Abstract
Although clinical trials of food-protein-derived peptides in the management of hypertension have been published, the results are controversial, which compelled us to conduct a meta-analysis to evaluate the pooled effect of peptide intervention. In this study, we searched for studies published between 2010 and 2021 and selected 12 eligible studies for a meta-analysis. The pooled effect of peptide intervention for systolic blood pressure (SBP) and diastolic blood pressure (DBP) was −3.28 mmHg (95% CI: −4.54, −2.03, p < 0.001) and −1.82 mmHg (95% CI: −3.46, −0.18, p = 0.03), respectively. Sub-group analyses showed that the reduction in BP in participants with higher basal BP (>140/85 mmHg) was greater (p = 0.007 for SBP and p = 0.01 for DBP), and the effect was stronger in Asian participants as compared with non-Asian participants (p = 0.01 for SBP and p = 0.04 for DBP). In addition, the effect of peptide intervention was more pronounced on SBP in participant groups with a lower ratio of male to female (≤0.5) as well as in participants with a mean age ≥50 years old. In conclusion, food-protein-derived antihypertensive peptides can significantly reduce BP in prehypertensive and hypertensive patients. Findings from this study could provide guidance for the design of clinical trials of antihypertensive peptides.
1. Introduction
Hypertension ranks as the top cause of cardiovascular disease. Although the global mean blood pressure has remained constant in recent decades due to antihypertensive medications, the prevalence of hypertension has continued to increase []. In addition, an upward trend of hypertension in young populations has also been observed over the past two decades []. Thus, hypertension is considered a global health challenge. As cardiovascular diseases, caused by hypertension, are the leading contributor to mortality worldwide [], the cost of healthcare associated with hypertension and other complications has become a social economic burden. In addition, the prolonged use of synthetic antihypertensive drugs always has side-effects. Therefore, it is necessary to develop novel strategies with fewer adverse effects and lower costs to manage hypertension. There is a consensus that controlling high blood pressure (BP) using dietary and natural products is highly encouraged. Phytochemicals including soy isoflavones and resveratrol are typical examples within the recommended class of natural products [,]. However, the availability of other natural products, in addition to phytochemicals, that contain strong clinical evidence in reducing BP is an open question.
Bioactive peptides are oligopeptides liberated from food proteins, which can exert physiological activities in addition to their nutritional values. Activities of the peptides are present once they are produced from their parent proteins by hydrolysis or fermentation. As bioactive peptides are naturally derived, they are considered promising alternatives for the management of chronic diseases, including hypertension []. Various peptides with the potential for antihypertensive activity have been characterized from animal- or plant-based food protein sources []. Previously, an abundance of work on antihypertensive peptides has concentrated on protein hydrolysate preparation, peptide identification, animal work-based activity evaluation, and mechanistic studies [].
Although clinical evidence of food-protein-derived peptides in reducing BP has been reported, most of the previous clinical studies of antihypertensive peptides evaluated the effect of milk-protein-derived peptides, and related meta-analyses have already been published [,]. Notably, clinical trials of antihypertensive peptides derived from food proteins other than milk proteins have been published in the last decade []. However, most of these clinical studies are randomized clinical trials (RCTs), recruiting specified participants. The outcomes of some studies were controversial []. A comprehensive overview of the antihypertensive activity of bioactive peptides in humans was found to be lacking, which has been a major factor impeding the commercialization of antihypertensive peptides. Since antihypertensive peptides have been identified from a number of food proteins, it is necessary to carry out a comprehensive review on the recent research progress of clinical trials of antihypertensive peptides and conduct a meta-analysis.
It is necessary to further investigate the effect of antihypertensive peptides and identify the key factors that may affect their effect size. Thus, we carried out a quantitative synthesis of evidence since 2010 and conducted a meta-analysis to assess the effect of antihypertensive peptides in humans.
2. Materials and Methods
This meta-analysis followed the recommendations of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [].
2.1. Search Strategy and the Inclusion and Exclusion Criteria
Database including PubMed and Web of Science were used to search for clinical trials investigating the antihypertensive effects of food-protein-derived bioactive peptides published between 2010 and up to 15 July 2021. The search was performed using the following strings: “Bioactive peptides” OR “Hydrolysate” AND “Blood pressure” OR “Hypertension” AND “Trial”. In addition, potentially eligible studies from review articles were manually searched.
The selection of studies to be included in this meta-analysis was based on the following eligibility criteria: RCT or a cross-over study that assessed the effects of bioactive peptides on blood pressure in adults (aged 18 or above); the primary outcome measurement was data from either office blood pressure measurement or ambulatory blood pressure monitoring; the subjects should be defined as “prehypertension” or “hypertension” according to the most recent guidelines [] and the intervention period should be at least 1 week. The retrieved studies were screened based on the title and abstract. Trials excluded in the screening step included studies that used animal models; studies that evaluated the effect of an intact food protein or single amino acid instead of a peptide; studies that investigated acute effects instead of chronic effects of the peptide; and studies that were not relevant to the scope of this meta-analysis. After the first step of screening, the full texts of the remaining studies were reviewed to determine if they were eligible to be included in this meta-analysis following the criteria mentioned above. All of the studies were reviewed by two investigators independently. When there was disagreement, a third reviewer joined the discussion until an agreement was reached.
2.2. Data Extraction
The title and abstract of each piece of literature was screened to determine whether the trial was eligible to be included. The following information of the included studies was extracted: authors; publication year; country of the study; study design; protein source of the peptide; intervention dosage; intervention duration; mean age of the subjects; the ratio of male to female of the participants; basal systolic/diastolic BP (SBP/DBP); change of systolic/diastolic pressure and the approach to BP measurement. The treatment effect was defined as the mean difference in BP change between the active and control groups. For trials with the cross-over design, the first-period data were collected to avoid disturbance from the wash-out period. The information from the highest dose group was used when there was more than one dose involved.
2.3. Statistical Analyses
The random effect model was applied to measure the difference of the effect size. The effect size of the treatment was calculated by subtracting the basal SBP or DBP from the corresponding SBP or DBP at the end point. The sub-group analyses were also conducted by using the random effect model, which included analyses of the effects of basal BP on the participants, the age of the participants, the ratio of male to female in each trial, the trial size, the duration of the treatment, the delivery vehicle of the peptides, and the protein source of the peptides. All of the analyses were run with a 5% level of significance. All of the data analyses were run via Review Manager 5.4 and Stat 11.0 software.
The heterogeneity was evaluated via the I2 statistics. An I2 > 75% was considered as a high level of heterogeneity. Furthermore, the publication bias was evaluated by a funnel plot and Egger’s test. The quality assessment of each individual study was examined based on the Cochrane risk of bias tool []. Each study was evaluated according to each item and scored as a high, unclear, or low risk of bias.
3. Results
3.1. Study Characteristics
In total, 2307 potentially relevant publications were identified. After removing the duplications (n = 736) and reviewing the titles and abstracts, we excluded 2286 studies. The full texts of the remaining 21 publications were reviewed, and 12 studies were included in this meta-analysis according to the inclusion and exclusion criteria (Figure 1).
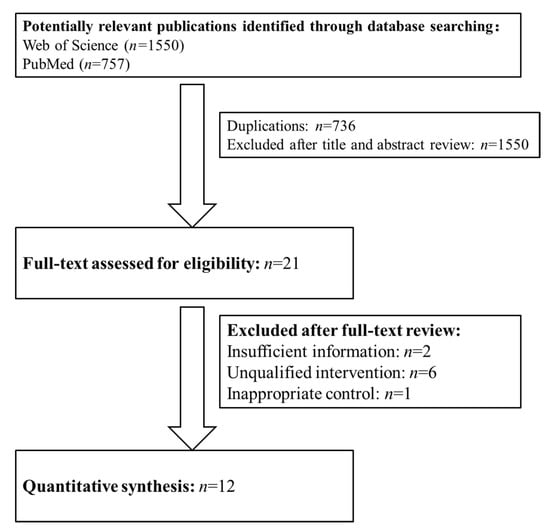
Figure 1.
The flow diagram of trial selection.
With regard to the included studies, all 12 of these studies were published in English in peer-reviewed journals as original research articles (Table 1). In total, the 12 reported trials included 761 participants. All of the included studies were randomized and double blind. For the intervention of each study, milk-protein-derived peptides were used in eight studies [,,,,,,,], egg protein-derived bioactive peptides were used in two trials [,], and a chicken collagen-derived peptide was used in one study []. Only one study used plant-derived peptides that were from rice bran []. Although there were two studies that investigated the blood-pressure-lowering effect of legume-derived peptides in humans, these trials were excluded due to the insufficient information of the participants [] or the fact that the treatment was also combined with other nature compounds []. From the primary literature, there was one trial that evaluated the effect of marine collagen-derived peptides, while the recruited participants were patients with type 2 diabetes as well as hypertension []. Therefore, the reduction in blood pressure after the peptide intervention might be due to the mitigation of type 2 diabetes instead of the direct effect on blood pressure. Thus, this study was also excluded. Protein hydrolysis and fermentation are major approaches for the production of bioactive peptides in large scale. For the 12 included studies, 8 of them used protein hydrolysates and 4 used peptides via fermentation.

Table 1.
Characteristics of the included trials.
3.2. The Effects of Bioactive Peptide Intervention
The results of the primary meta-analysis showed that the intervention of bioactive peptides reduced SBP and DBP by 3.28 mmHg (95% CI: −4.54, −2.03, p < 0.001) and 1.82 mmHg (95% CI: −3.46, −0.18, p = 0.03), respectively. This result suggested a significant effect of the intervention of bioactive peptides on the reductions in both SBP and DBP. Both of the pooled effects for SBP (I2 = 70%, Tau2 = 0.17, Chi2 = 37.20, df = 11, p = 0.0001) and DBP (I2 = 55%, Tau2 = 0.80, Chi2 = 24.49, df = 11, p = 0.003) were heterogeneous (Figure 2 and Figure 3).
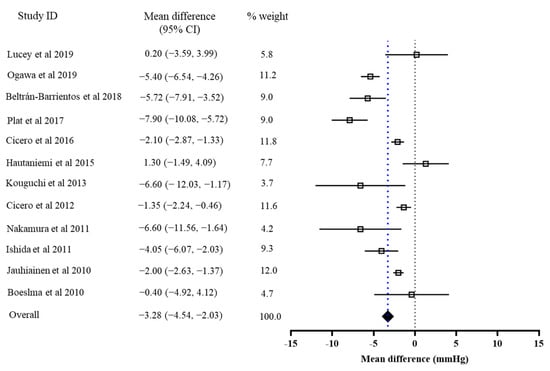
Figure 2.
Overall change in SBP (mmHg) after peptide intervention.
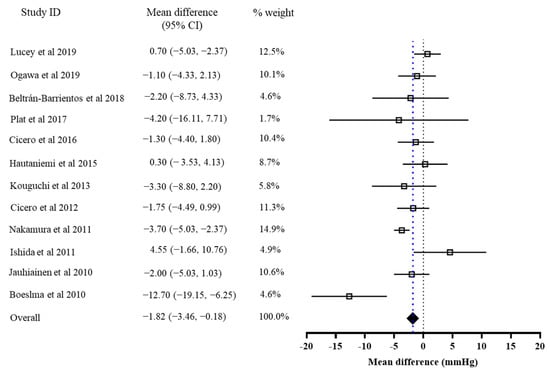
Figure 3.
Overall change in DBP (mmHg) after the peptide intervention.
We then conducted sub-group analyses. As shown by Table 2, it was found that the basal BP of the participants affected the effect sizes of both SBP (p = 0.007) and DBP (p = 001). Higher basal BP (>140/85 mmHg) was associated with a greater effect size (Table 2). The ratio of male to female of the participants affected the effect size of SBP significantly (p = 0.04), in which a lower ratio (≤0.5) indicated a more pronounced effect size. A similar trend was also present in the effect size of DBP, although the effect did not have any statistical significance (p = 0.06). In line with a previous study, we found that the Asian participants had a stronger response to the peptide intervention (SBP: p = 0.01 DBP: p = 0.04)as compared with the participants from other countries (seven studies recruited participants from European countries and one study recruited a participant from Mexico). We also found that the peptide intervention in the participants with a mean age above 50 years old had a more pronounced effect on the reduction in SBP (p = 0.001) but not the reduction in DBP (p = 0.88). In fact, the trial size could significantly affect the effect size of DBP (p = 0.04) but not SBP (p = 0.35). Most of the included trials used milk-protein-derived peptides. However, the origin of the peptide might not significantly affect the effect size of the intervention (SBP: p = 0.07, DBP: p = 0.20). In addition, neither the duration nor the delivery vehicle of peptides affected the effect size of the peptide intervention.

Table 2.
Sub-group analyses of the included trials.
3.3. Publication Bias
The publication bias was evaluated using the funnel plot and Egger’s test. There was no visual asymmetry in the funnel plots (Figure 4A,B). The p values of Egger’s tests for SBP and DBP were 0.43 and 0.79, respectively. Collectively, the above results indicated that publication bias existed in the trials involved in this analysis.
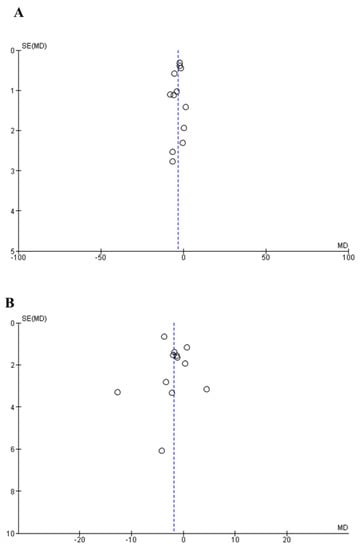
Figure 4.
Funnel plot measuring publication bias and the effects of peptide intervention on SBP (A) and DBP (B). The X axis represents mean difference (MD) and the Y axis represents standard error (SE) of the MD.
We then assessed the risk of bias of each included study based on the Cochrane guidelines. As shown in Table 3, a high risk was present in the blinding of both participants and personnel, as well as in the creation of incomplete outcome data of some studies, which indicated selection bias, performance bias, and attrition bias, respectively. In addition, the bias of random sequence generation and allocation concealment in most of the included studies were unknown, which suggested a potential selection bias in these trials.

Table 3.
Quality assessments of included studies based on the Cochrane guidelines.
4. Discussion
Bioactive peptides from food proteins have attracted enormous attention in the past few years due to their potential use in the management of chronic metabolic diseases, including hypertension. Since the first identification of an antihypertensive peptide from snake venom four decades ago, great efforts have been placed on the identification of more antihypertensive peptides as well as on cellular and animal studies to evaluate their activities and mechanisms []. The activity of antihypertensive peptides from milk, egg, and chicken proteins has also been investigated. However, the effects of these peptides on hypertensive subjects are controversial [,]. Thus, collection of evidence and meta-analyses are warranted to generate a comprehensive view on the activity of antihypertensive peptides.
To date, most clinical trials of antihypertensive peptides have concentrated on lactopeptides, and several meta-analyses that assessed the effects of these lactopeptides have been published [,,]. Notably, clinical trials of antihypertensive peptides derived from proteins other than milk proteins have appeared in recent years. Therefore, we included the qualified clinical trials published since 2010 concerning the antihypertensive peptides derived from various food protein sources and conducted a meta-analysis in the present study. Although the number of total participants involved in this meta-analysis was not as large as previous meta-analyses [,,], this meta-analysis is important to the relevant field since we included the most recent trials of peptides derived from various food protein sources instead of milk proteins only. We found that peptides derived from egg, chicken collagen, and rice proteins showed a comparable BP-lowering effect to the effect size of milk-protein-derived peptides. Therefore, it will be helpful to explore the BP-lowering activity of peptides originating from different types of protein sources in future clinical trials.
In this study, we found that antihypertensive peptides exerted a significant effect in reducing both SBP and DBP. The intervention of antihypertensive peptides was indicated to reduce the body weight of hypertensive patients by some studies [,], but such an effect is still ambiguous. As such, it will be helpful to collect more types of evidence, such as body weight and blood lipid profiles, in future clinical trials, which may be helpful in unveiling the mechanisms of peptides underlying the antihypertensive activity in humans.
The results from sub-group analyses suggested that the basal BP of the participants could be a key factor affecting the effect size. The effect of peptide intervention in participants with a higher basal BP (SBP > 140 mmHg and DBP < 85 mmHg) was more pronounced than in participants with a lower basal BP, which is consistent with a previous study that reported the effect of lactotripeptides was stronger in hypertensive subjects than in non-hypertensive subjects []. This finding also suggested that food protein-derived antihypertensive peptides may reduce BP by normalizing the dysregulated metabolic system.
Surprisingly, we found in the present meta-analysis that the ratio of male to female also affected the effect size of the peptide intervention. The peptide intervention had a stronger effect on SBP in participants with the ratio of male to female being less than 0.50. Similar trends also existed in the effect of peptides on DBP. Such findings indicated that antihypertensive peptides may favor female subjects over their male counterparts. Differential regulatory roles of bioactive compounds in males and females have attracted a considerable amount of attention in recent years [,]. Previous research of food-protein-derived bioactive peptides did not consider this important point. For antihypertensive peptides in particular, as the prevalence of hypertension in males is higher than in age-matched females before menopause, spontaneously hypertensive male rats were used as the model animal in most of the animal studies. In clinical trials of antihypertensive peptides, the data of male and female participants were always pooled together. Since we found that males and females might have different responses to peptide intervention, future clinical trials of antihypertensive peptides are encouraged to separate different genders and explore whether the effects and underlying mechanisms of peptides are different.
In this meta-analysis, we also found that the effect size of the peptide intervention could be affected by the age of the participant, which was a factor ignored in previous meta-analyses of antihypertensive peptides. Interestingly, peptide intervention had a significantly stronger effect on SBP in subjects with the mean age > 50 years. This finding may explain a previous study reporting the non-significant effect of lactotripeptides in hypertensive patients, in which the age of the recruited participants ranged from 35 to 70 years old []. Such a broad age range might mask the effect of peptides on the younger group. However, only four trials with an average age ≤50 were collected in this meta-analysis. More evidence is required to demonstrate that antihypertensive peptides have a better effect on older patients.
Although sub-group analyses involved the intervention duration and delivery vehicle of the peptides, neither of these two factors had a significant effect on the change in BP. Future trials are suggested to contain designs with a wider dose range and different intervention periods. In addition, the molecular mechanisms of a food-protein-derived bioactive peptide underlying its blood-pressure-lowering effect have been explored, which included inhibition of the angiotensin-converting enzyme activity to reduce the concentration of vasoconstrictor angiotensin II, activation of the angiotensin converting enzyme 2 activity to mitigate vascular inflammation and oxidative stress, and activation of the endothelial nitric oxide-signaling to enhance vascular relaxation [,,]. However, as these mechanistic studies were conducted on animal models, we suggest further exploring the blood-pressure-reducing mechanisms in humans in future trials.
5. Conclusions
In conclusion, food-protein-derived antihypertensive peptides can reduce BP in hypertensive individuals significantly. The basal BP, age, and gender of the participants may alter the outcome of the intervention. The findings of this meta-analysis shed light on the factors that may impact the activity of antihypertensive peptides in humans. In addition, the findings of this study could provide guidance for the design of clinical trials of antihypertensive peptides. It must be admitted that publication bias is present in the studies involved in this meta-analysis, which compels us to conduct more clinical trials of antihypertensive peptides.
Author Contributions
W.L. and G.S. conceptualized the study, contributed to data acquisition, and wrote the manuscript. D.X., Y.W., Y.L. and J.S. contributed to data acquisition and interpretation. H.X. and S.W. provided critical review and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the grant from the National Natural Science Foundation of China (No. 82103834), the Fundamental Research Funds for the Central Universities and Faculty Start-up Funds from the Southeast University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zhang, Y.; Yu, J.; Zha, M.; Zhu, Y.; Rahimi, K.; Rudan, I. Global prevalence of hypertension in children: A systematic review and meta-analysis. JAMA Pediatr. 2019, 173, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Dahlöf, B. Cardiovascular disease risk factors: Epidemiology and risk assessment. Am. J. Cardiol. 2010, 105 (Suppl. 1), 3A–9A. [Google Scholar] [CrossRef] [PubMed]
- Borghi, C.; Tsioufis, K.; Agabiti-Rosei, E.; Burnier, M.; Cicero, A.F.G.; Clement, D.; Coca, A.; Desideri, G.; Grassi, G.; Lovic, D.; et al. Nutraceuticals and blood pressure control: A European Society of Hypertension position document. J. Hypertens. 2020, 38, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Grassi, D.; Tocci, G.; Galletti, F.; Borghi, C.; Ferri, C. Nutrients and nutraceuticals for the management of high normal blood pressure: An evidence-based consensus document. High Blood Press. Cardiovasc. Prev. 2019, 26, 9–25. [Google Scholar] [CrossRef]
- Liao, W.; Wu, J. The ACE2/Ang (1–7)/MasR axis as an emerging target for antihypertensive peptides. Crit. Rev. Food Sci. Nutr. 2021, 61, 2572–2586. [Google Scholar] [CrossRef]
- Wu, J.; Liao, W.; Udenigwe, C.C. Revisiting the mechanisms of ACE inhibitory peptides from food proteins. Trends Food Sci. Technol. 2017, 69, 214–219. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Aubin, F.; Azais-Braesco, V.; Borghi, C. Do the lactotripeptides Isoleucine–Proline–Proline and Valine–Proline–Proline reduce systolic blood pressure in European Subjects? A Meta-analysis of randomized controlled trials. Am. J. Hypertens. 2013, 26, 442–449. [Google Scholar] [CrossRef]
- Fekete, Á.A.; Givens, D.I.; Lovegrove, J.A. Casein-derived lactotripeptides reduce systolic and diastolic blood pressure in a meta-analysis of randomised clinical trials. Nutrients 2015, 7, 659–681. [Google Scholar] [CrossRef]
- Majumder, K.; Wu, J. Molecular targets of antihypertensive peptides: Understanding the mechanisms of action based on the pathophysiology of hypertension. Int. J. Mol. Sci. 2015, 16, 256–283. [Google Scholar] [CrossRef]
- van der Zander, K.; Bots, M.L.; Bak, A.A.; Koning, M.M.; de Leeuw, P.W. Enzymatically hydrolyzed lactotripeptides do not lower blood pressure in mildly hypertensive subjects. Am. J. Clin. Nutr. 2008, 88, 1697–1702. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. J Am Coll Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Jauhiainen, T.; Rönnback, M.; Vapaatalo, H.; Wuolle, K.; Kautiainen, H.; Groop, P.H.; Korpela, R. Long-term intervention with Lactobacillus helveticus fermented milk reduces augmentation index in hypertensive subjects. Eur. J. Clin. Nutr. 2010, 64, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Boelsma, E.; Kloek, J. IPP-rich milk protein hydrolysate lowers blood pressure in subjects with stage 1 hypertension, a randomized controlled trial. Nutr. J. 2010, 9, 52. [Google Scholar] [CrossRef]
- Ishida, Y.; Shibata, Y.; Fukuhara, I.; Yano, Y.; Takehara, I.; Kaneko, K. Effect of an excess intake of casein hydrolysate containing Val-Pro-Pro and Ile-Pro-Pro in subjects with normal blood pressure, high-normal blood pressure, or mild hypertension. Biosci. Biotechnol. Biochem. 2011, 75, 427–433. [Google Scholar] [CrossRef]
- Nakamura, T.; Mizutani, J.; Ohki, K.; Yamada, K.; Yamamoto, N.; Takeshi, M.; Takazawa, K. Casein hydrolysate containing Val-Pro-Pro and Ile-Pro-Pro improves central blood pressure and arterial stiffness in hypertensive subjects: A randomized, double-blind, placebo-controlled trial. Atherosclerosis 2011, 219, 298–303. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Rosticci, M.; Ferroni, A.; Bacchelli, S.; Veronesi, M.; Strocchi, E.; Borghi, C. Predictors of the short-term effect of Isoleucine–Proline–Proline/Valine–Proline–Proline Lactotripeptides from casein on office and ambulatory blood pressure in subjects with pharmacologically untreated high-normal blood pressure or first-degree hypertension. Clin. Exp. Hypertens. 2012, 34, 601–605. [Google Scholar]
- Hautaniemi, E.J.; Tikkakoski, A.J.; Tahvanainen, A.; Nordhausen, K.; Kähönen, M.; Mattsson, T.; Luhtala, S.; Turpeinen, A.M.; Niemelä, O.; Vapaatalo, H.; et al. Effect of fermented milk product containing lactotripeptides and plant sterol esters on haemodynamics in subjects with the metabolic syndrome—A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2015, 114, 376–386. [Google Scholar] [CrossRef]
- Arrigo, F.G.; Cicero, A.C.; Martina, R.; Marcella, C.; Riccardo, U.; Marina, G.; Claudio, B.; D’Addato, S. Effect of lactotripeptides (Isoleucine–Proline–Proline/Valine–Proline–Proline) on blood pressure and arterial stiffness changes in subjects with suboptimal blood pressure control and metabolic syndrome: A double-blind, randomized, crossover clinical trial. Metab. Syndr. Relat. Disord. 2016, 14, 161–166. [Google Scholar]
- Beltrán-Barrientos, L.M.; González-Córdova, A.F.; Hernández-Mendoza, A.; Torres-Inguanzo, E.H.; Astiazarán-García, H.; Esparza-Romero, J.; Vallejo-Cordoba, B. Randomized double-blind controlled clinical trial of the blood pressure–lowering effect of fermented milk with Lactococcus lactis: A pilot study. J. Dairy Sci. 2018, 101, 2819–2825. [Google Scholar] [CrossRef]
- Plat, J.; Severins, N.; Morrison, S.; Mensink, R.P. Effects of NWT-03, an egg-protein hydrolysate, on blood pressure in normotensive, high-normotensive and mild-hypertensive men and women: A dose-finding study. Br. J. Nutr. 2017, 117, 942–950. [Google Scholar] [CrossRef][Green Version]
- Lucey, A.J.; Heneghan, C.; Manning, E.; Kroon, P.A.; Kiely, M.E. Effect of an egg ovalbumin-derived protein hydrolysate on blood pressure and cardiovascular risk in adults with a mildly elevated blood pressure: A randomized placebo-controlled crossover trial. Eur. J. Nutr. 2019, 58, 2823–2833. [Google Scholar] [CrossRef] [PubMed]
- Kouguchi, T.; Ohmori, T.; Shimizu, M.; Takahata, Y.; Maeyama, Y.; Suzuki, T.; Morimatsu, F.; Tanabe, S. Effects of a chicken collagen hydrolysate on the circulation system in subjects with mild hypertension or high-normal blood pressure. Biosci. Biotechnol. Biochem. 2013, 77, 691–696. [Google Scholar] [CrossRef]
- Ogawa, Y.; Shobako, N.; Fukuhara, I.; Satoh, H.; Kobayashi, E.; Kusakari, T.; Suwa, M.; Matsumoto, M.; Ishikado, A. Rice bran supplement containing a functional substance, the novel peptide Leu-Arg-Ala, has anti-hypertensive effects: A double-blind, randomized, placebo-controlled study. Nutrients 2019, 11, 726. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Prairie, N.; Udenigwe, C.C.; Adebiyi, A.P.; Tappia, P.S.; Aukema, H.M.; Jones, P.J.H.; Aluko, R.E. Blood pressure lowering effect of a pea protein hydrolysate in hypertensive rats and humans. J. Agric. Food Chem. 2011, 59, 9854–9860. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-M.; Ho, S.C.; Chen, Y.-M.; Woo, J. Effect of soy protein and isoflavones on blood pressure and endothelial cytokines: A 6-month randomized controlled trial among postmenopausal women. J. Hypertens. 2013, 31, 384–392. [Google Scholar] [CrossRef]
- Zhu, C.F.; Li, G.Z.; Peng, H.B.; Zhang, F.; Chen, Y.; Li, Y. Therapeutic effects of marine collagen peptides on Chinese patients with type 2 diabetes mellitus and primary hypertension. Am. J. Med Sci. 2010, 340, 360–366. [Google Scholar] [CrossRef]
- Qin, L.Q.; Xu, J.Y.; Dong, J.Y.; Zhao, Y.; van Bladeren, P.; Zhang, W. Lactotripeptides intake and blood pressure management: A meta-analysis of randomised controlled clinical trials. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Chanson-Rolle, A.; Aubin, F.; Braesco, V.; Hamasaki, T.; Kitakaze, M. Influence of the lactotripeptides Isoleucine–Proline–Proline and Valine–Proline–Proline on systolic blood pressure in Japanese subjects: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2015, 10, e014223. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wheeler, C.T.; Alberico, T.; Sun, X.; Seeberger, J.; Laslo, M.; Spangler, E.; Kern, B.; de Cabo, R.; Zou, S. The effect of resveratrol on lifespan depends on both gender and dietary nutrient composition in Drosophila melanogaster. AGE 2013, 35, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Shiina, K.; Tomiyama, H.; Matsumoto, C.; Komatsu, S.; Natsume, M.; Oba, C.; Ohshiba, Y.; Yamaji, T.; Chikamori, T.; Yamashina, A. Gender difference in the effects of cacao polyphenols on blood pressure and glucose/lipid metabolism in prediabetic subjects: A double-blinded, randomized, placebo-controlled crossover trial. Hypertens. Res. 2019, 42, 1083–1085. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).