The Impact of the Fermentation Strategy on the Flavour Formation of Ilzer Rose (Malus domestica Borkh.) Apple Wine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Material
2.2. Preparation of Juice Samples
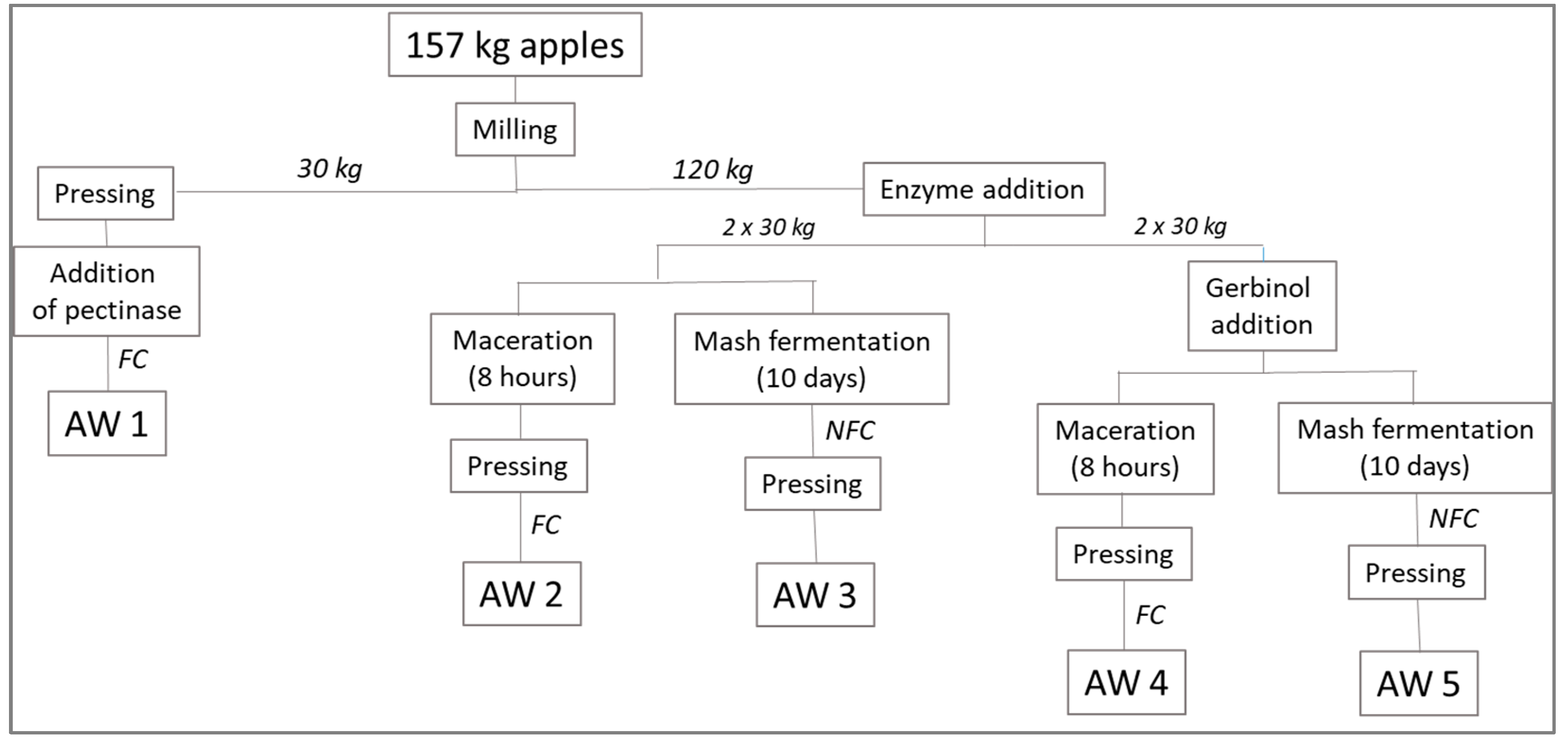
2.3. Preparation of the Apple Wines
2.4. Analysis of Basic Fruit Wine Parameters
2.4.1. Sugar Concentration
2.4.2. Acidity
2.4.3. Sample Preparation and GC-MS Analysis
2.4.4. Sensory Evaluation
2.4.5. Statistical Analysis
3. Results and Discussion
3.1. Volatile Compounds Obtained from Apple Juice and Dependence on the Maceration Time
3.2. Investigation of the Apple Wines
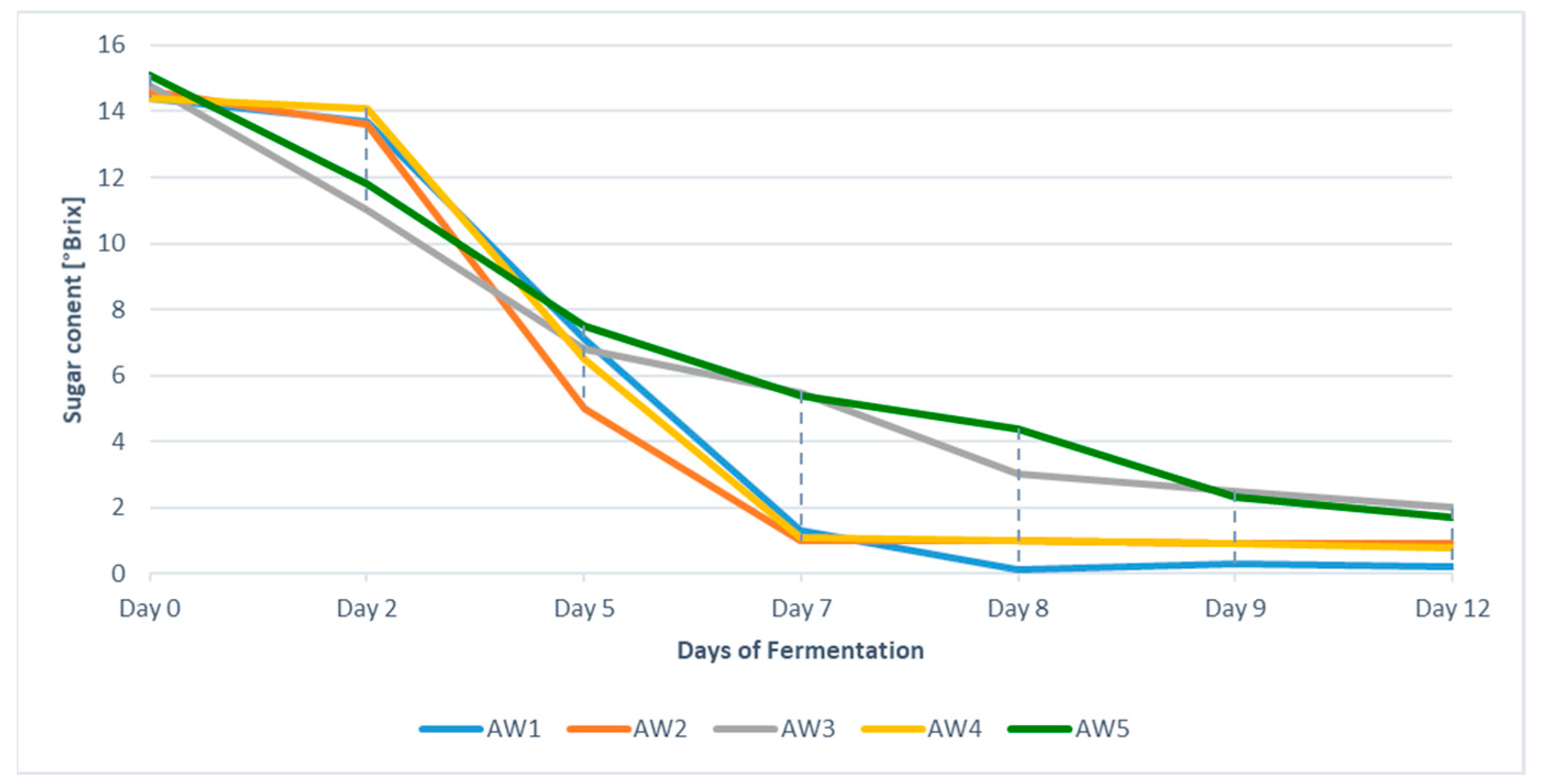
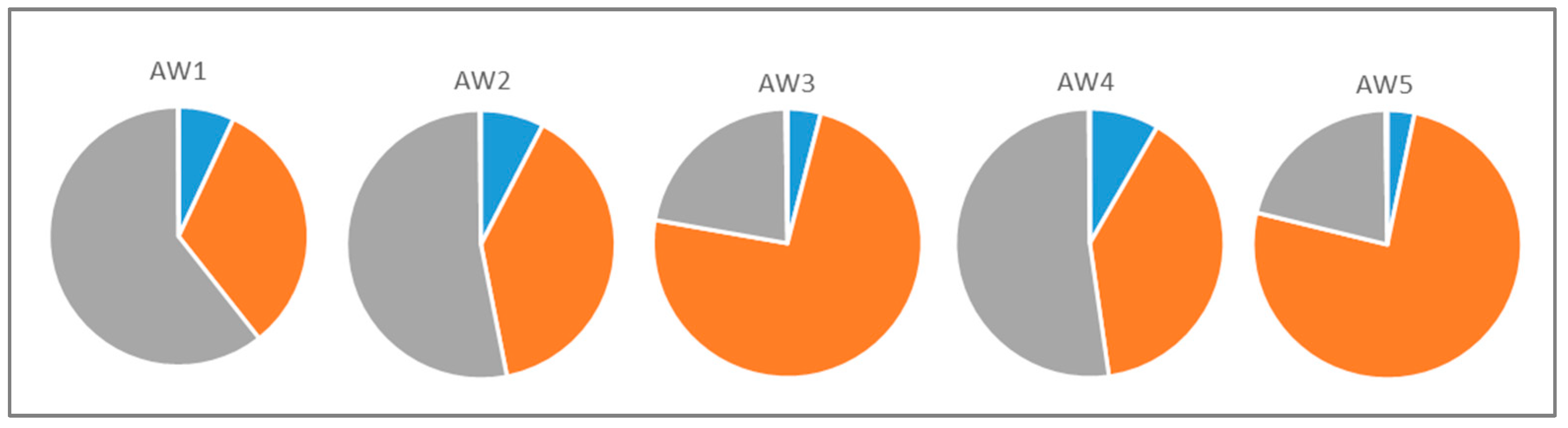
3.2.1. Fermentation and Basic Apple Wine Parameters
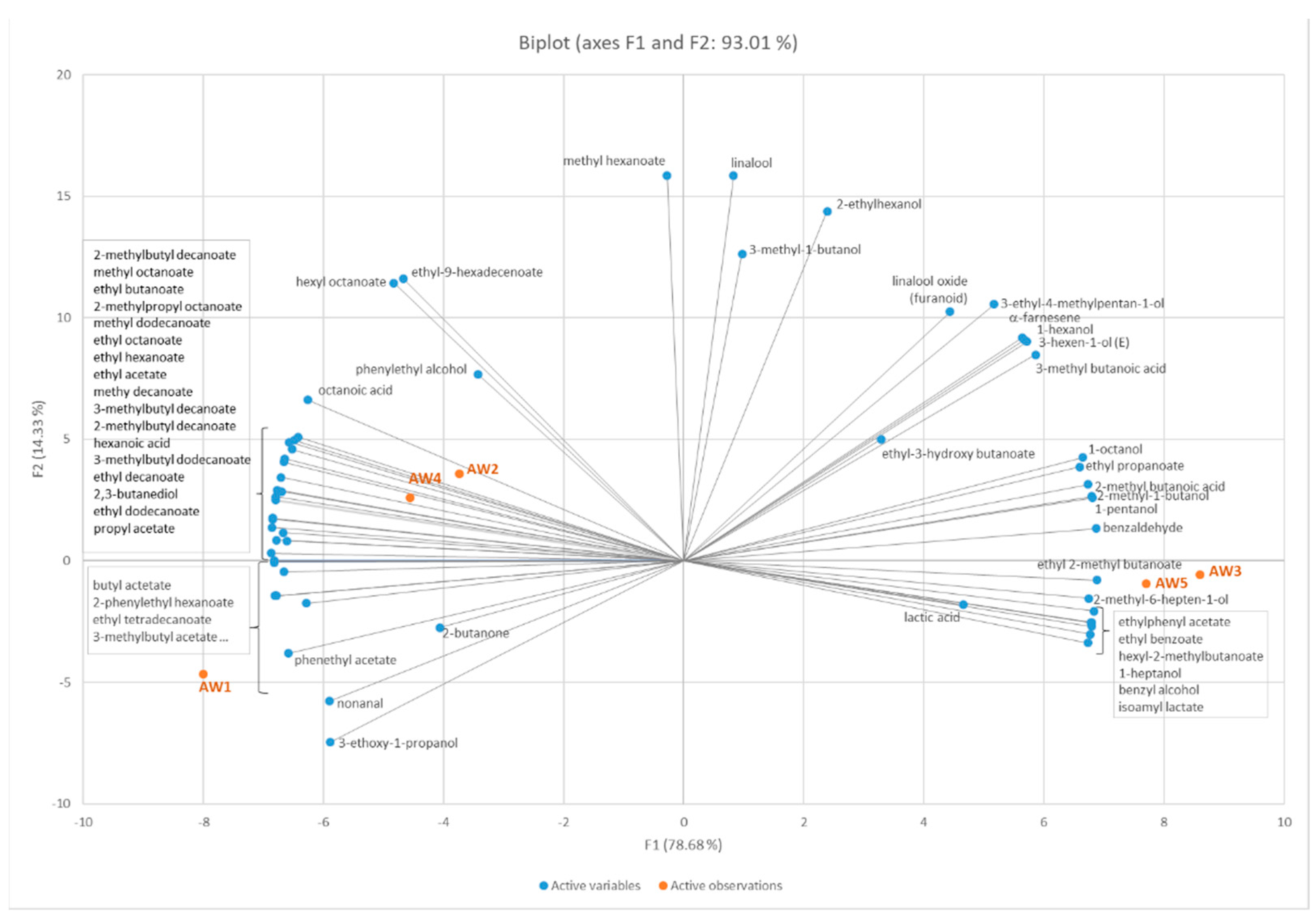
3.2.2. Volatile Compounds from Apple Wines
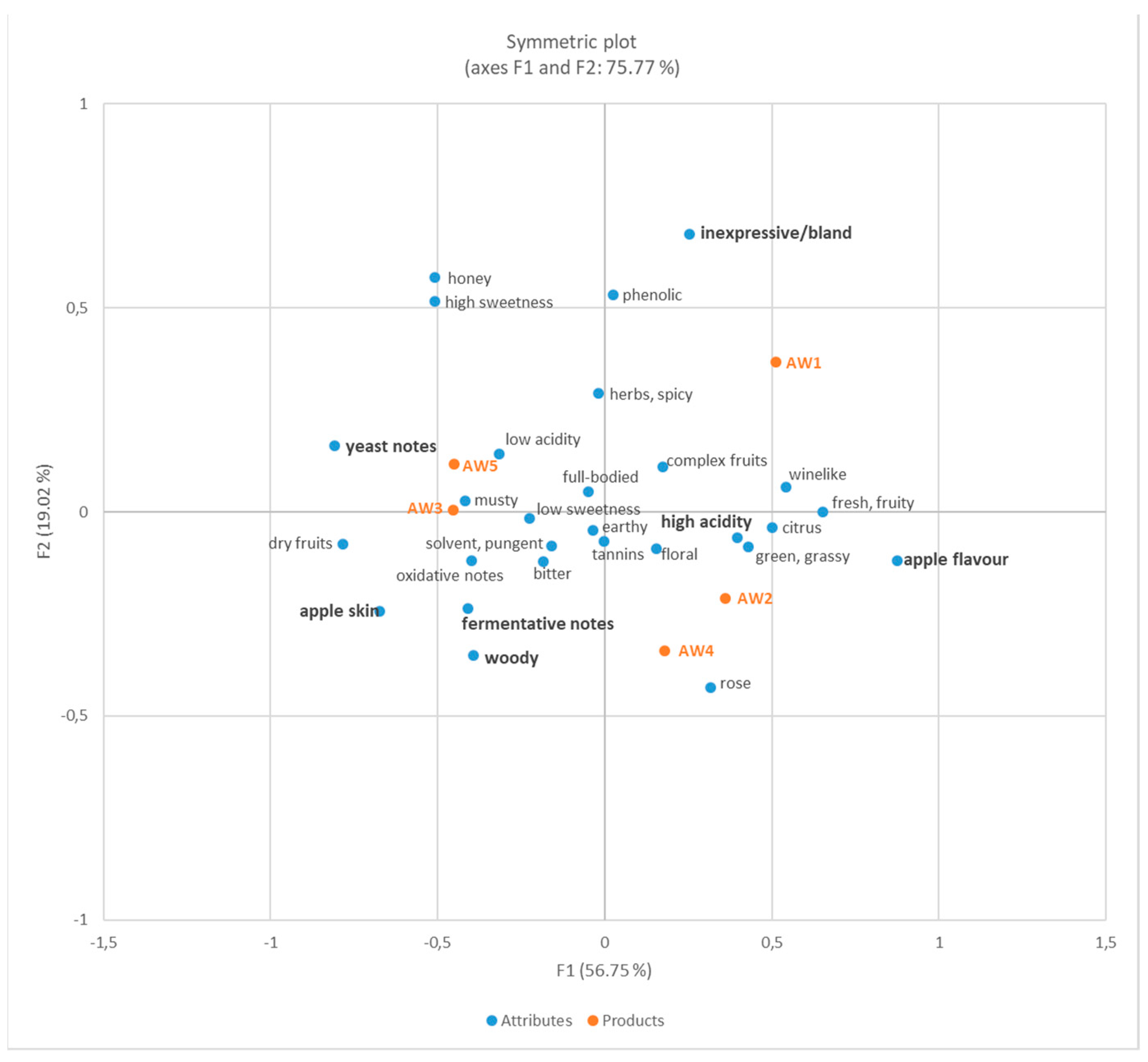
3.2.3. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AICV. The European Cider & Fruit Wine Association, European Cider Trends. 2020. Available online: https://aicv.org/en/news/aicv-european-cider-trends-2020-now-published (accessed on 2 July 2021).
- Picinelli Lobo, A.; Antón-Díaz, M.J.; Mangas Alonso, J.J.; Suárez Valles, B. Characterization of Spanish ciders by means of chemical and olfactometric profiles and chemometrics. Food Chem. 2016, 213, 505–513. [Google Scholar] [CrossRef]
- Qin, Z.; Petersen, M.A.; Bredie, W.L.P. Flavor profiling of apple ciders from the UK and Scandinavian region. Food Res. Int. 2019, 105, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Nespor, J.; Karabin, M.; Stuulikova, K.; Dostalek, P. An HS-SPME-GC-MS Method for Profiling Volatile Compounds as Related to Technology Used in Cider Production. Molecules 2019, 24, 2117. [Google Scholar] [CrossRef] [Green Version]
- Wilson, A.; Johnson, J.B.; Batley, R.; Lal, P.; Wakeling, L.; Naiker, M. Authentication Using Volatile Composition: A Proof-of-Concept Study on the Volatile Profiles of Fourteen Queensland Ciders. Beverages 2021, 7, 28. [Google Scholar] [CrossRef]
- Alberti, A.; Machado dos Santos, T.P.; Ferreira Zielinski, A.A.; Eleuterio dos Santos, C.M.; Braga, C.M.; Demiate, I.M.; Nogueira, A. Impact on chemical profile in apple juice and cider made from unripe, ripe and senescent dessert varieties. LWT Food Sci. Technol. 2016, 65, e436–e443. [Google Scholar] [CrossRef]
- Vidrih, R.; Hribar, J. Synthesis of higher alcohols during cider processing. Food Chem. 1999, 67, 287–294. [Google Scholar] [CrossRef]
- Laaksonen, O.; Kuldjärv, R.; Paalme, T.; Virkki, M.; Yang, B. Impact of apple cultivar, ripening stage, fermentation type and yeast strain on phenolic composition of apple ciders. Food Chem. 2017, 233, 29–37. [Google Scholar] [CrossRef]
- Antón-Díaz, M.J.; Suárez Valles, B.; Mangas-Alonso, J.J.; Fernández-García, O.; Picinelli-Lobo, A. Impact of different techniques involving contact with lees on the volatile composition of cider. Food Chem. 2016, 190, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Lukic, I.; Horvat, I.; Radeka, S.; Damijanic, K.; Staver, M. Effect of different levels of skin disruption and contact with oxygen during grape processing on phenols, volatile aromas, and sensory characteristics of white wine. J. Food Process. Preserv. 2019, 43, e13960. [Google Scholar] [CrossRef]
- Rosend, J.; Kuldjarv, R.; Arju, G.; Nisamedtinov, I. Yeast performance characterisation in different cider fermentation matrices. Agron. Res. 2019, 17, 2040–2053. [Google Scholar] [CrossRef]
- Wicklund, T.; Skotthei, E.R.; Rember, S.F. Various Factors Affect Product Properties in Apple Cider Production. Int. J. Food Stud. 2020, 9, SI84–SI96. [Google Scholar] [CrossRef] [Green Version]
- Calugar, P.C.; Coldea, T.E.; Salanta, L.C.; Pop, C.R.; Pasqualone, A.; Burja-Udrea, C.; Zhao, H.; Mudura, E. An Overview of the Factors Influencing Apple Cider Sensory and Microbial Quality from Raw Materials to Emerging Processing Technologies. Processes 2021, 9, 502. [Google Scholar] [CrossRef]
- Wurm, L.; Wendelin, S.; Gössinger, M.; Kieler, M.; Sigl, K.; Patzl, W.; Kickenweiz, M.; Rühmer, T.; Klöckl, V.; Brandes, W.; et al. Ertrag, Fruchtqualität, Inhaltsstoffe und Geschmacksqualität alter Apfelsorten unter biologischen und integrierten Anbaubedingungen. Mitt. Klosterneubg. 2014, 64, 63–81. [Google Scholar]
- Bingman, M.T.; Stellick, C.E.; Pelkey, J.P.; Scot, J.M.; Cole, C.A. Monitoring Cider Aroma Development throughout the Fermentation Process by Headspace Solid Phase Microextraction (HS-SPME) Gas Chromatography–Mass Spectrometry (GC-MS) Analysis. Beverages 2020, 6, 40. [Google Scholar] [CrossRef]
- Rühmer, T.; Voit, J. Most und Saft—Die veredelte Form von Äpfeln. Haidegger Perspekt. 2020, 3, 6–9. [Google Scholar]
- Siegmund, B.; Leitner, E. Characterisation of the Flavour of the old Austrian Apple variety ‘Ilzer Rose’. In Flavour Science: Proceedings of the XV Weurman Flavour Research Symposium; Siegmund, B.; Leitner, E. Technischen Universität Graz: Graz, Austria, 2018; pp. 135–138. [Google Scholar] [CrossRef]
- Hjelmeland, K.; Ebeler, S.E. Glycosidically Bound Volatile Aroma Compounds in Grapes and Wine: A Review. Am. J. Enol. Vitic. 2015, 66, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ruppert, V.; Innerhofer, G.; Voit, J.; Hiden, P.; Siegmund, B. Enzymatic Release of Flavour Compounds from Heritage Apple Varieties. In Flavour Science—Proceeding of the 16th Weurman Flavour Research Symposium; Le Quéré, J.L., Guichard, E., Eds.; INRAE: Dijon, France, 2021; under review. [Google Scholar]
- Galati, A.; Schifani, G.; Crescimanno, M.; Migliore, G. “Natural wine” consumers and interest in label information: An analysis of willingness to pay in a new Italian wine market segment. J. Clean. Prod. 2019, 227, e405–e413. [Google Scholar] [CrossRef]
- Urdapilleta, I.; Demarchi, S.; Parr, W.V. Influence of culture on social representation of wines produced by various methods: Natural, organic and conventional. Food Qual. Prefer. 2021, 87, 104034. [Google Scholar] [CrossRef]
- Ancient Georgian Traditional Qvevri Wine-Making Method. Available online: https://ich.unesco.org/en/RL/ancient-georgian-traditional-qvevri-wine-making-method-00870 (accessed on 12 July 2021).
- Gonzalez, G.A.; Parga-Dans, E. Natural wine: Do consumers know what it is, and how natural it really is? J. Clean. Prod. 2020, 251, 119635. [Google Scholar] [CrossRef]
- Sancho-Galán, P.; Amores-Arrocha, A.; Jiménez-Cantizano, A.; Palacios, V. Influence of the Presence of Grape Skins during White Wine Alcoholic Fermentation. Agronomy 2021, 11, 452. [Google Scholar] [CrossRef]
- OIV. Resolution OIV/OENO 390/2010, Guidelines on Infrared Analysers in Oenology. 2010. Available online: https://www.oiv.int/public/medias/1239/oiv-oeno-390-2010-en.pdf (accessed on 27 September 2021).
- Johnson, L.G.; Skou, P.B.; Khakimov, B.; Bro, R. Gas chromatography—Mass spectrometry data processing made easy. J. Chromatogr. A 2017, 1503, 57–64. [Google Scholar] [CrossRef]
- Risum, A.B.; Bro, R. Using deep learning to evaluate peaks in chromatographic data. Talanta 2019, 204, 255–260. [Google Scholar] [CrossRef]
- DIN EN ISO 8586: 2014-05. Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors; DIN Deutsches Institut für Normung. e.V., Beuth Verlags GmbH: Berlin, Germany, 2014. [Google Scholar]
- Elmore, J.S. Aroma extract analysis. In Flavour Development, Analysis and Perception in Food and Beverages; Parker, J.K., Elmore, J.S., Methven, M., Eds.; Woodhead Publishing: Cambridge, UK, 2015; pp. 47–62. [Google Scholar] [CrossRef]
- Schwab, W.; Wüst, M. Understanding the Constitutive and Induced Biosynthesis of Mono- and Sesquiterpenes in Grapes (Vitis vinifera): A Key to Unlocking the Biochemical Secrets of Unique Grape Aroma Profiles. J. Agric. Food Chem. 2015, 63, 10591–10603. [Google Scholar] [CrossRef]
- Alexandre, H.; Costello, P.J.; Remize, F.; Guzzo, J.; Guilloux-Benatier, M. Saccharomyces cerevisiae–Oenococcus oeni interactions in wine: Current knowledge and perspectives. Int. J. Food Microbiol. 2004, 93, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Antón, M.J.; Valle, B.S.; Hevia, A.G.; Lobo, A.P. Aromatic Profile of Ciders by Chemical Quantitative, Gas Chromatography-Olfactometry, and Sensory Analysis. J. Food Sci. 2014, 79, S92–S99. [Google Scholar] [CrossRef] [PubMed]
- Hirst, M.B.; Richter, C.L. Review of Aroma Formation through Metabolic Pathways of Saccharomyces cerevisiae in Beverage Fermentations. Am. J. Enol. Vitic. 2016, 67, 4. [Google Scholar] [CrossRef] [Green Version]
- Marullo, P.; Trujillo, M.; Viannais, R.; Hercman, L.; Guillaumie, S.; Colonna-Ceccaldi, B.; Albertin, W.; Barbe, J.C. Metabolic, Organoleptic and Transcriptomic Impact of Saccharomyces cerevisiae Genes Involved in the Biosynthesis of Linear and Substituted Esters. Int. J. Mol. Sci. 2021, 22, 4026. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rollero, S.; Bloem, A.; Camarasa, C.; Sanchez, I.; Ortiz-Julien, A.; Sablayrolles, J.M.; Dequin, S.; Mouret, J.R. Combined effects of nutrients and temperature on the production of fermentative aromas by Saccharomyces cerevisiae during wine fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 2291–2304. [Google Scholar] [CrossRef]
- Campo, E.; Ballester, J.; Langlois, J.; Dacremont, C.; Valentin, D. Comparison of conventional descriptive analysis and a citation frequency-based descriptive method for odor profiling: An application to Burgundy Pinot noir wines. Food Qual. Prefer. 2010, 21, 44–55. [Google Scholar] [CrossRef]
- Buck, D.; Kemp, S.E. Check-All-That-Apply and Free Choice Description. In Descriptive Analysis in Sensory Evaluation; Kemp, S.E., Hort, J., Hollowood, T., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2018; pp. 579–607. [Google Scholar]
| Compound | RI [DB5] exp § | RI [DB5] lit. $ | Juice 0 h Maceration Mean ± SD [µg L−1] | Juice 2 h Maceration Mean ± SD [µg L−1] | Juice 5 h Maceration Mean ± SD [µg L−1] | Juice 8 h Maceration Mean ± SD [µg L−1] | Significance |
|---|---|---|---|---|---|---|---|
| acids | |||||||
| acetic acid | 612 | 625 | 1.53 ± 0.3 | 0.72 ± 0.6 | 2.56 ± 0.2 | 1.37 ± 1.2 | No |
| 2-methyl butanoic acid | 842 | 856 | 25.3 ± 1.3 a | 15.9 ± 1.3 b | 17.7 ± 1.5 b | 20.4 ± 0.2 a,b | * |
| hexanoic acid | 967 | 980 | 3.0 ± 0.0 c | 7.1 ± 0.1 b | 7.0 ± 0.2 b | 10.3 ± 0.3 a | ** |
| alcohols | |||||||
| 1-propanol t | <600 | 548 | 1.94 ± 0.1 | 2.00 ± 0.7 | 2.96 ± 0.0 | 2.36 ± 0.6 | No |
| 2-butanol | 601 | 605 | 0.91 ± 0.0 | 0.81 ± 0.3 | 1.25 ± 0.0 | 1.17 ± 0.3 | No |
| 2-methyl Propanol | 628 | 654 | 2.92 ± 0.1 | 0.71 ± 0.8 | 1.20 ± 0.3 | 1.03 ± 1.1 | No |
| 1-butanol | 665 | 660 | 40.3 ± 3.1 b | 51.4 ± 2.7 a,b | 60.9 ± 1.6 a | 61.9 ± 2.7 a | * |
| 1-penten-3-ol | 684 | 686 | 0.34 ± 0.0 b | 0.92 ± 0.1 a | 0.95 ± 0.0 a | 1.14 ± 0.1 a | ** |
| 2-methyl-1-butanol | 739 | 743 | 67.5 ± 3.8 a | 84.1 ± 4.5 a,b | 78.0 ± 0.6 a,b | 93.6 ± 3.3 b | * |
| 1-pentanol | 767 | 766 | 0.36 ± 1.9 | 3.35 ± 0.5 | 3.68 ± 0.1 | 5.07 ± 0.2 | No |
| 2,3-butanediol | 780 | 773 | 1.38 ± 0.4 | 3.37 ± 1.7 | 4.31 ± 1.1 | 4.02 ± 1.1 | No |
| 3-hexen-1-ol (Z)- | 851 | 858 | 0.89 ± 0.5 c | 20.5 ± 0.5 b | 21.0 ± 0.1 b | 34.1 ± 2.5 a | ** |
| 2-hexen-1-ol (E)- | 866 | 887 | 12.6 ± 2.1 c | 356 ± 13.9 a,b | 317 ± 11.3 b | 394 ± 19.6 c | ** |
| 1-hexanol | 868 | 867 | 106.9 ± 0.2 b | 623 ± 43.4 a | 617 ± 23.1 a | 821 ± 60.6 a | ** |
| 1-heptanol | 967 | 970 | 8.02 ± 1.0 b | 24.0 ± 4.7 a | 27.3 ± 0.4 a | 29.6 ± 0.7 a | * |
| 1-octen-3-ol | 979 | 979 | 0.55 ± 0.0 b | 1.93 ± 0.1 a | 1.72 ± 0.0 a | 2.07 ± 0.1 a | ** |
| methionol | 982 | 980 | 5.46 ± 0.2 a | 3.91 ± 0.2 b | 3.97 ± 0.3 a,b | 5.12 ± 0.3 a,b | * |
| 6-methyl-5-hepten-2-ol | 993 | 994 | 15.6 ± 1.0 c | 29.2 ± 1.5 b | 33.9 ± 0.9 a,b | 40.0 ± 2.2 a | ** |
| 2-ethyl hexanol | 1029 | 1028 | 2.01 ± 0.1 c | 3.58 ± 0.1 a | 3.00 ± 0.0 b | 3.74 ± 0.0 a | ** |
| benzyl alcohol | 1042 | 1032 | 3.04 ± 0.3 d | 8.06 ± 0.2 c | 10.4 ± 0.3 b | 21.5 ± 0.2 a | ** |
| 1-octanol | 1069 | 1069 | 1.14 ± 0.1 d | 5.14 ± 0.1 c | 7.70 ± 0.1 b | 13.06 ± 0.3 a | ** |
| 3-octen-1-ol (Z)- | 1071 | 1047 | 1.85 ± 0.2 b | 5.96 ± 0.1 a | 6.30 ± 0.2 a | 8.03 ± 0.9 a | * |
| phenylethyl alcohol | 1127 | 1113 | 2.29 ± 0.3 c | 3.12 ± 0.1 b,c | 3.69 ± 0.2 b | 6.04 ± 0.1 a | ** |
| 1,3-octanediol | 1264 | 1275 | 451 ± 24.3 b | 544 ± 20.3 a,b | 688 ± 24.4 a | 703 ± 71.1 a | * |
| 1-dodecanol | 1477 | 1475 | 6.60 ± 2.8 | 7.69 ± 1.6 | 6.18 ± 1.4 | 5.82 ± 1.0 | No |
| carbonyls | |||||||
| 2,3-butanedione | 615 | 623 | 0.15 ± 0.0 c | 0.25 ± 0.0 b | 0.37 ± 0.0 a | 0.39 ± 0.0 a | ** |
| butanal | 602 | 601 | 51.2 ± 0.0 | 1.45 ± 0.1 | 1.13 ± 0.0 | 1.33 ± 0.1 | No |
| 2-butanone | 600 | 600 | 2.79 ± 0.4 | 3.47 ± 0.4 | 3.54 ± 0.1 | 4.41 ± 0.7 | No |
| 1-penten-3-one | 687 | 687 | 0.55 ± 0.1 | 0.74 ± 0.1 | 0.65 ± 0.0 | 0.50 ± 0.0 | No |
| 2-pentanone | 688 | 695 | 2.76 ± 0.1 | 2.52 ± 0.3 | 2.69 ± 0.1 | 2.70 ± 0.3 | No |
| 2-pentenal (E)- | 756 | 748 | 0.62 ± 0.0 b | 1.10 ± 0.1 a | 0.61 ± 0.0 b | 0.51 ± 0.1 b | * |
| 3-hexenal (Z) | 797 | 795 | 10.3 ± 0.5 a | 6.44 ± 0.5 b | 2.78 ± 0.1 c | 2.67 ± 0.3 c | ** |
| hexanal | 799 | 801 | 51.4 ± 2.2 a | 49.2 ± 3.0 a | 18.6 ± 0.1 b | 18.3 ± 1.2 b | ** |
| 2,4-hexadienal (E,E)- | 911 | 916 | 12.03 ± 0.8 a | 11.3 ± 1.1 a | 5.28 ± 0.3 b | 5.24 ± 0.3 b | ** |
| 2-heptenal (E)- | 960 | 964 | 1.47 ± 0.2 b | 3.40 ± 0.1 a | 3.16 ± 0.0 a | 2.83 ± 0.1 a | ** |
| benzaldehyde | 972 | 961 | 5.03 ± 0.2 d | 25.5 ± 0.4 a | 13.7 ± 0.6 d | 22.0 ± 0.3 c | ** |
| 6-methyl-5-hepten-2-one | 988 | 988 | 4.03 ± 0.1 c | 10.7 ± 0.3 b | 13.3 ± 0.5 a,b | 14.8 ± 0.8 a | ** |
| phenylacetaldehyde | 1055 | 1047 | 0.29 ± 0.1 b | 1.01 ± 0.1 a | 1.14 ± 0.0 a | 1.05 ± 0.0 a | * |
| 2-octenal (E)- | 1063 | 1063 | 5.69 ± 0.2 c | 9.54 ± 0.0 b | 9.88 ± 0.2 a,b | 10.7 ± 0.1 a | ** |
| nonanal | 1107 | 1102 | 3.63 ± 1.8 | 6.45 ± 0.6 | 4.69 ± 0.4 | 4.98 ± 0.4 | No |
| esters | |||||||
| methyl acetate t | <600 | 522 | 0.22 ± 0.0 | 0.21 ± 0.0 | 0.34 ± 0.0 | 0.28 ± 0.1 | No |
| ethyl acetate | 615 | 628 | 15.2 ± 0.4 | 15.9 ± 1.5 b | 20.9 ± 0.4 a,b | 23.3 ± 1.4 a | * |
| propyl acetate | 715 | 695 | 0.50 ± 0.0 b | 0.59 ± 0.1 b | 0.64 ± 0.0 a,b | 1.02 ± 0.1 a | * |
| methyl butanoate | 724 | 710 | 1.00 ± 0.1 | 0.81 ± 0.1 | 0.88 ± 0.0 | 0.84 ± 0.1 | No |
| butyl acetate | 812 | 802 | 8.02 ± 0.1 b | 9.52 ± 0.9 b | 12.43 ± 0.4 a | 17.63 ± 1.2 a | * |
| 2-methyl butyl acetate | 877 | 880 | 24.5 ± 0.6 b | 36.7 ± 2.7 a,b | 40.8 ± 1.2 a | 49.3 ± 3.3 a | * |
| pentyl acetate | 911 | 926 | 1.04 ± 0.1 d | 3.16 ± 0.1 c | 5.72 ± 0.0 b | 7.22 ± 0.3 a | ** |
| ethyl-3-hydroxy-butanoate | 935 | 945 | 2.75 ± 0.1 b | 2.93 ± 0.1 a | 3.20 ± 0.3 a | 1.58 ± 0.1 a | * |
| butyl butanoate | 995 | 1002 | 2.03 ± 0.1 b | 8.17 ± 0.8 a | 7.15 ± 0.1 a | 7.59 ± 0.7 a | * |
| 3-hexen-1-yl acetate (Z) | 1001 | 996 | 1.04 ± 0.4 d | 11.5 ± 0.5 c | 22.6 ± 0.4 b | 29.8 ± 2.2 a | ** |
| 2-hexen-1-yl acetate (E) | 1013 | 1017 | 5.83 ± 2.1 c | 195 ± 9.6 b | 244 ± 2.7 a | 237 ± 12.4 a,b | ** |
| hexyl acetate | 1011 | 1011 | 18.5 ± 1.4 c | 153 ± 9.3 b | 279 ± 5.7 a | 315 ± 20.7 a | ** |
| 2-methyl butyl butanoate | 1042 | 1041 | 3.88 ± 0.6 b | 11.0 ± 0.9 a | 12.3 ± 0.3 a | 10.8 ± 1.1 a | * |
| methyl octanoate | 1123 | 1129 | 0.18 ± 0.1 b | 2.10 ± 0.6 a | 0.57 ± 0.1 a,b | 0.41 ± 0.0 a,b | * |
| hexyl-2-methylpropanoate | 1147 | 1138 | 0.30 ± 0.0 b | 1.92 ± 0.1 a | 2.23 ± 0.0 a | 2.54 ± 0.3 a | ** |
| hexyl-2-methyl butanoate | 1238 | 1236 | 9.27 ± 0.8 c | 65.4 ± 0.1 b | 104 ± 0.4 a | 101 ± 5.9 a | ** |
| pentyl hexanoate | 1287 | 1282 | 0.29 ± 0.0 b | 3.01 ± 0.0 a | 3.53 ± 0.1 a | 3.20 ± 0.3 a | ** |
| butyl octanoate | 1387 | 1393 | 0.63 ± 0.1 c | 3.06 ± 0.1 b | 4.45 ± 0.1 a | 4.21 ± 0.0 a | ** |
| hexyl hexanoate | 1386 | 1386 | 4.19 ± 0.3 c | 28.7 ± 1.5 b | 48.7 ± 1.7 a | 48.5 ± 0.6 a | ** |
| 3-methylbutyl octanoate | 1451 | 1450 | 0.16 ± 0.1 c | 0.67 ± 0.0 b,c | 1.43 ± 0.2 a | 1.11 ± 0.1 a,b | * |
| methyljasmonate | 1672 | 1647 | 1.29 ± 0.2 | 0.89 ± 0.1 | 1.38 ± 0.2 | 1.15 ± 0.0 | No |
| terpenoids | |||||||
| 2-methyl 1,3-pentadiene (E)- t | 669 | n/a | 0.12 ± 0.0 b | 0.88 ± 0.1 a | 0.87 ± 0.1 a | 1.11 ± 0.1 a | * |
| linalool oxide isomer I t | 1084 | n/a | 11.6 ± 0.6 b | 22.2 ± 1.6 a | 26.8 ± 0.0 a | 26.9 ± 1.3 a | * |
| linalool oxide isomer II t | 1099 | n/a | 11.5 ± 0.5 c | 19.9 ± 0.5 b | 23.4 ± 0.6 a | 23.2 ± 0.6 a | ** |
| β-damascenone | 1408 | 1400 | 4.06 ± 0.2 | 4.25 ± 0.8 | 4.50 ± 0.9 | 5.72 ± 0.3 | No |
| β-ionone | 1511 | 1493 | 4.74 ± 0.4 b | 7.25 ± 0.1 a | 6.27 ± 0.1 a | 7.32 ± 0.1 a | * |
| α-farnesene | 1517 | 1508 | 21.4 ± 13.5 | 32.6 ± 4.5 | 60.0 ± 9.6 | 48.0 ± 4.4 | No |
| miscellaneous | |||||||
| 2-ethylfuran | 703 | 702 | 1.52 ± 0.2 a | 1.28 ± 0.1 a,b | 0.84 ± 0.1 b | 0.73 ± 0.0 b | * |
| 2-pentylfuran | 995 | 991 | 0.67 ± 0.1 | 1.86 ± 0.5 | 1.59 ± 0.4 | 1.11 ± 0.3 | No |
| hexanal dimethyl acetal | 978 | 980 | 6.95 ± 0.9 a | 7.02 ± 0.4 a | 1.81 ± 0.3 b | 1.90 ± 0.3 b | * |
| Ethanol [%v/v] | Residual Sugar [g L−1] | Titratable Acid § [g L−1] | Malic Acid [g L−1] | Lactic Acid [g L−1] | |
|---|---|---|---|---|---|
| AW1 | 7.4 | <1.7 | 6.2 | 7.0 | n.d. |
| AW2 | 7.3 | 2.5 | 6.8 | 7.4 | n.d. |
| AW3 | 7.4 | 8.3 | 5.9 | 2.2 | 2.3 |
| AW4 | 7.3 | 3.0 | 6.8 | 7.2 | n.d. |
| AW5 | 7.2 | 8.1 | 5.8 | 2.1 | 2.3 |
| Compound | RI [DB5] exp § | RI [DB5] lit. $ | AW1 Mean ± SD [µg L−1] | AW2 Mean ± SD [µg L−1] | AW3 Mean ± SD [µg L−1] | AW4 mean ± SD [µg L−1] | AW5 mean ± SD [µg L−1] | Significance |
|---|---|---|---|---|---|---|---|---|
| acids | ||||||||
| 3-methyl butanoic acid | 823 | 835 | 8.0 ± 1.2 c | 12.1 ± 0.8 a,b | 13.6 ± 0.6 a | 11.25 ± 0.2 b | 13.2 ± 1.1 a,b | ** |
| 2-methyl butanoic acid | 835 | 846 | 10.1 ± 2.1 c | 18.6 ± 0.3 b | 30.7 ± 2.5 a | 17.30 ± 1.7 b | 27.4 ± 2.5 a | ** |
| hexanoic acid | 969 | 980 | 403 ± 45.7 a | 357 ± 22 a | 105 ± 8.7 b | 375.47 ± 12.4 a | 102 ± 12 b | ** |
| octanoic acid | 1164 | 1178 | 721 ± 158 a | 889 ± 177 a | 169 ± 10.3 b | 977.1 ± 211.4 a | 152 ± 15 b | ** |
| alcohols | ||||||||
| 2-methyl-1-butanol | 719 | 730 | 437 ± 38.3 c | 580 ± 16 b | 800 ± 84 a | 569 ± 22 b | 777 ± 15 a | ** |
| 3-methyl-1-butanol | 722 | 743 | 3526 ± 230 | 3 812 ± 417 | 3 648 ± 315 | 3 936 ± 87 | 3 864 ± 343 | no |
| 1-pentanol | 753 | 766 | 4.0 ± 0.2 c | 6.9 ± 0.3 b | 11.0 ± 1.2 c | 6.27 ± 0.6 b | 10.5 ± 0.3 c | ** |
| 2,3-butanediol | 779 | 773 | 54.0 ± 18.7 | 51.1 ± 27.9 | 24.7 ± 4.5 | 42.77 ± 13.5 | 18.3 ± 3.8 | no |
| 3-ethoxy-1-propanol | 836 | 816 | 58.7 ± 2.8 a | 27.7 ± 1.7 b | 5.9 ± 3.2 d | 18.73 ± 1.5 c | 4.1 ± 1.6 d | ** |
| 3-hexen-1-ol (E) | 848 | 858 | 3.8 ± 0.7 c | 17.2 ± 0.8 b | 22.0 ± 0.7 a | 16.14 ± 0.6 b | 21.3 ± 0.8 a | ** |
| 1-hexanol | 865 | 867 | 434 ± 31.4 c | 1 158 ± 48 b | 1 388 ± 78 a | 1 097 ± 23.8 b | 1 388 ± 59 a | ** |
| 1-heptanol | 968 | 970 | 0.9 ± 0.2 b | 1.5 ± 0.2 b | 19.6 ± 0.3 a | 1.38 ± 0.1 b | 18.9 ± 0.6 a | ** |
| 2-methyl-6-hepten-1-ol | 992 | 994 | 49.6 ± 2.0 c | 54.6 ± 2.3 c | 79.4 ± 5.0 a | 50.8 ± 2.3 c | 71.6 ± 1.8 b | ** |
| 3-ethyl-4-methylpentan-1-ol | 1024 | 1020 | 0.3 ± 0.1 c | 3.5 ± 0.1 b | 4.2 ± 0.3 c | 3.47 ± 0.1 b | 4.1 ± 0.1 c | ** |
| 2-ethylhexanol | 1029 | 1029 | 4.8 ± 0.4 | 5.8 ± 1.0 | 5.6 ± 0.2 | 5.98 ± 0.5 | 5.8 ± 0.2 | no |
| benzyl alcohol | 1041 | 1032 | 1.2 ± 0.2 c | 5.1 ± 0.6 c | 76.4 ± 3.4 a | 4.84 ± 0.4 c | 67.7 ± 2.8 b | ** |
| 1-octanol | 1069 | 1069 | 4.3 ± 0.5 d | 18.1 ± 0.7 c | 33.9 ± 0.8 a | 17.19 ± 0.8 c | 30.9 ± 1.3 b | ** |
| phenylethyl alcohol | 1127 | 1113 | 629 ± 48 a | 727 ± 55 a,b | 618 ± 17 b | 611 ± 39 b | 558 ± 20 b | * |
| carbonyls | ||||||||
| benzaldehyde | 971 | 961 | 6.8 ± 0.9 c | 11.1 ±1.3 b | 19.5 ±1.6 a | 10.3 ±0.3 b | 18.5 ± 0.8 a | ** |
| nonanal | 1106 | 1101 | 8.5 ± 3.4 | 7.3 ± 3.2 | 6.6 ± 1.3 | 7.0 ± 5.8 | 5.8 ± 1.2 | no |
| 2-butanone | 601 | 600 | 9.5 ± 1.3 | 8.3 ±1.3 | 7.5 ±1.1 | 9.8 ±0.9 | 9.1 ±1.2 | No |
| esters | ||||||||
| ethyl acetate | 614 | 628 | 315 ± 63 a | 311 ± 52 a | 143 ± 32 b | 309 ± 20 a | 168 ± 20 b | ** |
| ethyl propanoate | 692 | 706 | 1.4 ± 0.4 b | 2.5 ± 0.3 a,b | 3.3 ± 0.8 a | 2.0 ± 0.2 b | 3.12 ± 0.3 a | ** |
| propyl acetate | 694 | 695 | 4.5 ± 1.0 a | 3.9 ± 0.4 a | 1.2 ± 0.2 b | 3.7 ± 0.4 a | 1.2 ± 0.2 b | ** |
| ethyl butanoate | 791 | 808 | 31.3 ± 8.6 a | 36.5 ± 3.1 a | 6.4 ± 1.6 b | 30.5 ± 2.2 a | 5.5 ± 2.2 b | ** |
| butyl acetate | 805 | 802 | 29.4 ± 5.4 a | 26.0 ± 1.7 a | 9.9 ± 0.5 b | 23.2 ± 1.0 a | 11.4 ± 0.4 b | ** |
| ethyl 2-methyl butanoate | 846 | 850 | 0.9 ± 0.3 b | 2.1 ± 0.2 b | 5.9 ± 1.3 a | 1.5 ± 0.2 b | 5.6 ± 0.9 a | ** |
| 2-methylbutyl acetate | 876 | 880 | 114 ± 33 a | 95.4 ± 6.3 a | 13.1 ± 1.2 b | 81.7 ± 7.2 a | 15.7 ± 1.9 b | ** |
| 3-methylbutyl acetate | 873 | 876 | 1028 ± 254 a | 770 ± 46 a,b | 106 ± 9.5 c | 670 ± 48 b | 121 ± 12 c | ** |
| methyl hexanoate | 923 | 936 | 7.5 ± 2.6 c | 18.7 ± 2.1 a,b | 12.8 ± 1.9 b,c | 19.7 ± 3.3 a | 13.4 ± 1.6 b,c | ** |
| ethyl-3-hydroxy butanoate | 934 | 945 | 1.0 ± 0.1 c | 2.1 ± 0.2 b | 2.4 ± 0.1 a | 1.0 ± 0.1 c | 1.2 ± 0.2 c | ** |
| ethyl hexanoate | 997 | 999 | 956 ± 258 a | 980 ± 47 a | 587 ± 15 b | 846 ± 46 a | 275 ± 37 b | ** |
| hexyl acetate | 1010 | 1011 | 1072 ± 290 a | 795 ± 44 a,b | 47.0 ± 1.5 c | 657 ± 32 b | 55.4 ± 6.3 c | ** |
| isoamyl lactate | 1071 | 1065 | 0.3 ± 0.1 c | 0.2 ± 0.1 c | 23.8 ± 1.4 a | 0.3 ± 0.1 c | 20.5 ± 1.1 b | ** |
| methyl octanoate | 1123 | 1129 | 216 ± 78 a | 233 ± 63 a | 69.9 ± 17.2 b | 241 ± 69 a | 66.8 ± 15.6 b | * |
| ethyl benzoate | 1183 | 1170 | 0.7 ± 0.1 c | 1.4 ± 0.0 c | 12.8 ± 0.3 a | 1.6 ± 0.1 c | 10.8 ± 0.7 b | ** |
| ethyl octanoate | 1196 | 1194 | 2083 ± 479 a | 2148 ± 123 a | 561 ± 46 b | 1 994 ± 101 a | 483 ± 77 b | ** |
| hexyl-2-methylbutanoate | 1238 | 1234 | 0.5 ± 0.1 b | 3.1 ± 0.1 b | 36.9 ± 2.8 a | 2.2 ± 0.2 b | 32.4 ± 4.9 a | ** |
| ethylphenyl acetate t | 1255 | n/a | 0.8 ± 0.1 c | 2.0 ± 0.2 b | 11.6 ± 0.5 a | 1.9 ± 0.2 b | 11.4 ± 0.6 a | ** |
| phenethyl acetate | 1269 | 1256 | 222 ± 16.8 a | 140.1 ± 6.0 b | 16.7 ± 0.9 d | 113 ± 4.2 c | 15.5 ± 0.4 d | ** |
| methyl decanoate | 1324 | 1324 | 387 ± 81 a | 368 ± 81 a | 56.8 ± 13 b | 363 ± 70 a | 51.3 ± 11 b | ** |
| 2-methylpropyl octanoate | 1348 | 1345 | 6.0 ± 1.7 a | 6.3 ± 0.8 a | 1.1 ± 0.2 b | 6.4 ± 1.1 a | 1.0 ± 0.2 b | ** |
| ethyl decanoate | 1395 | 1392 | 2177 ± 445 a | 1832 ± 160 a | 279 ± 41 b | 1 941 ± 245 a | 230 ± 40 b | ** |
| 3-methylbutyl octanoate | 1448 | 1450 | 104 ± 30 a | 88.7 ± 10.8 a | 9.2 ± 1.6 b | 94.8 ± 18.1 a | 7.2 ± 1.2 b | ** |
| methyl dodecanoate | 1525 | 1526 | 276 ± 16.6 a | 291 ± 41 a | 61.8 ± 7.0 b | 285 ± 26.2 a | 52.8 ± 5.2 b | ** |
| hexyl octanoate | 1584 | 1571 | 3.3 ± 1.2 b | 8.5 ± 1.0 a | 0.9 ± 0.2 b | 9.0 ± 2.2 a | 0.8 ± 0.2 b | ** |
| ethyl dodecanoate | 1594 | 1597 | 547 ± 194 a | 395 ± 61 a | 53.3 ± 12.2 b | 533 ± 134 a | 43.4 ± 10.4 b | ** |
| 3-methylbutyl decanoate | 1647 | 1649 | 108.9 ± 31.2 a | 96.0 ± 10.5 a | 6.4 ± 1.5 b | 109 ± 18.4 a | 5.1 ± 1.1 b | ** |
| 2-methylbutyl decanoate | 1651 | 1647 | 16.4 ± 5.4 a | 17.0 ± 2.1 a | 2.5 ± 0.2 b | 21.5 ± 4.5 a | 2.3 ± 0.2 b | ** |
| 2-phenylethyl hexanoate | 1664 | 1650 | 5.4 ± 0.7 a | 4.5 ± 0.2 b | 0.6 ± 0.1 c | 3.8 ± 0.2 b | 0.6 ± 0.1 c | ** |
| ethyl tetradecanoate | 1794 | 1794 | 26.9 ± 8.8 a | 17.7 ± 2.6 a,b | 3.6 ± 1.6 b | 26.1 ± 5.8 a | 5.7 ± 0.8 b | ** |
| 3-methylbutyl dodecanoate | 1848 | 1844 | 6.8 ± 1.6 ab | 4.8 ± 0.9 b | 0.9 ± 0.2 c | 7.2 ± 0.7 a | 0.8 ± 0.1 c | ** |
| methyl hexadecanoate | 1927 | 1933 | 132 ± 32 | 135 ± 38 | 86 ± 12 | 147 ± 41 | 89 ± 19 | no |
| ethyl-9-hexadecenoate | 1979 | 1977 | 7.2 ± 1.2 c | 16.6 ± 1.8 a | 4.2 ± 0.7 d | 13.3 ± 0.9 b | 2.9 ± 0.2 d | ** |
| ethyl hexadecanoate | 1994 | 1978 | 53.1 ± 3.1 a | 35.7 ± 4.3 b | 25.8 ± 2.2 c | 53.0 ± 5.3 a | 24.2 ± 1.3 c | ** |
| terpenoids | ||||||||
| linalool oxide (furanoid) | 1083 | 1073 | 5.9 ± 0.1 c | 9.0 ± 0.5 a | 9.2 ± 0.5 a | 7.4 ± 0.0 b | 8.1 ± 0.2 b | ** |
| linalool | 1103 | 1101 | 3.6 ± 0.5 d | 5.5 ± 0.1 a | 4.8 ± 0.1 b,c | 5.1 ± 0.1 b,c | 4.4 ± 0.2 a,b | ** |
| α-farnesene | 1517 | 1508 | 2.0 ± 0.3 c | 7.3 ± 0.5 a,b | 9.2 ± 1.7 a | 6.3 ± 0.8 b | 8.1 ± 1.0 a,b | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruppert, V.; Innerhofer, G.; Voit, J.; Hiden, P.; Siegmund, B. The Impact of the Fermentation Strategy on the Flavour Formation of Ilzer Rose (Malus domestica Borkh.) Apple Wine. Foods 2021, 10, 2348. https://doi.org/10.3390/foods10102348
Ruppert V, Innerhofer G, Voit J, Hiden P, Siegmund B. The Impact of the Fermentation Strategy on the Flavour Formation of Ilzer Rose (Malus domestica Borkh.) Apple Wine. Foods. 2021; 10(10):2348. https://doi.org/10.3390/foods10102348
Chicago/Turabian StyleRuppert, Valerie, Georg Innerhofer, Jörg Voit, Peter Hiden, and Barbara Siegmund. 2021. "The Impact of the Fermentation Strategy on the Flavour Formation of Ilzer Rose (Malus domestica Borkh.) Apple Wine" Foods 10, no. 10: 2348. https://doi.org/10.3390/foods10102348
APA StyleRuppert, V., Innerhofer, G., Voit, J., Hiden, P., & Siegmund, B. (2021). The Impact of the Fermentation Strategy on the Flavour Formation of Ilzer Rose (Malus domestica Borkh.) Apple Wine. Foods, 10(10), 2348. https://doi.org/10.3390/foods10102348





