Coculture Strategy for Developing Lactobacillus paracasei PS23 Fermented Milk with Anti-Colitis Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of L. paracasei PS23
2.2. Preparation of FM with PS23
2.3. Determination of Physicochemical Properties of FM
2.4. Caco-2 Epithelial Monolayer
2.5. Effect of PS23 and PS23 FM on the Integrity of Caco-2 Epithelial Monolayer
2.6. DSS-Induced Colitis Animal Model
2.7. Assessment of Intestinal Bleeding and Determination of Fecal Calprotectin
2.8. Histological Evaluation
2.9. Colon Organ Culture and Cytokine Production
2.10. Determination of Occludin and Myeloperoxidase
2.11. Determination of Specific Cecal Bacteria
2.12. Statistical Analysis
3. Results
3.1. Coculture with PS23 Improved the Microbial and Physicochemical Properties of FM Products
3.2. PS23 FM Enhanced Intestinal Epithelial Barrier Function In Vitro
3.3. Low-Dose PS23 FM Ameliorated DSS-Induced Colitis In Vivo
3.4. Low-Dose PS23 FM Regulated Intestinal Tight Junction Protein, Cytokine Secretion in Mesenteric Lymph Modes, Cecal Luminal Bacteria, and Cecal Short-Chain Fatty Acid
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, H.Y.; Ning, M.X.; Chen, D.K.; Ma, W.T. Interactions Between the Gut Microbiota and the Host Innate Immune Response against Pathogens. Front. Immunol. 2019, 10, 607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, O.; Kiyoshi, T. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017, 49, 1–8. [Google Scholar]
- Lycke, N.Y.; Bemark, M. The regulation of gut mucosal IgA B-cell responses: Recent developments. Mucosal Immunol. 2017, 10, 1361–1374. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, F.; Polk, D.B. Probiotics and probiotic-derived functional factors—Mechanistic insights into applications for intestinal homeostasis. Front. Immunol. 2020, 11, 1428. [Google Scholar] [CrossRef] [PubMed]
- Gareau, M.G.; Sherman, P.M.; Walker, W.A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef] [Green Version]
- Fusco, A.; Savio, V.; Donniacuo, M.; Perfetto, B.; Donnarumma, G. Antimicrobial peptides human beta-defensin-2 and -3 protect the gut during candida albicans infections enhancing the intestinal barrier integrity: In vitro study. Front. Cell. Infect. Microbiol. 2021, 11, 666900. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Osaka, T.; Moriyama, E.; Date, Y.; Kikuchi, J.; Tsuneda, S. Strengthening of the intestinal epithelial tight junction by Bifidobact. Bifidum. Physiol. Rep. 2015, 3, e12327. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.W.; Hong, R.; Choi, E.Y.; Yu, K.; Kim, N.; Hyeon, J.Y.; Cho, K.K.; Choi, I.S.; Yun, C.H. A probiotic mixture regulates T cell balance and reduces atopic dermatitis symptoms in mice. Front. Microbiol. 2018, 9, 2414. [Google Scholar] [CrossRef]
- Chen, Y.P.; Hsu, C.A.; Hung, W.T.; Chen, M.J. Effects of Lactobacillus paracasei 01 fermented milk beverage on protection of intestinal cell in vitro. Sci. Food Agric. 2016, 96, 2154–2160. [Google Scholar] [CrossRef]
- Huang, S.Y.; Chen, L.H.; Wang, M.F.; Hsu, C.C.; Chan, C.H.; Li, J.X.; Huang, H.Y. Lactobacillus paracasei PS23 delays progression of age-related cognitive decline in senescence accelerated mouse prone 8 (SAMP8) mice. Nutrients 2018, 10, 894. [Google Scholar] [CrossRef] [Green Version]
- Liao, J.F.; Hsu, C.C.; Chou, G.T.; Hsu, J.S.; Liong, M.T.; Tsai, Y.C. Lactobacillus paracasei PS23 reduced early-life stress abnormalities in maternal separation mouse model. Benef. Microbes. 2019, 10, 425–436. [Google Scholar] [CrossRef]
- Obrenovich, M.E.M. Leaky gut, leaky brain? Microorganisms 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashraf, R.; Shah, N.P. Selective and differential enumerations of Lactobacillus delbrueckii subsp bulgaricus Streptococcus thermophilus, Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium spp. in yoghurt. Int. J. Food Microbiol. 2011, 149, 194–208. [Google Scholar] [PubMed]
- Rimada, P.S.; Abraham, A. Comparative study of different methodologies to determine the exopolysaccharide produced by kefir grains in milk and whey. Le Lait 2003, 83, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.Y.; Ho, Y.F.; Chen, Y.P.; Chen, M.J. Effects of a novel encapsulating technique on the temperature tolerance and anti-colitis activity of the probiotic bacterium Lactobacillus kefiranofaciens M1. Food Microbiol. 2015, 46, 494–500. [Google Scholar] [CrossRef]
- Chen, Y.P.; Hsiao, P.J.; Hong, W.S.; Dai, T.Y.; Chen, M.J. Lactobacillus kefiranofaciens M1 isolated from milk kefir grains ameliorates experimental colitis in vitro and in vivo. J. Dairy Sci. 2012, 95, 63–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klingberg, T.D.; Pedersen, M.H.; Cencic, A.; Budde, B.B. Application of measurements of transepithelial electrical resistance of intestinal epithelial cell monolayers to evaluate probiotic activity. Appl. Environ. Microbiol. 2005, 71, 7528–7530. [Google Scholar] [CrossRef] [Green Version]
- Araki, Y.; Sugihara, H.; Hattori, T. In vitro effects of dextran sulfate sodium on a Caco-2 cell line and plausible mechanisms for dextran sulfate sodium-induced colitis. Oncol. Rep. 2006, 16, 1357–1362. [Google Scholar] [CrossRef]
- Wirtz, S.; Neufert, C.; Weigmann, B.; Neurath, M.F. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007, 2, 541–546. [Google Scholar] [CrossRef]
- Ton, H.; Brandsnes, O.; Dale, S.; Holtlund, J.; Skuibina, E.; Schjonsby, H.; Johne, B. Improved assay for fecal calprotectin. Clin. Chim. Acta 2000, 292, 41–54. [Google Scholar] [CrossRef]
- Lubbs, D.C.; Vester, B.M.; Fastinger, N.D.; Swanson, K.S. Dietary protein concentration affects intestinal microbiota of adult cats: A study using DGGE and qPCR to evaluate differences in microbial populations in the feline gastrointestinal tract. J. Anim. Physiol. Anim. Nutr. 2007, 93, 113–121. [Google Scholar] [CrossRef]
- Krych, L.; Kot, W.; Bendtsen, K.M.; Hansen, A.K.; Vogensen, F.K.; Nielsen, D.S. Have you tried spermine? A rapid and cost-effective method to eliminate dextran sodium sulfate inhibition of PCR and RT-PCR. J. Microbiol. Methods 2018, 144, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sodini, I.; Lucas, A.; Oliveira, M.N.; Remeuf, F.; Corrieu, G. Effect of milk base and starter culture on acidification, texture, and probiotic cell counts in fermented milk processing. J. Dairy Sci. 2002, 85, 2479–2488. [Google Scholar] [CrossRef]
- Bergamini, C.V.; Palma, S.B.; Sabbag, N.G.; Zalazar, C.A. Proteolytic activity of three probiotic strains in semi-hard cheese as single and mixed cultures: Lactobacillus acidophilus, Lactobacillus paracasei and Bifidobacterium lactis. Int. Dairy J. 2009, 19, 467–475. [Google Scholar] [CrossRef]
- Saxelin, M.; Grenov, B.; Svensson, U.; Fonden, R.; Reniero, R.; Mattila-Sandholm, T. The technology of probiotics. Trends Food Sci. Technol. 1999, 10, 387–392. [Google Scholar] [CrossRef]
- Kristo, E.; Biliaderis, C.G.; Tzanetakis, N. Modelling of rheological, microbiological and acidification properties of a fermented milk product containing a probiotic strain of Lactobacillus paracasei. Int. Dairy J. 2003, 13, 517–528. [Google Scholar] [CrossRef]
- Saarela, M.; Mogensen, G.; Fonden, R.; Matto, J.; Mattila-Sandholm, T. Probiotic bacteria: Safety, functional and technological properties. J. Biotechnol. 2000, 84, 197–215. [Google Scholar] [CrossRef]
- Bengoa, A.A.; Llamas, M.G.; Iraporda, C.; Duenas, M.T.; Abraham, A.G.; Garrote, G.L. Impact of growth temperature on exopolysaccharide production and probiotic properties of Lactobacillus paracasei strains isolated from kefir grains. Food Microbiol. 2018, 69, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I. Bacterial exopolysaccharides. Adv. Microb. Physiol. 1972, 8, 143–213. [Google Scholar] [PubMed]
- Kumar, A.S.; Mody, K.; Jha, B. Bacterial exopolysaccharidesea perception. J. Basic Microbiol. 2007, 47, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.T.; Cheng, P.C.; Liao, J.; Pan, T.M. Effects of administration of Lactobacillus paracasei subsp. paracasei NTU 101 on Peyer’s patch-mediated mucosal immunity. Int. Immunopharmacol. 2010, 10, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Bosco, N.; Perruisseau, G.; Nicolas, J.; Segura-Roggero, I.; Duboux, S.; Briand, M.; Blum, S.; Benyacoub, J. Lactobacillus paracasei reduces intestinal inflammation in adoptive transfer mouse model of experimental colitis. Clin. Dev. Immunol. 2011, 11, 807483. [Google Scholar]
- Bermudez-Brito, M.; Munoz-Quezada, S.; Gomez-Llorente, C.; Matencio, E.; Bernal, M.J.; Romero, F.; Gil, A. Human intestinal dendritic cells decrease cytokine release against Salmonella infection in the presence of Lactobacillus paracasei upon TLR activation. PLoS ONE 2012, 7, e43197. [Google Scholar] [CrossRef] [Green Version]
- Thoreux, K.; Senegas-Balas, F.; Bernard-Perrone, F.; Giannarelli, S.; Denariaz, G.; Bouley, C.; Balas, D. Modulation of proliferation, second messenger levels, and morphotype expression of the rat intestinal epithelial cell line IEC-6 by fermented milk. J. Dairy Sci. 1996, 79, 33–43. [Google Scholar] [CrossRef]
- Hegazy, S.K.; El-Bedewy, M.M. Effect of probiotics on pro-inflammatory cytokines and NF-κB activation in ulcerative colitis. World J. Gastroenterol. 2010, 16, 4145–4151. [Google Scholar] [CrossRef]
- Ho, G.T.; Lee, H.M.; Brydon, G.; Ting, T.; Hare, N.; Drummond, H.; Shand, A.G.; Bartolo, D.C.; Wilson, R.G.; Dunlop, M.G.; et al. Fecal calprotectin predicts the clinical course of acute severe ulcerative colitis. Am. J. Gastroenterol. 2009, 104, 673–678. [Google Scholar]
- Roseth, A.G.; Schmidt, P.N.; Fagerhol, M.K. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with in ammatory bowel disease. Scand. J. Gastroenterol. 1999, 34, 50–54. [Google Scholar]
- Vasseur, P.; Devaure, I.; Sellier, J.; Delwail, A.; Chagneau-Derrode, C.; Charier, F.; Tougeron, D.; Tasu, J.; Rabeony, H.; Lecron, J.; et al. High plasma levels of the proinflammatory cytokine IL-22 and the anti-inflammatory cytokines IL-10 and IL-1ra in acute pancreatitis. Pancreatology 2014, 14, 465–469. [Google Scholar] [CrossRef]
- Mizoguchi, A.; Yano, A.; Himuro, H.; Ezaki, Y.; Sadanaga, T.; Mizoguchi, E. Clinical importance of IL-22 cascade in IBD. J. Gastroenterol. 2017, 53, 465–474. [Google Scholar] [CrossRef] [Green Version]
- Agus, A.; Denizot, J.; Thévenot, J.; Martinez-Medina, M.; Massier, S.; Sauvanet, P. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive, E. coli infection and intestinal inflammation. Sci. Rep. 2016, 6, 19032. [Google Scholar] [CrossRef] [Green Version]
- Mennigen, R.; Nolte, K.; Rijcken, E.; Utech, M.; Loeffler, B.; Senninger, N.; Bruewer, M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1140–G1149. [Google Scholar] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microb. 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Offermanns, S. Free fatty acid (FFA) and hydroxy carboxylic acid (HCA) receptors. Annu. Rev. Pharm. Toxicol. 2014, 54, 407–434. [Google Scholar] [CrossRef]
- Voth, D.E.; Ballard, J.D. Clostridium difficile toxins: Mechanism of action and role in disease. Clin. Microbiol. Rev. 2005, 18, 247–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhodes, J.M. The role of Escherichia coli in inflammatory bowel disease. Gut 2007, 56, 610–612. [Google Scholar] [CrossRef]
- Zhang, S.; Dogan, B.; Guo, C.; Herlekar, D.; Stewart, K.; Scherl, E.J.; Simpson, K.W. Short chain fatty acids modulate the growth and virulence of pathosymbiont Escherichia coli and host response. Antibiotics 2020, 9, 462. [Google Scholar] [CrossRef]
- Fachi, J.L.; Felipe, J.S.; Pral, L.P.; Silva, B.K.; Corrêa, R.O.; Andrade, M.C.P.; Fonseca, D.M.; Basso, P.J.; Câmara, N.O.S.; Souza, E.L.S.; et al. Butyrate protects mice from Clostridium difficile-Induced colitis through an HIF-1-dependent mechanism. Cell Rep. 2019, 27, 750–761. [Google Scholar] [CrossRef] [Green Version]
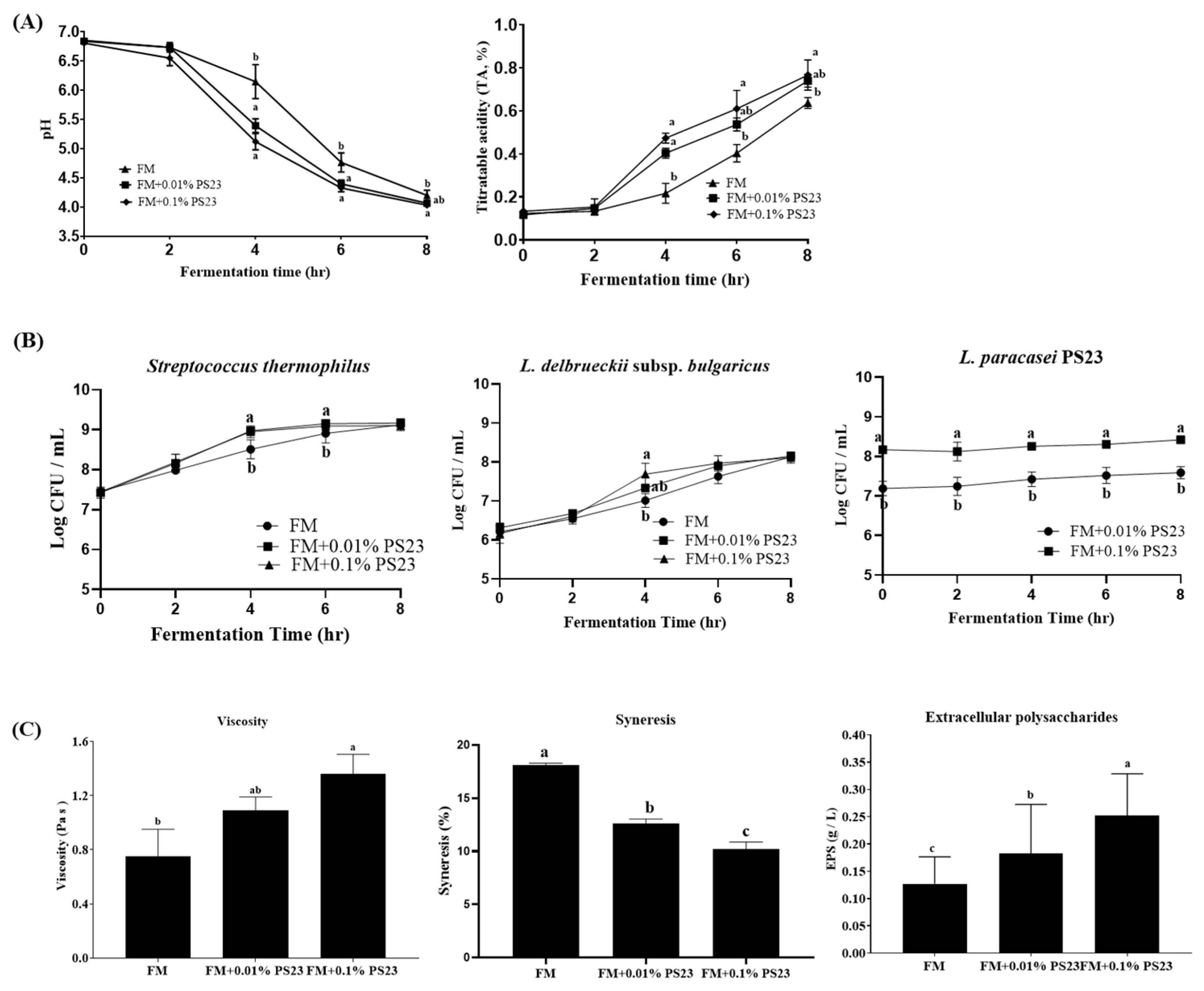
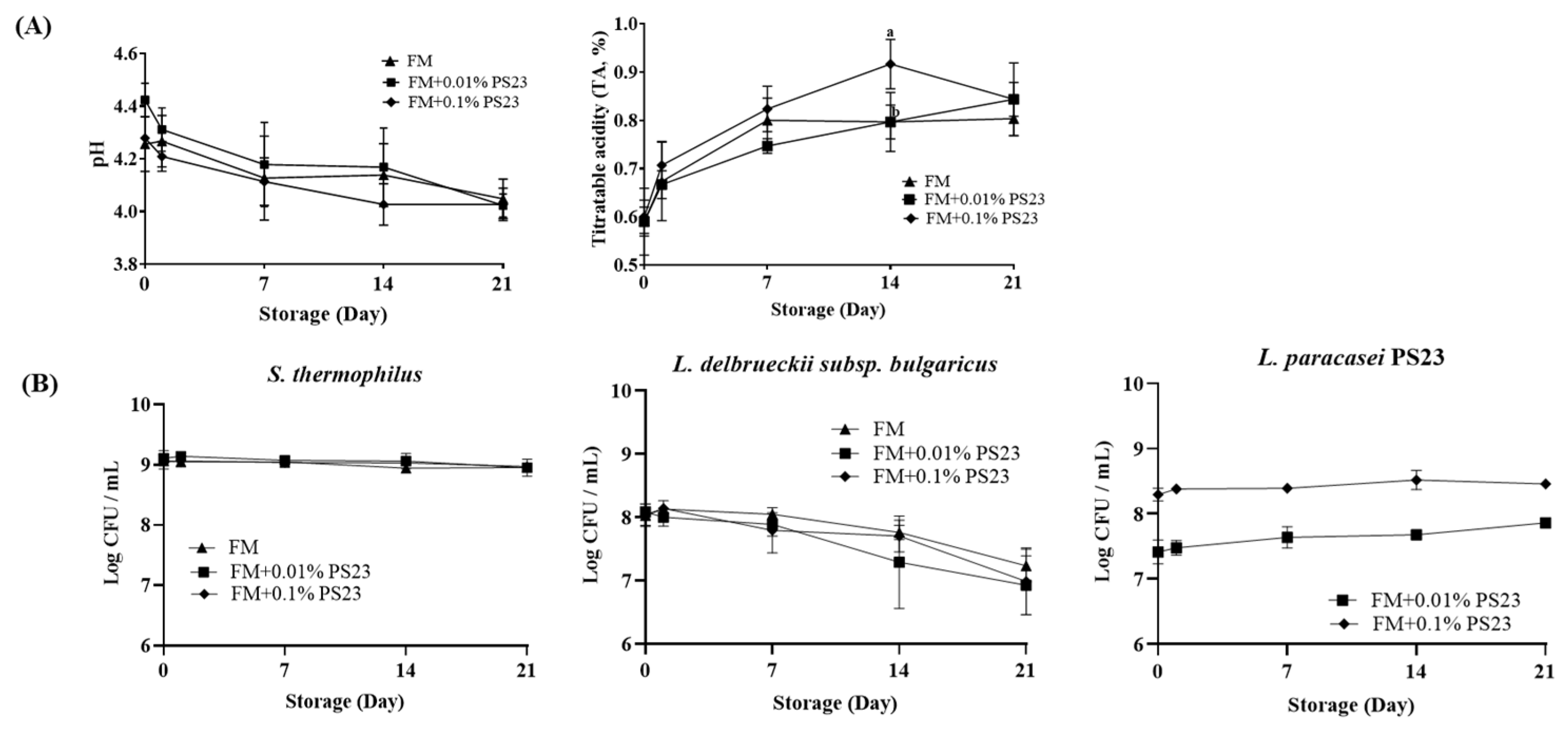

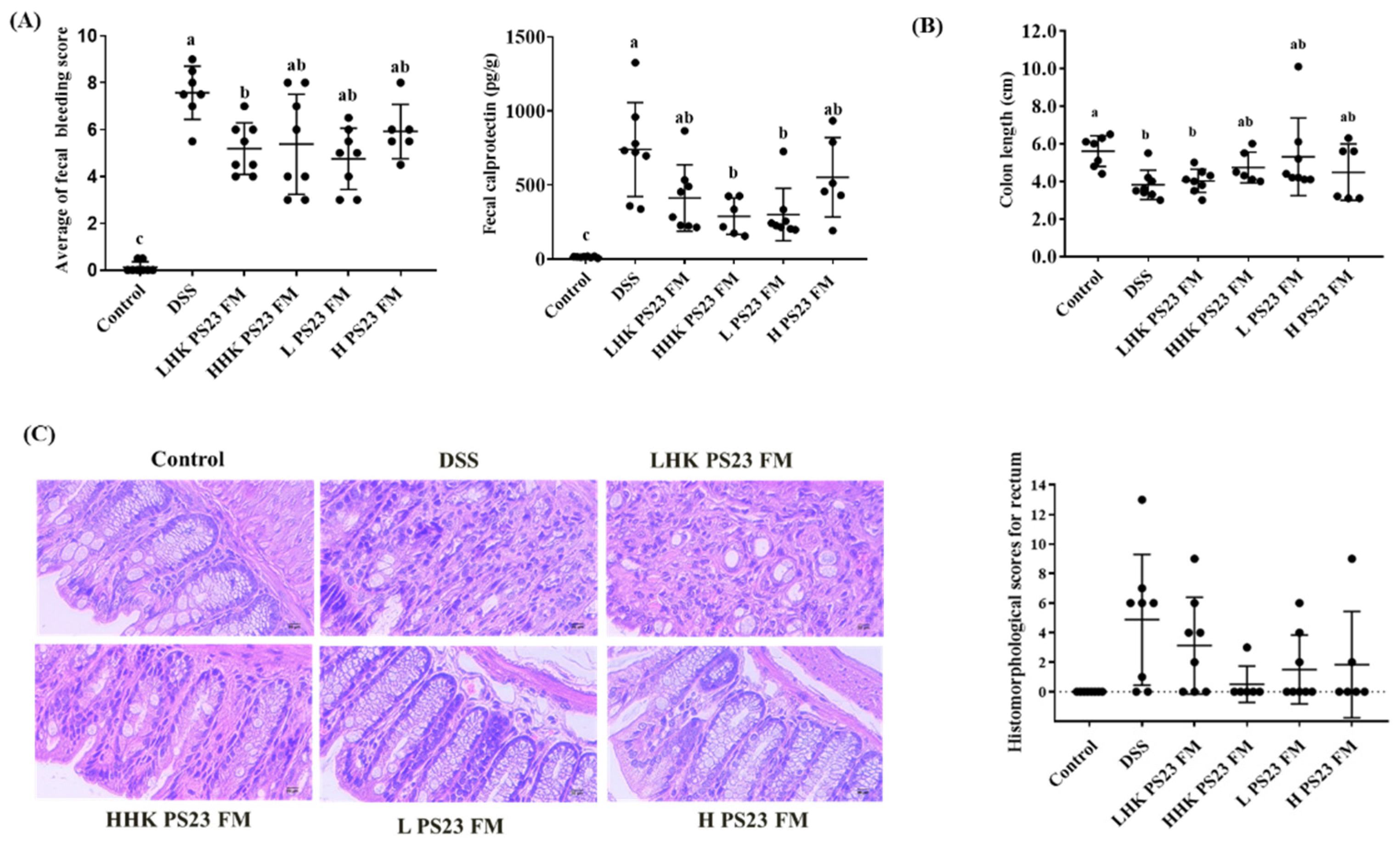
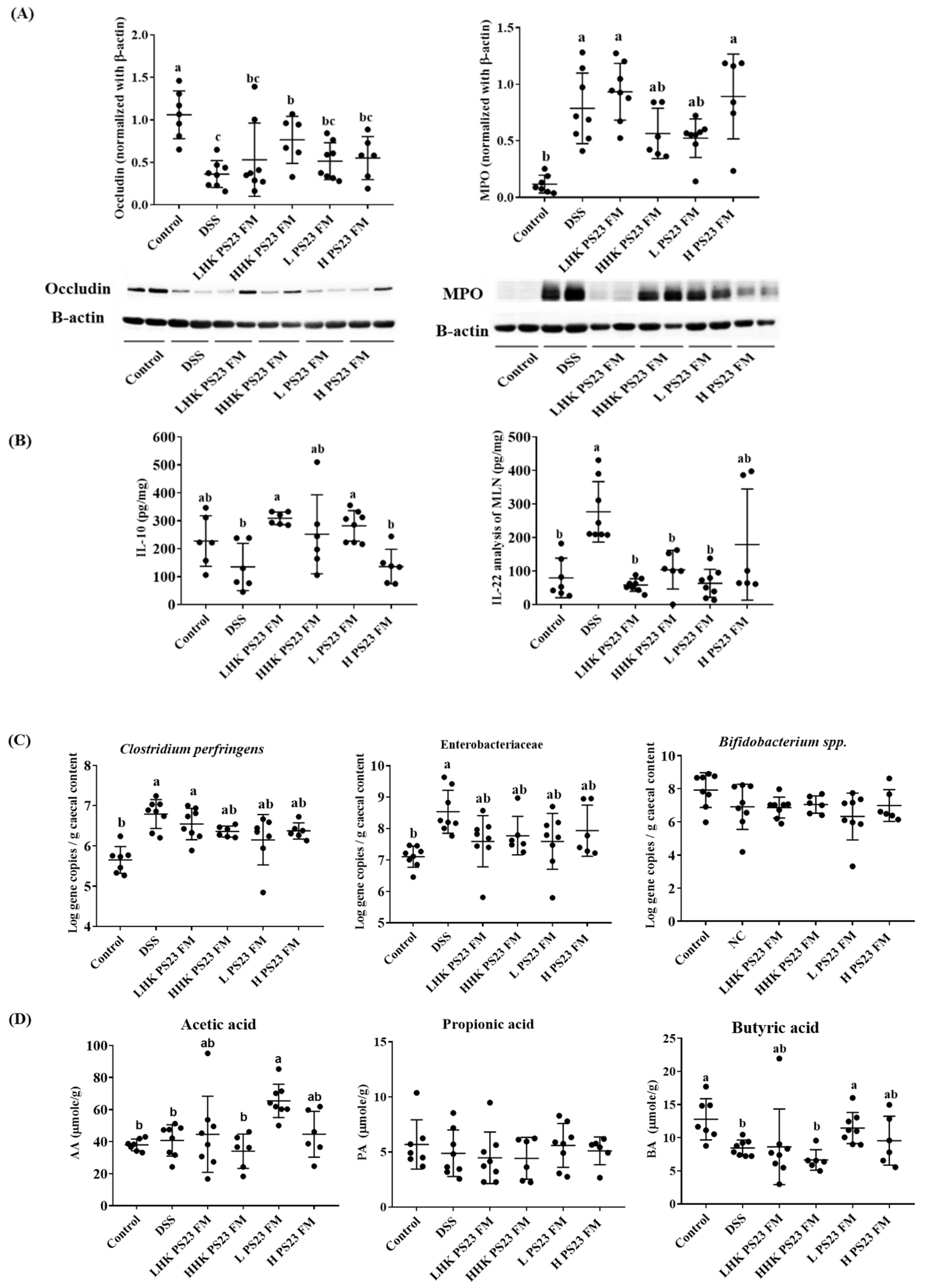
| Textural Properties of Fermented Milk | ||||
|---|---|---|---|---|
| Groups | Firmness (g) | Consistency (g.s) | Cohesiveness (g) | Viscosity Index (g.s) |
| FM | 191.52 ± 8.84 b | 4060.10 ± 155.42 b | 135.29 ± 8.42 c | 236.46 ± 12.96 c |
| FM+0.01% PS23 | 202.51 ± 8.21 ab | 4296.43 ± 175.73 a | 179.32 ± 6.15 b | 283.04 ± 12.73 b |
| FM+0.1% PS23 | 209.02 ± 5.26 a | 4390.41 ± 125.64 a | 219.97 ± 8.03 a | 349.65 ± 12.52 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-Y.; Tsai, Y.-C.; Wang, S.-Y.; Chen, Y.-P.; Chen, M.-J. Coculture Strategy for Developing Lactobacillus paracasei PS23 Fermented Milk with Anti-Colitis Effect. Foods 2021, 10, 2337. https://doi.org/10.3390/foods10102337
Lee K-Y, Tsai Y-C, Wang S-Y, Chen Y-P, Chen M-J. Coculture Strategy for Developing Lactobacillus paracasei PS23 Fermented Milk with Anti-Colitis Effect. Foods. 2021; 10(10):2337. https://doi.org/10.3390/foods10102337
Chicago/Turabian StyleLee, Kai-Yi, Ying-Chieh Tsai, Sheng-Yao Wang, Yen-Po Chen, and Ming-Ju Chen. 2021. "Coculture Strategy for Developing Lactobacillus paracasei PS23 Fermented Milk with Anti-Colitis Effect" Foods 10, no. 10: 2337. https://doi.org/10.3390/foods10102337
APA StyleLee, K.-Y., Tsai, Y.-C., Wang, S.-Y., Chen, Y.-P., & Chen, M.-J. (2021). Coculture Strategy for Developing Lactobacillus paracasei PS23 Fermented Milk with Anti-Colitis Effect. Foods, 10(10), 2337. https://doi.org/10.3390/foods10102337






