSD | Average | RSD | |
| δ18O | 25.48 a | 0.018 | 25.42 a | 0.03 | 24.20 b | 0.018 |
| Mn | 17.71 c | 0.26 | 12.74 d | 0.23 | 12.82 cd | 0.10 |
| Co | 0.085 e | 0.89 | 0.039 f | 2.03 | 0.033 ef | 0.45 |
| Rb | 8.72 g | 0.34 | 4.30 h | 0.22 | 4.19 h | 0.28 |
| Mo | 0.55 i | 0.25 | 0.49 ij | 0.32 | 0.32 j | 0.34 |
| Classification | YP | RP | SP | Overall |
|---|---|---|---|---|
| LDA (%) | Elemental composition only | |||
| 85.7 | 75.0 | 100.0 | 82.1 | |
| Cross-validated | 71.4 | 75.0 | 80.0 | 75.0 |
| LDA (%) | Combination of elemental and stable isotope compositions | |||
| 85.7 | 100.0 | 100.0 | 96.4 | |
| Cross-validated | 71.4 | 100.0 | 100.0 | 92.9 |
| Origin | Prediction in 3 Provincial Origins | |||||
|---|---|---|---|---|---|---|
| YP | RP | SP | Total | |||
| Classification | count | YP | 6 | 1 | 0 | 7 |
| RP | 0 | 16 | 0 | 16 | ||
| SP | 0 | 0 | 5 | 5 | ||
| % | YP | 85.7 | 14.3 | 0.0 | 100.0 | |
| RP | 0.0 | 100.0 | 0.0 | 100.0 | ||
| SP | 0.0 | 0.0 | 100.0 | 100.0 | ||
| Cross-validated | count | YP | 5 | 1 | 1 | 7 |
| RP | 0 | 16 | 0 | 16 | ||
| SP | 0 | 0 | 5 | 5 | ||
| % | YP | 71.4 | 14.3 | 14.3 | 100.0 | |
| RP | 0.0 | 100.0 | 0.0 | 100.0 | ||
| SP | 0.0 | 0.0 | 100.0 | 100.0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kongsri, S.; Sricharoen, P.; Limchoowong, N.; Kukusamude, C. Tracing the Geographical Origin of Thai Hom Mali Rice in Three Contiguous Provinces of Thailand Using Stable Isotopic and Elemental Markers Combined with Multivariate Analysis. Foods 2021, 10, 2349. https://doi.org/10.3390/foods10102349
Kongsri S, Sricharoen P, Limchoowong N, Kukusamude C. Tracing the Geographical Origin of Thai Hom Mali Rice in Three Contiguous Provinces of Thailand Using Stable Isotopic and Elemental Markers Combined with Multivariate Analysis. Foods. 2021; 10(10):2349. https://doi.org/10.3390/foods10102349
Chicago/Turabian StyleKongsri, Supalak, Phitchan Sricharoen, Nunticha Limchoowong, and Chunyapuk Kukusamude. 2021. "Tracing the Geographical Origin of Thai Hom Mali Rice in Three Contiguous Provinces of Thailand Using Stable Isotopic and Elemental Markers Combined with Multivariate Analysis" Foods 10, no. 10: 2349. https://doi.org/10.3390/foods10102349
APA StyleKongsri, S., Sricharoen, P., Limchoowong, N., & Kukusamude, C. (2021). Tracing the Geographical Origin of Thai Hom Mali Rice in Three Contiguous Provinces of Thailand Using Stable Isotopic and Elemental Markers Combined with Multivariate Analysis. Foods, 10(10), 2349. https://doi.org/10.3390/foods10102349





