Oxidative Quality of Dairy Powders: Influencing Factors and Analysis
Abstract
:1. Introduction
2. Bovine Milk Lipids
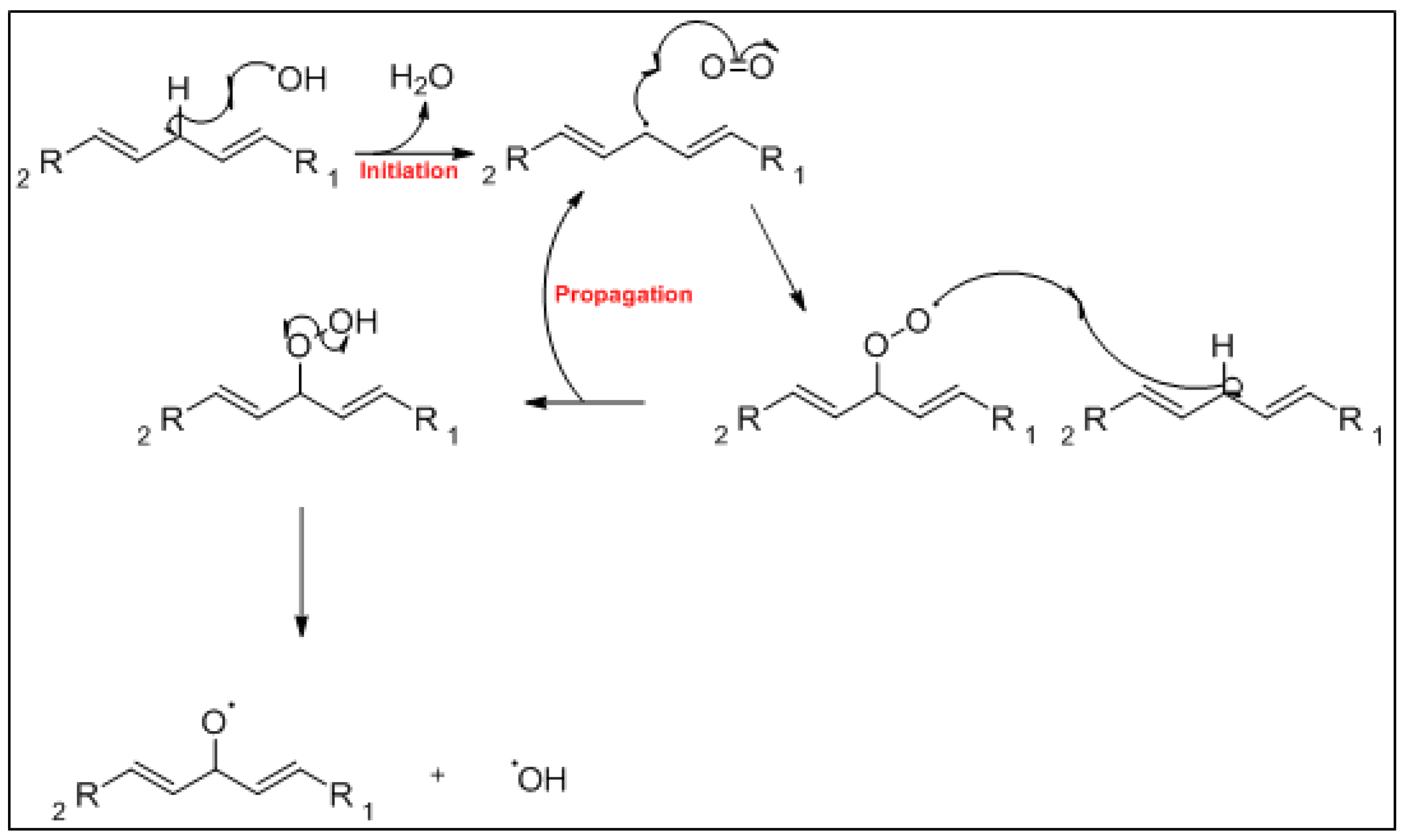
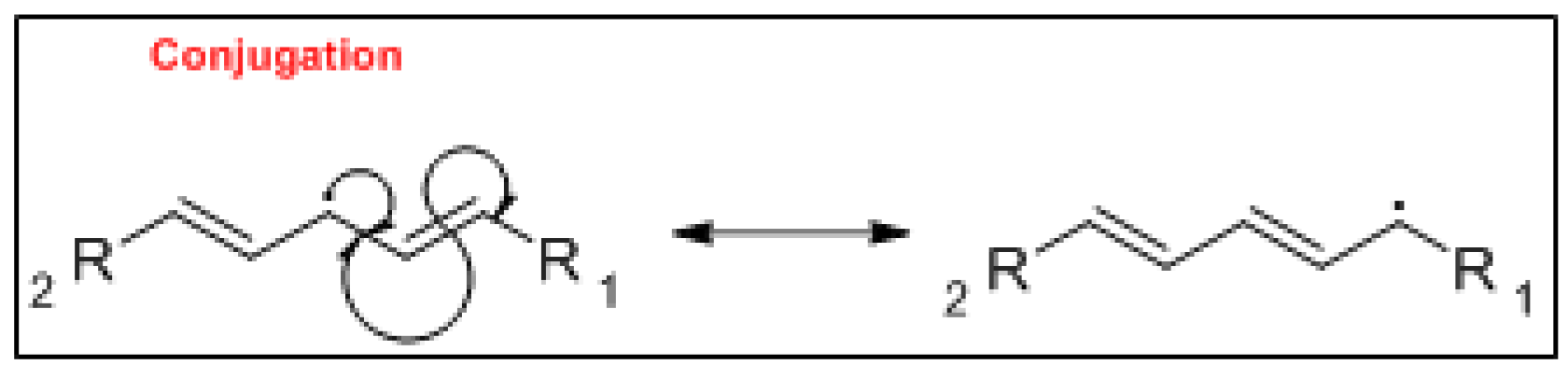
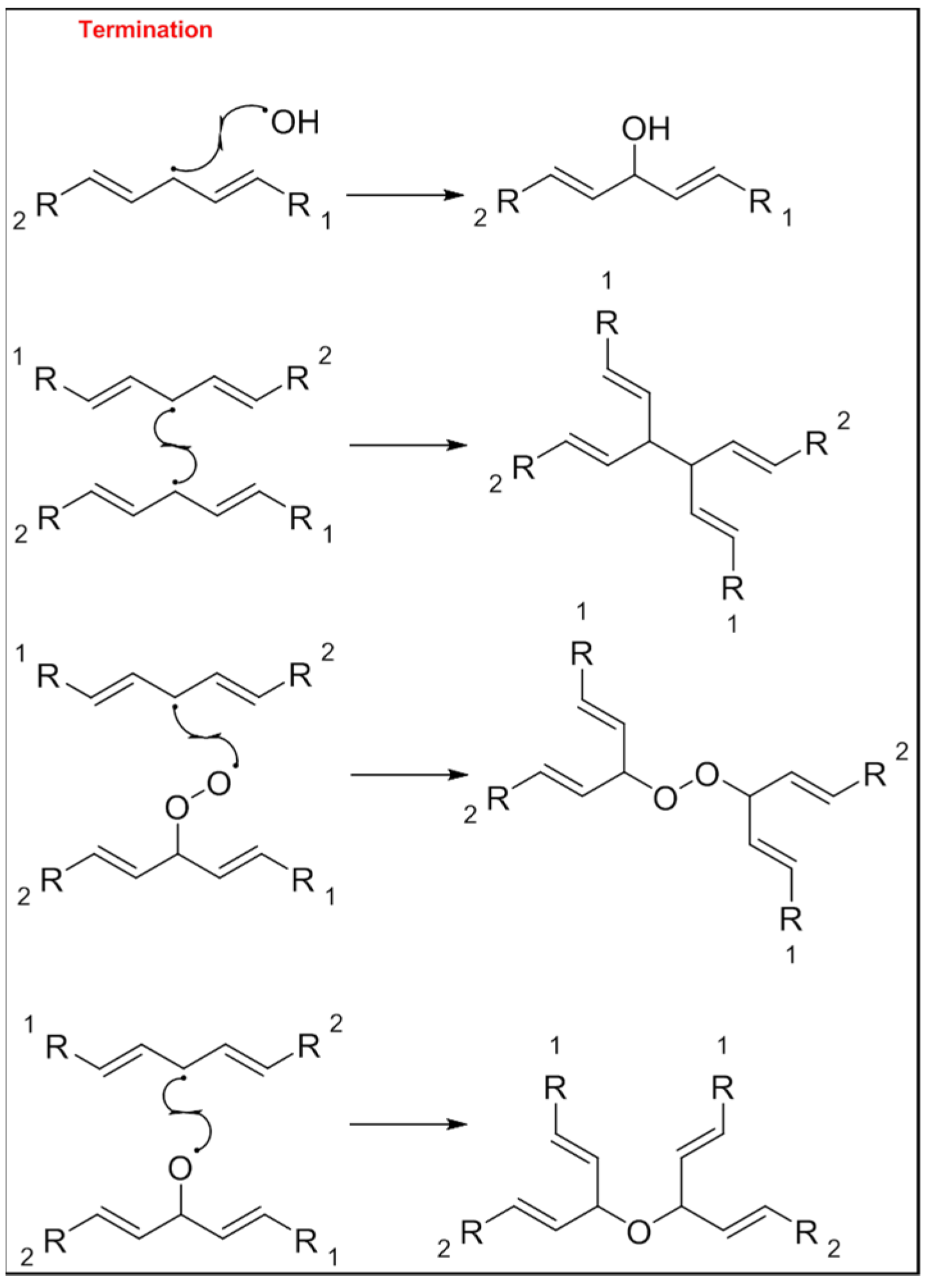
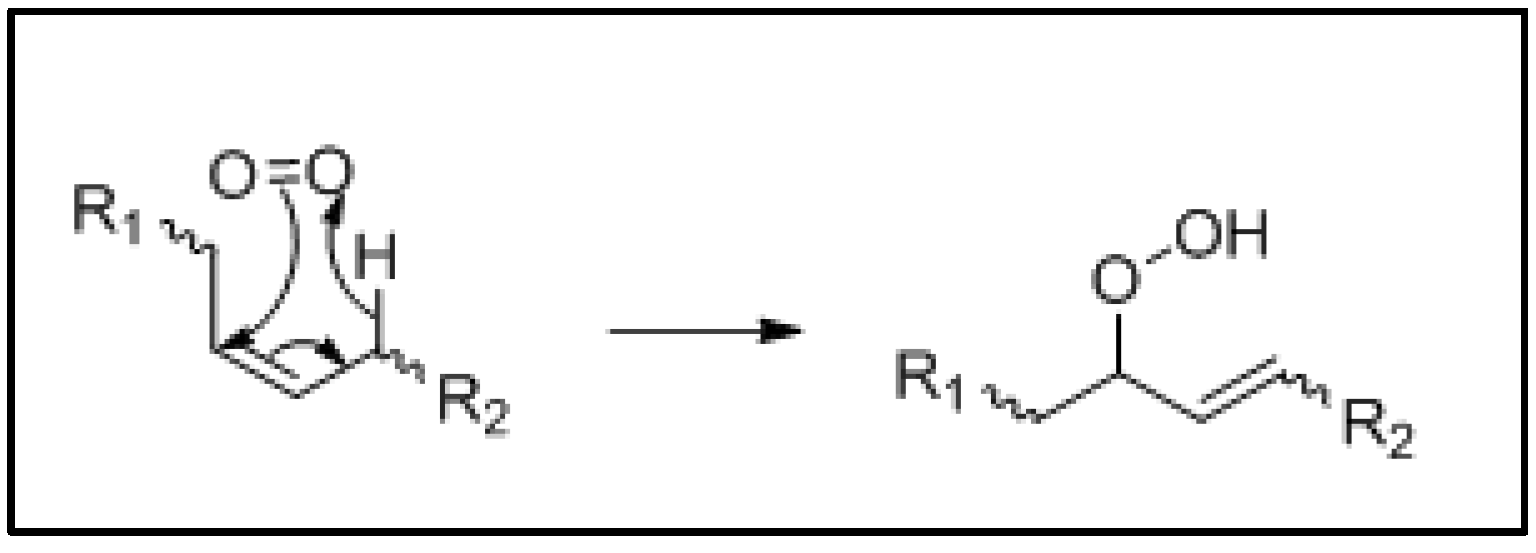
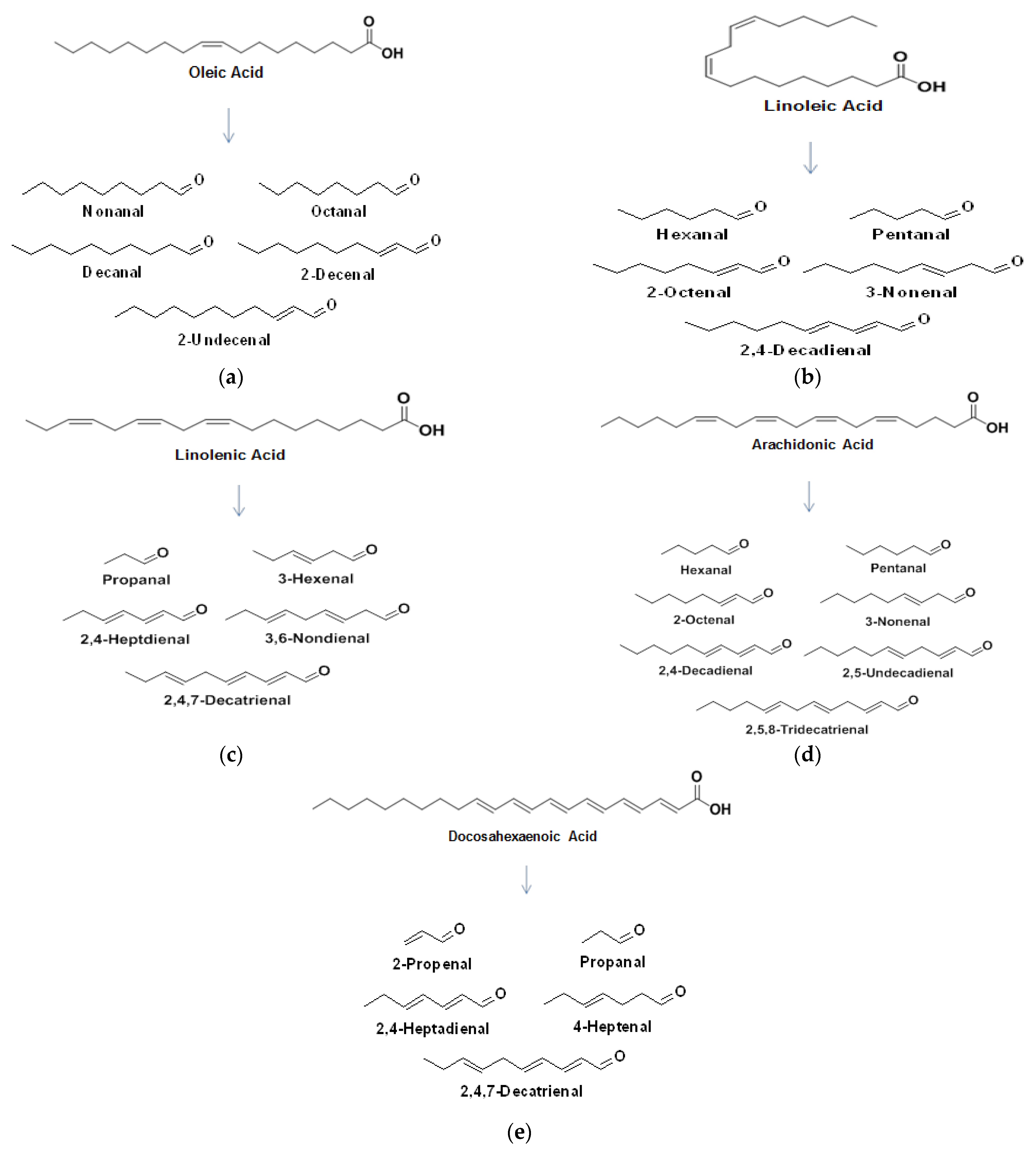
3. Mechanism of Lipid Oxidation
4. Secondary Reactions Associated with Lipid Oxidation
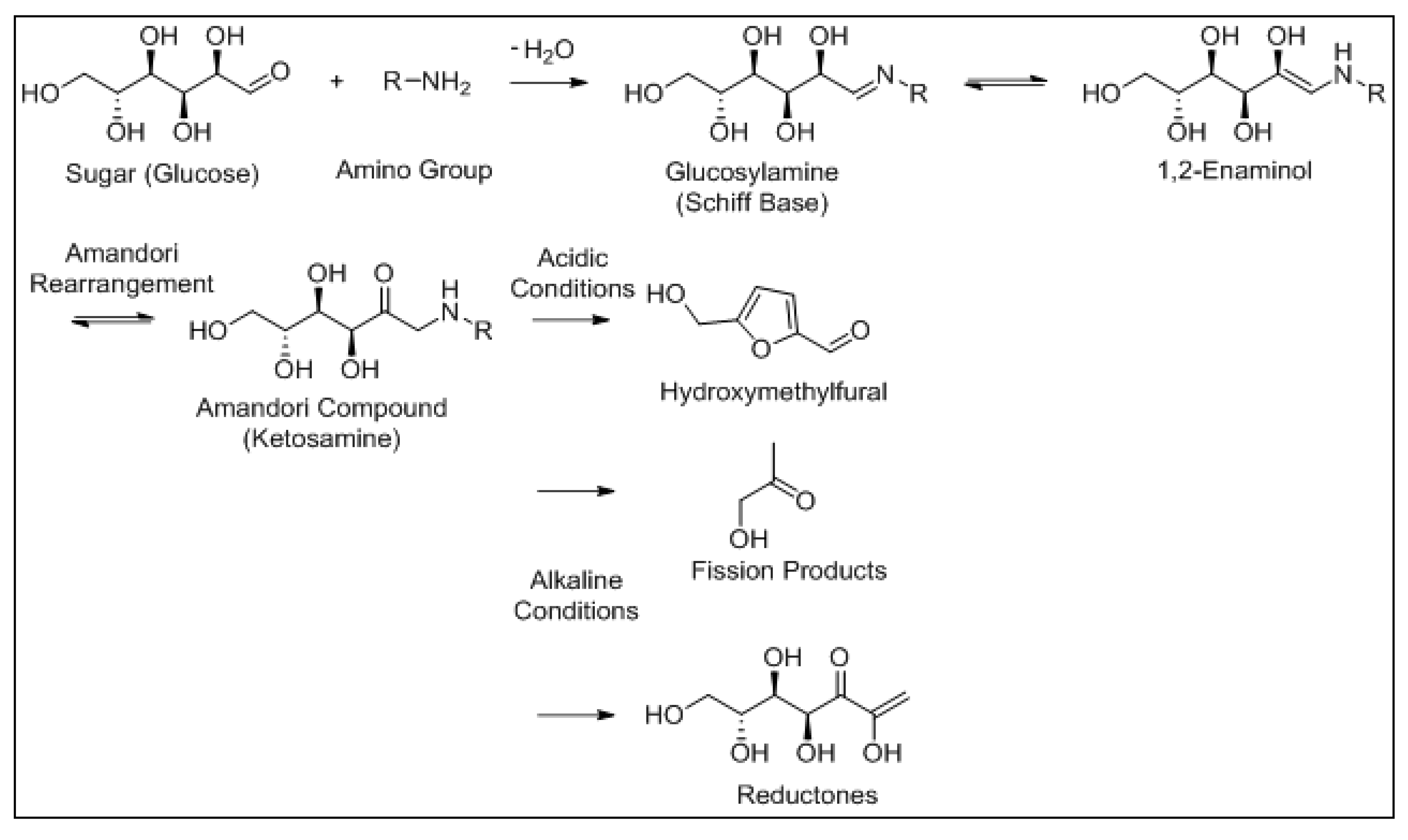
4.1. The Maillard Reaction
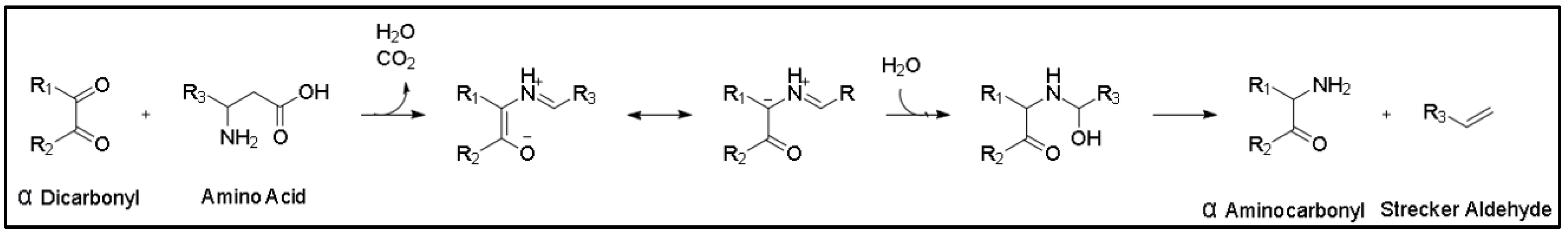
4.2. The Strecker Reaction
5. Lipid Oxidation in Dairy Powders
5.1. Whole Milk Powder
5.2. Skim Milk Powder
5.3. Infant Milk Formula
5.4. Whey Protein Concentrate and Whey Protein Isolate
6. Main Factors Influencing Lipid Oxidation in Dairy Powders
7. Impact of Processing Conditions on Dairy Powders
8. Volatile Organic Compounds Associated with Lipid Oxidation in Milk
| Compound | Associated Aroma Descriptors | LRI | Odour Reference | LRI Reference |
|---|---|---|---|---|
| Aldehyde | [71] | |||
| Pentanal | Fermented, bready, fruity | 735 | * | |
| Propanal | Alcohol, earthy | 506 | * | |
| Hexanal | Cardboard like, metallic off flavour, green | 837 | * | |
| (E)-2-Nonenal | Green, fatty | 1160 | * | [104] |
| Heptanal | Fatty, oily, green, woody | 901 | * | [71] |
| (Z)-4-Heptenal | Oily, fatty, green, milky, dairy | 901 | * | [105] |
| 2,4-Decadienal | Fatty, oily, green, chicken skin-like, fried | 1300 | * | [106] |
| Undecanal | Soapy, aldehydic, waxy, floral | 1311 | * | [107] |
| Ketone | ||||
| Acetone | Earthy, strong fruity, wood pulp, hay | 532 | [108] | [71] |
| 2-Nonanone | Malty, fruity, hot milk, smoked cheese | 1092 | [109] | |
| 2-Heptanone | Blue cheese, spicy, Roquefort cheese | 890 | ||
| 2-Pentanone | Orange peel, sweet, fruity | 727 | [71] | |
| 3-Octen-2-one | Earth, oily, ketonic, sweet, hay, mushroom-like | 1096 | * | [51] |
| 2,3-Octanedione | Dill, herbal, buttery | 981 | * | [110] |
| 1-Octen-3-one | Metallic, mushroom-like | 1294 | * | [111] |
| 3,5-Octadien-2-one | Mushroom-like, fatty | 1030 | * | [71] |
| Alcohol | ||||
| 1-Heptanol | Sweet, green, woody | 972 | * | [71] |
| 1-Octanol | Waxy, green, citrus, floral, sweet, fatty, coconut | 1116 | * | |
| 1-Pentanol | Fermented, sweet, balsam, yeasty, solvent-like | 794 | * | |
| 1-Hexanol | Green, herbal, alcohol, sweet | 894 | * |
9. Qualitative and Quantitative Measurement of Lipid Oxidation Compounds in Dairy Products
9.1. Peroxide Value
9.2. Thiobarbituric Acid Reactive Substances
9.3. KREIS Test
9.4. Physical Evaluation Methods
9.5. Analysis of Volatile Organic Compounds by Gas Chromatography
9.6. Gas Chromatography Olfactometry
10. Sensory Analysis
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boroski, M.; Giroux, H.J.; Sabik, H.; Petit, H.V.; Visentainer, J.V.; Matumoto-Pintro, P.T.; Britten, M. Use of oregano extract and oregano essential oil as antioxidants in functional dairy beverage formulations. LWT—Food Sci. Technol. 2012, 47, 167–174. [Google Scholar] [CrossRef]
- Kochhar, S. Oxidative pathways to the formation of off-flavours. In Food Taints and Off-Flavours; Springer: Berlin/Heidelberg, Germany, 1996; pp. 168–225. [Google Scholar]
- Kolanowski, W.; Jaworska, D.; Weißbrodt, J. Importance of instrumental and sensory analysis in the assessment of oxidative deterioration of omega-3 long-chain polyunsaturated fatty acid-rich foods. J. Sci. Food Agric. 2007, 87, 181–191. [Google Scholar] [CrossRef]
- Kubow, S. Routes of formation and toxic consequences of lipid oxidation products in foods. Radic. Biol. Med. 1992, 12, 63–81. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W. Formation of oxidized flavor compounds in concentrated milk and distillate during milk concentration. J. Dairy Sci. 2016, 99, 9647–9651. [Google Scholar] [CrossRef]
- Lloyd, M.; Drake, M.; Gerard, P. Flavor Variability and Flavor Stability of US-Produced Whole Milk Powder. J. Food Sci. 2009, 74, S334–S343. [Google Scholar] [CrossRef]
- Romeu-Nadal, M.; Chavez-Servin, J.; Castellote, A.; Rivero, M.; Lopez-Sabater, M. Oxidation stability of the lipid fraction in milk powder formulas. Food Chem. 2007, 100, 756–763. [Google Scholar] [CrossRef]
- Hall, G.; Andersson, J.; Lingnert, H.; Olofssono, B. Flavor changes in whole milk powder during storage. J. Food Qual. 1985, 7, 153–190. [Google Scholar] [CrossRef]
- Hougaard, A.B.; Vestergaard, J.S.; Varming, C.; Bredie, W.L.; Ipsen, R.H. Composition of volatile compounds in bovine milk heat treated by instant infusion pasteurisation and their correlation to sensory analysis. Int. J. Dairy Technol. 2011, 64, 34–44. [Google Scholar] [CrossRef]
- Kilcawley, K.N.; Faulkner, H.; Clarke, H.J.; O’Sullivan, M.G.; Kerry, J.P. Factors Influencing the Flavour of Bovine Milk and Cheese from Grass Based versus Non-Grass Based Milk Production Systems. Foods 2018, 7, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Callaghan, T.; Hennessy, D.; McAuliffe, S.; Kilcawley, K.; O’Donovan, M.; Dillon, P.; Ross, R.; Stanton, C. Effect of pasture versus indoor feeding systems on raw milk composition and quality over an entire lactation. J. Dairy Sci. 2016, 99, 9424–9440. [Google Scholar] [CrossRef] [PubMed]
- Roesch, M.; Doherr, M.; Blum, J. Performance of dairy cows on Swiss farms with organic and integrated production. J. Dairy Sci. 2005, 88, 2462–2475. [Google Scholar] [CrossRef]
- Heck, J.; Van Valenberg, H.; Dijkstra, J.; Van Hooijdonk, A. Seasonal variation in the Dutch bovine raw milk composition. J. Dairy Sci. 2009, 92, 4745–4755. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.K.; Fretté, X.C.; Kristensen, T.; Eriksen, J.; Søegaard, K.; Nielsen, J.H. Fatty acid, tocopherol and carotenoid content in herbage and milk affected by sward composition and season of grazing. J. Sci. Food Agric. 2012, 92, 2891–2898. [Google Scholar] [CrossRef]
- Craninx, M.; Steen, A.; Van Laar, H.; Van Nespen, T.; Martin-Tereso, J.; De Baets, B.; Fievez, V. Effect of lactation stage on the odd-and branched-chain milk fatty acids of dairy cattle under grazing and indoor conditions. J. Dairy Sci. 2008, 91, 2662–2677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palladino, R.; Buckley, F.; Prendiville, R.; Murphy, J.; Callan, J.; Kenny, D. A comparison between Holstein-Friesian and Jersey dairy cows and their F1 hybrid on milk fatty acid composition under grazing conditions. J. Dairy Sci. 2010, 93, 2176–2184. [Google Scholar] [CrossRef] [Green Version]
- Jensen, R.G.; Clark, R.W. Lipid Composition and Properties. In Fundamentals of Dairy Chemistry; Springer: Berlin/Heidelberg, Germany, 1988; pp. 171–213. [Google Scholar]
- Lindmark Månsson, H. Fatty acids in bovine milk fat. Food Nutr. Res. 2008, 52, 1821. [Google Scholar] [CrossRef] [Green Version]
- Birdi, K. Surface and Colloid Chemistry: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Danthine, S.; Blecker, C.; Paquot, M.; Innocente, N.; Deroanne, C. Progress in milk fat globule membrane research: A review. Lait 2000, 80, 209–222. [Google Scholar] [CrossRef] [Green Version]
- Markiewicz-Kęszycka, M.; Czyżak-Runowska, G.; Lipińska, P.; Wójtowski, J. Fatty acid profile of milk—A review. Bull. Vet. Inst. Pulawy 2013, 57, 135–139. [Google Scholar] [CrossRef] [Green Version]
- Romeu-Nadal, M.; Castellote, A.; López-Sabater, M. Headspace gas chromatographic method for determining volatile compounds in infant formulas. J. Chromatogr. A 2004, 1046, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.M. Effects of Lipid Oxidation Initiators and Antioxidants on the Total Antioxidant Capacity of Milk and Oxidation Products during Storage. Master’s Thesis, Iowa State University, Ames, IA, USA, 2014. [Google Scholar]
- Shahidi, F.; Zhong, Y. Lipid oxidation: Measurement methods. In Bailey’s Industrial Oil and Fat Products; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Frankel, E. Frying fats. In Lipid Oxidation; The Oily Press: Dundee, UK, 1998; pp. 227–248. [Google Scholar]
- Miyashita, K. Polyunsaturated lipids in aqueous systems do not follow our preconceptions of oxidative stability. Lipid Technol. Newsl. 2002, 8, 35–41. [Google Scholar]
- Rawls, H.R.; Van Santen, P. A possible role for singlet oxygen in the initiation of fatty acid autoxidation. J. Am. Oil Chem. Soc. 1970, 47, 121–125. [Google Scholar] [CrossRef]
- Simkovsky, N.M.; Ecker, A. Einfluß von Licht und Tocopherolgehalt auf die Oxidationsstabilität von Fettsäuremethylestern. Lipid Fett. 1998, 100, 534–538. [Google Scholar] [CrossRef]
- Wishner, L.A. Light-induced oxidations in milk. J. Dairy Sci. 1964, 47, 216–221. [Google Scholar] [CrossRef]
- Brothersen, C.; McMahon, D.; Legako, J.; Martini, S. Comparison of milk oxidation by exposure to LED and fluorescent light. J. Dairy Sci. 2016, 99, 2537–2544. [Google Scholar] [CrossRef] [Green Version]
- Stull, J. The Effect of Light on Activated Flavor Development and on the Constituents of Milk and its Products: A Review. J. Dairy Sci. 1953, 36, 1153–1164. [Google Scholar] [CrossRef]
- Jung, M.; Yoon, S.; Lee, H.; Min, D. Singlet Oxygen and Ascorbic Acid Effects on Dimethyl Disulfide and Off-Flavor in Skim Milk Exposed to Light. J. Food Sci. 1998, 63, 408–412. [Google Scholar] [CrossRef]
- Hedegaard, R.; Kristensen, D.; Nielsen, J.H.; Frøst, M.B.; Østdal, H.; Hermansen, J.E.; Kröger-Ohlsen, M.; Skibsted, L.H. Comparison of descriptive sensory analysis and chemical analysis for oxidative changes in milk. J. Dairy Sci. 2006, 89, 495–504. [Google Scholar] [CrossRef] [Green Version]
- Silcock, P.; Alothman, M.; Zardin, E.; Heenan, S.; Siefarth, C.; Bremer, P.; Beauchamp, J. Microbially induced changes in the volatile constituents of fresh chilled pasteurised milk during storage. Food Packag. Shelf Life 2014, 2, 81–90. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Coordinate contribution of lipid oxidation and Maillard reaction to the nonenzymatic food browning. Crit. Rev. Food Sci. Nutr. 2005, 45, 49–59. [Google Scholar] [CrossRef]
- Chen, X.-M.; Kitts, D.D. Characterization of antioxidant and anti-inflammatory activities of bioactive fractions recovered from a glucose− lysine Maillard reaction model system. Mol. Cell. Biochem. 2012, 364, 147–157. [Google Scholar] [CrossRef]
- Nunes, L.; Martins, E.; Perrone, Í.T.; de Carvalho, A.F. The Maillard Reaction in Powdered Infant Formula. J. Food Nutr. Res. 2019, 7, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Whetstine, M.C.; Croissant, A.; Drake, M. Characterization of dried whey protein concentrate and isolate flavor. J. Dairy Sci. 2005, 88, 3826–3839. [Google Scholar] [CrossRef]
- Tunick, M.H.; Thomas-Gahring, A.; Van Hekken, D.L.; Iandola, S.K.; Singh, M.; Qi, P.X.; Ukuku, D.O.; Mukhopadhyay, S.; Onwulata, C.I.; Tomasula, P.M. Physical and chemical changes in whey protein concentrate stored at elevated temperature and humidity. J. Dairy Sci. 2016, 99, 2372–2383. [Google Scholar] [CrossRef]
- Sienkiewicz, T.; Riedel, C. Whey and Whey Utilization: Possibilities for Utilization in Agriculture and Foodstuffs Production; Verlag Th. Mann: Gelsenkirchen-Buer, Germany, 1990. [Google Scholar]
- Atasoy, A.F.; Hayaloglu, A.A.; Kırmacı, H.; Levent, O.; Türkoğlu, H. Effects of partial substitution of caprine for ovine milk on the volatile compounds of fresh and mature Urfa cheeses. Small Rumin. Res. 2013, 115, 113–123. [Google Scholar] [CrossRef]
- Kondyli, E.; Massouras, T.; Katsiari, M.; Voutsinas, L. Lipolysis and volatile compounds of Galotyri-type cheese made using different procedures. Small Rumin. Res. 2013, 113, 432–436. [Google Scholar] [CrossRef]
- Mottram, D.S. Flavour formation in meat and meat products: A review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- Estévez, M.; Ventanas, S.; Heinonen, M. Formation of Strecker aldehydes between protein carbonyls–α-aminoadipic and γ-glutamic semialdehydes–and leucine and isoleucine. Food Chem. 2011, 128, 1051–1057. [Google Scholar] [CrossRef]
- Zhou, Q.; Wintersteen, C.L.; Cadwallader, K.R. Identification and quantification of aroma-active components that contribute to the distinct malty flavor of buckwheat honey. J. Agric. Food Chem. 2002, 50, 2016–2021. [Google Scholar] [CrossRef]
- Delgado, F.J.; González-Crespo, J.; Cava, R.; García-Parra, J.; Ramírez, R. Characterisation by SPME–GC–MS of the volatile profile of a Spanish soft cheese PDO Torta del Casar during ripening. Food Chem. 2010, 118, 182–189. [Google Scholar] [CrossRef]
- Zamora, R.; Gallardo, E.; Hidalgo, F.J. Strecker degradation of phenylalanine initiated by 2,4-decadienal or methyl 13-oxooctadeca-9,11-dienoate in model systems. J. Agric. Food Chem. 2007, 55, 1308–1314. [Google Scholar] [CrossRef]
- Zamora, R.; Gallardo, E.; Hidalgo, F.J. Model studies on the degradation of phenylalanine initiated by lipid hydroperoxides and their secondary and tertiary oxidation products. J. Agric. Food Chem. 2008, 56, 7970–7975. [Google Scholar] [CrossRef]
- Whetstine, M.E.C.; Drake, M. The Flavor and Flavor Stability of Skim and Whole Milk Powders; ACS Publications: Washington, DC, USA, 2007. [Google Scholar]
- Cadwallader, K.; Singh, T. Flavours and off-flavours in milk and dairy products. In Advanced Dairy Chemistry; Springer: Berlin/Heidelberg, Germany, 2009; pp. 631–690. [Google Scholar]
- Clarke, H.J.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Correlating Volatile Lipid Oxidation Compounds with Consumer Sensory Data in Dairy Based Powders during Storage. Antioxidants 2020, 9, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, H.; Griffin, C.; Hennessy, D.; O’Callaghan, T.; O’Sullivan, M.; Kerry, J.; Kilcawley, K. Effect of bovine feeding system (pasture or concentrate) on the oxidative and sensory shelf life of whole milk powder. J. Dairy Sci. 2021, 104, 10654–10668. [Google Scholar] [CrossRef] [PubMed]
- Palmquist, D.; Beaulieu, A.D.; Barbano, D. Feed and animal factors influencing milk fat composition. J. Dairy Sci. 1993, 76, 1753–1771. [Google Scholar] [CrossRef]
- Caudle, A.D.; Yoon, Y.; Drake, M. Influence of flavor variability in skim milk powder on consumer acceptability of ingredient applications. J. Food Sci. 2005, 70, s427–s431. [Google Scholar] [CrossRef]
- Wolf, I.V.; Bergamini, C.V.; Perotti, M.C.; Hynes, E.R. Sensory and Flavor Characteristics of Milk. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781118534168.ch15 (accessed on 28 September 2021).
- Banavara, D.; Anupama, D.; Rankin, S. Studies on physicochemical and functional properties of commercial sweet whey powders. J. Dairy Sci. 2003, 86, 3866–3875. [Google Scholar] [CrossRef] [Green Version]
- Abdalla, A.; Smith, K.; Lucey, J. Sensory Evaluation of Nonfat Dry Milk and Skim Milk Powder. Int. J. Dairy Sci. 2017, 12, 190–196. [Google Scholar] [CrossRef] [Green Version]
- Shiratsuchi, H.; Shimoda, M.; Imayoshi, K.; Noda, K.; Osajima, Y. Volatile flavor compounds in spray-dried skim milk powder. J. Agric. Food Chem. 1994, 42, 984–988. [Google Scholar] [CrossRef]
- Frøst, M.B.; Janhøj, T. Understanding creaminess. Int. Dairy J. 2007, 17, 1298–1311. [Google Scholar] [CrossRef] [Green Version]
- Lopez, C.; Cauty, C.; Guyomarc’h, F. Organization of lipids in milks, infant milk formulas and various dairy products: Role of technological processes and potential impacts. Dairy Sci. Technol. 2015, 95, 863–893. [Google Scholar] [CrossRef]
- Saphier, O.; Silberstein, T. Lipid peroxidation of infant milk formula. In Handbook of Dietary and Nutritional Aspects of Bottle Feeding; Wageningen Academic Publishers: Wageningen, The Netherlands, 2014; p. 137. [Google Scholar]
- Cesa, S.; Casadei, M.; Cerreto, F.; Paolicelli, P. Infant milk formulas: Effect of storage conditions on the stability of powdered products towards autoxidation. Foods 2015, 4, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.-x.; Chen, W.-L.; Qi, X.-Y.; Su, M.-Y. The stability of milk-based infant formulas during accelerated storage. CyTA J. Food 2019, 17, 96–104. [Google Scholar] [CrossRef]
- Morr, C.V.; Ha, E. Whey protein concentrates and isolates: Processing and functional properties. Crit. Rev. Food Sci. Nutr. 1993, 33, 431–476. [Google Scholar] [CrossRef] [PubMed]
- USAID. Whey Protein Concentrate Commodity Fact Sheet. Available online: https://www.usaid.gov/what-we-do/agriculture-and-food-security/food-assistance/resources/whey-protein-concentrate (accessed on 19 August 2021).
- Tomaino, R.; Turner, L.; Larick, D. The effect of Lactococcus lactis starter cultures on the oxidative stability of liquid whey. J. Dairy Sci. 2004, 87, 300–307. [Google Scholar] [CrossRef] [Green Version]
- Jensen, B.M.; Sørensen, J.; Mortensen, G.; Dalsgaard, T.K. Oxidation of whey concentrates during long-term storage. Milchwissenschaft 2012, 67, 195. [Google Scholar]
- Berton-Carabin, C.C.; Schröder, A.; Rovalino-Cordova, A.; Schroën, K.; Sagis, L. Protein and lipid oxidation affect the viscoelasticity of whey protein layers at the oil–water interface. Eur. J. Lipid Sci. Technol. 2016, 118, 1630–1643. [Google Scholar] [CrossRef]
- Wright, B.; Zevchak, S.; Wright, J.M.; Drake, M. The impact of agglomeration and storage on flavor and flavor stability of whey protein concentrate 80% and whey protein isolate. J. Food Sci. 2009, 74, S17–S29. [Google Scholar] [CrossRef]
- Russell, T.; Drake, M.; Gerard, P. Sensory properties of whey and soy proteins. J. Food Sci. 2006, 71, S447–S455. [Google Scholar] [CrossRef]
- Clarke, H.J.; Griffin, C.; Rai, D.K.; O’Callaghan, T.F.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Dietary Compounds Influencing the Sensorial, Volatile and Phytochemical Properties of Bovine Milk. Molecules 2020, 25, 26. [Google Scholar] [CrossRef] [Green Version]
- Coppa, M.; Martin, B.; Pradel, P.; Leotta, B.; Priolo, A.; Vasta, V. Effect of a hay-based diet or different upland grazing systems on milk volatile compounds. J. Agric. Food Chem. 2011, 59, 4947–4954. [Google Scholar] [CrossRef]
- Cheng, Z.; O’Sullivan, M.; Kerry, J.; Drake, M.; Miao, S.; Kaibo, D.; Kilcawley, K. A cross-cultural sensory analysis of skim powdered milk produced from pasture and non-pasture diets. Food Res. Int 2020, 138, 109749. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, T.F.; Faulkner, H.; McAuliffe, S.; O’Sullivan, M.G.; Hennessy, D.; Dillon, P.; Kilcawley, K.N.; Stanton, C.; Ross, R.P. Quality characteristics, chemical composition, and sensory properties of butter from cows on pasture versus indoor feeding systems. J. Dairy Sci. 2016, 99, 9441–9460. [Google Scholar] [CrossRef] [Green Version]
- O’Callaghan, T.F.; Mannion, D.T.; Hennessy, D.; McAuliffe, S.; O’Sullivan, M.G.; Leeuwendaal, N.; Beresford, T.P.; Dillon, P.; Kilcawley, K.N.; Sheehan, J.J. Effect of pasture versus indoor feeding systems on quality characteristics, nutritional composition, and sensory and volatile properties of full-fat Cheddar cheese. J. Dairy Sci. 2017, 100, 6053–6073. [Google Scholar] [CrossRef]
- Chilliard, Y.; Ferlay, A.; Doreau, M. Effect of different types of forages, animal fat or marine oils in cow’s diet on milk fat secretion and composition, especially conjugated linoleic acid (CLA) and polyunsaturated fatty acids. Livest. Prod. Sci. 2001, 70, 31–48. [Google Scholar] [CrossRef]
- Caroprese, M.; Mancino, R.; Ciliberti, M.G.; Di Luccia, A.; La Gatta, B.; Albenzio, M. Fatty acid profile and coagulating ability of milk from Jersey and Friesian cows fed whole flaxseed. J. Dairy Res. 2017, 84, 14–22. [Google Scholar] [CrossRef]
- Bodkowski, R.; Czyż, K.; Kupczyński, R.; Patkowska-Sokoła, B.; Nowakowski, P.; Wiliczkiewicz, A. Lipid complex effect on fatty acid profile and chemical composition of cow milk and cheese. J. Dairy Sci. 2016, 99, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Schöne, F.; Spörl, K.; Leiterer, M. Iodine in the feed of cows and in the milk with a view to the consumer’s iodine supply. J. Trace Elem. Med. Biol. 2017, 39, 202–209. [Google Scholar] [CrossRef]
- Glover, K.; Budge, S.; Rose, M.; Rupasinghe, H.; MacLaren, L.; Green-Johnson, J.; Fredeen, A. Effect of feeding fresh forage and marine algae on the fatty acid composition and oxidation of milk and butter. J. Dairy Sci. 2012, 95, 2797–2809. [Google Scholar] [CrossRef] [Green Version]
- Lejonklev, J.; Kidmose, U.; Jensen, S.; Petersen, M.A.; Helwing, A.; Mortensen, G.; Weisbjerg, M.R.; Larsen, M.K. Effect of oregano and caraway essential oils on the production and flavor of cow milk. J. Dairy Sci. 2016, 99, 7898–7903. [Google Scholar] [CrossRef]
- Yang, Y.; Ferreira, G.; Teets, C.; Corl, B.; Thomason, W.; Griffey, C. Effects of feeding hull-less barley on production performance, milk fatty acid composition, and nutrient digestibility of lactating dairy cows. J. Dairy Sci. 2017, 100, 3576–3583. [Google Scholar] [CrossRef] [PubMed]
- AbuGhazaleh, A.; Holmes, L. Diet supplementation with fish oil and sunflower oil to increase conjugated linoleic acid levels in milk fat of partially grazing dairy cows. J. Dairy Sci. 2007, 90, 2897–2904. [Google Scholar] [CrossRef]
- Villeneuve, M.-P.; Lebeuf, Y.; Gervais, R.; Tremblay, G.; Vuillemard, J.; Fortin, J.; Chouinard, P. Milk volatile organic compounds and fatty acid profile in cows fed timothy as hay, pasture, or silage. J. Dairy Sci. 2013, 96, 7181–7194. [Google Scholar] [CrossRef]
- Faulkner, H.; O’Callaghan, T.F.; McAuliffe, S.; Hennessy, D.; Stanton, C.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Effect of different forage types on the volatile and sensory properties of bovine milk. J. Dairy Sci. 2018, 101, 1034–1047. [Google Scholar] [CrossRef]
- Toso, B.; Procida, G.; Stefanon, B. Determination of volatile compounds in cows’ milk using headspace GC-MS. J. Dairy Res. 2002, 69, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Vanbergue, E.; Delaby, L.; Peyraud, J.-L.; Colette, S.; Gallard, Y.; Hurtaud, C. Effects of breed, feeding system, and lactation stage on milk fat characteristics and spontaneous lipolysis in dairy cows. J. Dairy Sci. 2017, 100, 4623–4636. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, L.; Wang, W. Formation of Aldehyde and Ketone Compounds during Production and Storage of Milk Powder. Molecules 2012, 17, 9900. [Google Scholar] [CrossRef] [Green Version]
- Stapelfeldt, H.; Nielsen, B.R.; Skibsted, L.H. Effect of heat treatment, water activity and storage temperature on the oxidative stability of whole milk powder. Int. Dairy J. 1997, 7, 331–339. [Google Scholar] [CrossRef]
- Oldfield, D.; Taylor, M.; Singh, H. Effect of preheating and other process parameters on whey protein reactions during skim milk powder manufacture. Int. Dairy J. 2005, 15, 501–511. [Google Scholar] [CrossRef]
- Baldwin, A.; Ackland, J. Effect of preheat treatment and storage on the properties of whole milk powder. Changes in physical and chemical properties. Neth. Milk Dairy J. 1991, 45, 169–181. [Google Scholar]
- Park, C.; Drake, M. The distribution of fat in dried dairy particles determines flavor release and flavor stability. J. Food Sci. 2014, 79, R452–R459. [Google Scholar] [CrossRef] [PubMed]
- Park, C.W.; Stout, M.A.; Drake, M. The effect of spray-drying parameters on the flavor of nonfat dry milk and milk protein concentrate 70%. J. Dairy Sci. 2016, 99, 9598–9610. [Google Scholar] [CrossRef]
- Drake, M.; Karagul-Yuceer, Y.; Cadwallader, K.; Civille, G.; Tong, P. Determination of the sensory attributes of dried milk powders and dairy ingredients. J. Sens. Stud. 2003, 18, 199–216. [Google Scholar] [CrossRef] [Green Version]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- WHO. Indoor air quality: Organic pollutants. In Report on a WHO Meeting, Berlin (West) 23–27 August 1987. Copenhagen, Denmark; World Health Organization Regional Office for Europe: Geneva, Switzerland, 1989. [Google Scholar]
- Moio, L.; Dekimpe, J.; Etievant, P.; Addeo, F. Neutral volatile compounds in the raw milks from different species. J. Dairy Res. 1993, 60, 199–213. [Google Scholar] [CrossRef]
- Ross, C.F. Sensory science at the human–machine interface. Trends Food Sci. Technol. 2009, 20, 63–72. [Google Scholar] [CrossRef]
- Tunick, M.H. Analyzing volatile compounds in dairy products. J. Sci. Food Agric. 2014, 94, 1701–1705. [Google Scholar] [CrossRef]
- Potts, D.M.; Peterson, D.G. Identification of objectionable flavors in purported spontaneous oxidized flavor bovine milk. J. Dairy Sci. 2018, 101, 10877–10885. [Google Scholar] [CrossRef] [Green Version]
- Al-Attabi, Z.; D’Arcy, B.R.; Deeth, H.C. Volatile sulfur compounds in pasteurised and UHT milk during storage. Dairy Sci. Technol. 2014, 94, 241–253. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Landaverde, P.A.; Torres, J.A.; Qian, M.C. Quantification of trace volatile sulfur compounds in milk by solid-phase microextraction and gas chromatography–pulsed flame photometric detection. J. Dairy Sci. 2006, 89, 2919–2927. [Google Scholar] [CrossRef]
- Kilic, M.; Lindsay, R. Distribution of conjugates of alkylphenols in milk from different ruminant species. J. Dairy Sci. 2005, 88, 7–12. [Google Scholar] [CrossRef]
- Marsili, R. Flavor, Fragrance, and Odor Analysis; CRC Press: Boca Raton, FL, USA, 2001; Volume 115. [Google Scholar]
- Zhao, L.-M.; Wu, W.; Tao, N.-P.; Li, Y.-Q.; Wu, N.; Qin, X. Characterization of important odorants in four steamed Coilia ectenes from China by gas chromatography–mass spectrometry–olfactometry. Fish Sci. 2015, 81, 947–957. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhao, J.; Xu, F.; Wu, X.; Hu, W.; Chang, Y.; Zhang, L.; Chen, J.; Liu, C. GC–MS, GC-O and OVA analyses of key aroma compounds in Jiaozi Steamed Bread. Grain Oil Sci. Technol. 2019, 3, 9–17. [Google Scholar] [CrossRef]
- Xu, B.M.; Baker, G.L.; Sarnoski, P.J.; Goodrich-Schneider, R.M. A comparison of the volatile components of cold pressed Hamlin and Valencia (Citrus sinensis (L.) Osbeck) orange oils affected by Huanglongbing. J. Food Qual. 2017, 2017, 6793986. [Google Scholar] [CrossRef]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L. Fundamentals of Cheese Science; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Rouseff, R.L.; Cadwallader, K.R. Headspace Analysis of Foods and Flavors: Theory and Practice. In Proceedings of the American Chemical Society, Boston, MA, USA, 23–27 August 1998. [Google Scholar]
- Serrano, E.; Cornu, A.; Kondjoyan, N.; Agabriel, J.; Micol, D. Traceability of grass feeding in beef: Terpenes, 2,3-octanedione and skatole accumulation in adipose tissue of young bulls. Animal 2011, 5, 641–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothe, M. Flavour Science. Recent Developments; Taylor, A.J., Mottram, D.S., Eds.; The Royal Society of Chemistry: Cambridge, UK, 1996; p. 41. [Google Scholar]
- The Good Scents Company Information System. Available online: http://www.thegoodscentscompany.com/ (accessed on 17 February 2020).
- Havemose, M.; Justesen, P.; Bredie, W.; Nielsen, J.H. Measurement of volatile oxidation products from milk using solvent-assisted flavour evaporation and solid phase microextraction. Int. Dairy J. 2007, 17, 746–752. [Google Scholar] [CrossRef]
- Zellner, B.d.A.; Dugo, P.; Dugo, G.; Mondello, L. Gas chromatography–olfactometry in food flavour analysis. J. Chromatogr. A 2008, 1186, 123–143. [Google Scholar] [CrossRef]
- Panseri, S.; Soncin, S.; Chiesa, L.M.; Biondi, P.A. A headspace solid-phase microextraction gas-chromatographic mass-spectrometric method (HS-SPME–GC/MS) to quantify hexanal in butter during storage as marker of lipid oxidation. Food Chem. 2011, 127, 886–889. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Association of Official Analytical Chemists, 17th ed.; Horwitz, W., Ed.; Official Methods of Analysis of the AOAC International, AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Østdal, H.; Andersen, H.J.; Nielsen, J.H. Antioxidative activity of urate in bovine milk. J. Agric. Food Chem. 2000, 48, 5588–5592. [Google Scholar] [CrossRef]
- Smet, K.; Raes, K.; De Block, J.; Herman, L.; Dewettinck, K.; Coudijzer, K. A change in antioxidative capacity as a measure of onset to oxidation in pasteurized milk. Int. Dairy J. 2008, 18, 520–530. [Google Scholar] [CrossRef]
- Saxby, M. Food Taints and Off-Flavours; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Decker, E.A.; Elias, R.J.; McClements, D.J. Oxidation in Foods and Beverages and Antioxidant Applications: Management in Different Industry Sectors; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Ross, C.F.; Smith, D.M. Use of volatiles as indicators of lipid oxidation in muscle foods. Compr. Rev. Food Sci. Food Saf. 2006, 5, 18–25. [Google Scholar] [CrossRef]
- Devasagayam, T.; Boloor, K.; Ramasarma, T. Methods for estimating lipid peroxidation: An analysis of merits and demerits. Indian J. Biochem. Biophys. 2003, 40, 300–308. [Google Scholar]
- O’Sullivan, A.M.; O’Callaghan, Y.C.; O’Grady, M.N.; Waldron, D.S.; Smyth, T.J.; O’Brien, N.M.; Kerry, J.P. An examination of the potential of seaweed extracts as functional ingredients in milk. Int. J. Dairy Technol. 2014, 67, 182–193. [Google Scholar] [CrossRef]
- Fenaille, F.; Mottier, P.; Turesky, R.J.; Ali, S.; Guy, P.A. Comparison of analytical techniques to quantify malondialdehyde in milk powders. J. Chromatogr. A 2001, 921, 237–245. [Google Scholar] [CrossRef]
- Cesa, S. Malondialdehyde contents in infant milk formulas. J. Agric. Food Chem. 2004, 52, 2119–2122. [Google Scholar] [CrossRef]
- Narasimhan, S.; Kumar, A.K.; Ravi, R.; Chand, N. Optimization of Kreis test for edible oils. J. Food Lipids 1999, 6, 107–115. [Google Scholar] [CrossRef]
- Gray, J. Measurement of lipid oxidation: A review. J. Am. Oil Chem. Soc. 1978, 55, 539–546. [Google Scholar] [CrossRef]
- Kerr, R.H.; Sorber, D. The Analytical Detection of Rancidity. J. Ind. Eng. Chem. 1923, 15, 383–386. [Google Scholar] [CrossRef]
- Mehlenbacher, V. The Analysis of Oils and Fats; The Garad Press Publisher: Champaign, IL, USA, 1960. [Google Scholar]
- Pignitter, M.; Somoza, V. Critical evaluation of methods for the measurement of oxidative rancidity in vegetable oils. J. Food Drug Anal. 2012, 20. [Google Scholar]
- Klein, M.S.; Almstetter, M.F.; Schlamberger, G.; Nürnberger, N.; Dettmer, K.; Oefner, P.J.; Meyer, H.; Wiedemann, S.; Gronwald, W. Nuclear magnetic resonance and mass spectrometry-based milk metabolomics in dairy cows during early and late lactation. J. Dairy Sci. 2010, 93, 1539–1550. [Google Scholar] [CrossRef]
- Sundekilde, U.K.; Frederiksen, P.D.; Clausen, M.R.; Larsen, L.B.; Bertram, H.C. Relationship between the metabolite profile and technological properties of bovine milk from two dairy breeds elucidated by NMR-based metabolomics. J. Agric. Food Chem. 2011, 59, 7360–7367. [Google Scholar] [CrossRef]
- Tsiafoulis, C.G.; Papaemmanouil, C.; Alivertis, D.; Tzamaloukas, O.; Miltiadou, D.; Balayssac, S.; Malet-Martino, M.; Gerothanassis, I.P. NMR-Based Μetabolomics of the Lipid Fraction of Organic and Conventional Bovine Milk. Molecules 2019, 24, 1067. [Google Scholar] [CrossRef] [Green Version]
- Siefarth, C.; Serfert, Y.; Drusch, S.; Buettner, A. Comparative evaluation of diagnostic tools for oxidative deterioration of polyunsaturated fatty acid-enriched infant formulas during storage. Foods 2014, 3, 30–65. [Google Scholar] [CrossRef]
- Arya, S.; Ramanujam, S.; Vijayaraghavan, P. Refractive index as an objective method for evaluation of rancidity in edible oils and fats. J. Am. Oil Chem. Soc. 1969, 46, 28–30. [Google Scholar] [CrossRef]
- Ahlers, N.; McTaggart, N. The determination of hydroxyl, ketone and ester groups in autoxidised fatty esters and related compounds by infra-red spectroscopy. Analyst 1954, 79, 70–76. [Google Scholar] [CrossRef]
- Qualley, A.V.; Dudareva, N. Metabolomics of plant volatiles. In Plant Systems Biology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 329–343. [Google Scholar]
- Heaven, M.W.; Nash, D. Recent analyses using solid phase microextraction in industries related to food made into or from liquids. Food Control 2012, 27, 214–227. [Google Scholar] [CrossRef]
- Vazquez-Landaverde, P.A.; Velazquez, G.; Torres, J.; Qian, M. Quantitative determination of thermally derived off-flavor compounds in milk using solid-phase microextraction and gas chromatography. J. Dairy Sci. 2005, 88, 3764–3772. [Google Scholar] [CrossRef]
- Mounchili, A.; Wichtel, J.; Bosset, J.; Dohoo, I.R.; Imhof, M.; Altieri, D.; Mallia, S.; Stryhn, H. HS-SPME gas chromatographic characterization of volatile compounds in milk tainted with off-flavour. Int. Dairy J. 2005, 15, 1203–1215. [Google Scholar] [CrossRef]
- Clarke, H.J.; Mannion, D.T.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Development of a headspace solid-phase microextraction gas chromatography mass spectrometry method for the quantification of volatiles associated with lipid oxidation in whole milk powder using response surface methodology. Food Chem. 2019, 292, 75–80. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, G.; Wei, X.; Ding, B.; Huang, C.; Xu, B. Determination of vanillin and ethyl-vanillin in milk powder by headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry. Food Anal. Methods 2016, 9, 3360–3366. [Google Scholar] [CrossRef]
- Chen, C.; Husny, J.; Rabe, S. Predicting fishiness off-flavour and identifying compounds of lipid oxidation in dairy powders by SPME-GC/MS and machine learning. Int. Dairy J. 2018, 77, 19–28. [Google Scholar] [CrossRef]
- García-Llatas, G.; Lagarda, M.J.; Romero, F.; Abellán, P.; Farré, R. A headspace solid-phase microextraction method of use in monitoring hexanal and pentane during storage: Application to liquid infant foods and powdered infant formulas. Food Chem. 2007, 101, 1078–1086. [Google Scholar] [CrossRef] [Green Version]
- Nie, S.-P.; Huang, J.-G.; Zhang, Y.-N.; Hu, J.-L.; Wang, S.; Shen, M.-Y.; Li, C.; Marcone, M.F.; Xie, M.-Y. Analysis of furan in heat-processed foods in China by automated headspace gas chromatography-mass spectrometry (HS-GC-MS). Food Control 2013, 30, 62–68. [Google Scholar] [CrossRef]
- Merkle, S.; Kleeberg, K.; Fritsche, J. Recent developments and applications of solid phase microextraction (SPME) in food and environmental analysis—A review. Chromatography 2015, 2, 293–381. [Google Scholar] [CrossRef] [Green Version]
- Valero, E.; Miranda, E.; Sanz, J.; Martinez-Castro, I. Automatic thermal desorption in GC analysis of dairy product volatiles. Chromatographia 1997, 44, 59–64. [Google Scholar] [CrossRef]
- Francesca, I.; Patrizia, P.; Luca, C.; Federico, M.; Annalisa, R. Analysis of volatile compounds in powdered milk for infant nutrition by direct desorption (CIS4–TDU) and GC–MS. Talanta 2015, 141, 195–199. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Zhu, R.-G.; Erichsen, H.; Soerensen, J.; Petersen, M.A.; Skibsted, L.H. High temperature storage of infant formula milk powder for prediction of storage stability at ambient conditions. Int. Dairy J. 2017, 73, 166–174. [Google Scholar] [CrossRef]
- Roberts, G.; Kelly, L.; Barden, D. Flavour Profiling of Milk and Premium Teas by HiSorb Sorptive Extraction with Thermal Desorption-GC-MS Analysis. LC GC Eur. 2016, 29, 699–700. [Google Scholar]
- Hoffmann, A.; Heiden, A. Determination of flavor and off flavor compounds in dairy products using stir bar sorptive extraction (SBSE) and thermal desorption GC/MSD/PFPD. In Proceedings of the 23rd International Symposium on Capillary Chromatography, Riva del Garda, Italy, 5–10 June 2000; pp. 5–10. [Google Scholar]
- Esteban, J.; Valero, E.; Miranda, E.; Jimenez, M.; Martinez-Castro, I.; Sanz, J.; Morales, R. Automatic thermal desorption in GC and GC-MS analysis of volatile food components using conventional and chiral capillary columns. LC GC 1997, 15, 264–275. [Google Scholar]
- Engel, W.; Bahr, W.; Schieberle, P. Solvent assisted flavour evaporation—A new and versatile technique for the careful and direct isolation of aroma compounds from complex food matrices. Eur. Food Res. Technol. 1999, 209, 237–241. [Google Scholar] [CrossRef]
- Bendall, J.G. Aroma compounds of fresh milk from New Zealand cows fed different diets. J. Agric. Food Chem. 2001, 49, 4825–4832. [Google Scholar] [CrossRef]
- High, R.; Bremer, P.; Kebede, B.; Eyres, G.T. Comparison of four extraction techniques for the evaluation of volatile compounds in spray-dried New Zealand sheep milk. Molecules 2019, 24, 1917. [Google Scholar] [CrossRef] [Green Version]
- Baltussen, E.; Sandra, P.; David, F.; Cramers, C. Stir bar sorptive extraction (SBSE), a novel extraction technique for aqueous samples: Theory and principles. J. Microcolumn Sep. 1999, 11, 737–747. [Google Scholar] [CrossRef]
- Prieto, A.; Basauri, O.; Rodil, R.; Usobiaga, A.; Fernández, L.; Etxebarria, N.; Zuloaga, O. Stir-bar sorptive extraction: A view on method optimisation, novel applications, limitations and potential solutions. J. Chromatogr. A 2010, 1217, 2642–2666. [Google Scholar] [CrossRef]
- McGorrin, R.J. Flavor analysis of dairy products. In Flavor of Dairy Products, 1st ed.; Cadwallader, K.R., Drake, M., McGorrin, R.J., Eds.; American Chemical Society: Washington, DC, USA, 2007; pp. 23–49. [Google Scholar]
- Park, C.W.; Drake, M. Condensed milk storage and evaporation affect the flavor of nonfat dry milk. J. Dairy Sci. 2016, 99, 9586–9597. [Google Scholar] [CrossRef]
- Ochiai, N.; Sasamoto, K.; David, F.; Sandra, P. Solvent-assisted stir bar sorptive extraction by using swollen polydimethylsiloxane for enhanced recovery of polar solutes in aqueous samples: Application to aroma compounds in beer and pesticides in wine. J. Chromatogr. A 2016, 1455, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Schiano, A.; Benoist, D.; Drake, M. Comparison of 3 rapid methods for analysis of vitamin degradation compounds in fluid skim milk. J. Dairy Sci. 2019, 102, 4906–4912. [Google Scholar] [CrossRef]
- Jochmann, M.A.; Yuan, X.; Schilling, B.; Schmidt, T.C. In-tube extraction for enrichment of volatile organic hydrocarbons from aqueous samples. J. Chromatogr. A 2008, 1179, 96–105. [Google Scholar] [CrossRef]
- Bader, N. Stir bar sorptive extraction as a sample preparation technique for chromatographic analysis: An overview. Asian J. Nanosci. Mater. 2018, 1, 54–60. [Google Scholar]
- Markes International. Flavour Profiling of Milk Using HiSorb Sorptive Extraction and TD–GC–MS. Application Note 120. 2016. Available online: https://kinesis-australia.com.au/media/wysiwyg/knowledebase/pdf/Flavour_profiling_of_various_drinks_using_HiSorb_sorptive_extraction_and_TD_GC_MS.pdf (accessed on 28 September 2021).
- Mannion, D.T.; Furey, A.; Kilcawley, K.N. Comparison and validation of 2 analytical methods for the determination of free fatty acids in dairy products by gas chromatography with flame ionization detection. J. Dairy Sci. 2016, 99, 5047–5063. [Google Scholar] [CrossRef] [Green Version]
- Karagül-Yüceer, Y.; Cadwallader, K.R.; Drake, M. Volatile flavor components of stored nonfat dry milk. J. Agric. Food Chem. 2002, 50, 305–312. [Google Scholar] [CrossRef]
- Tranchida, P.Q.; Salivo, S.; Bonaccorsi, I.; Rotondo, A.; Dugo, P.; Mondello, L. Analysis of the unsaponifiable fraction of lipids belonging to various milk-types by using comprehensive two-dimensional gas chromatography with dual mass spectrometry/flame ionization detection and with the support of high resolution time-of-flight mass spectrometry for structural elucidation. J. Chromatogr. A 2013, 1313, 194–201. [Google Scholar]
- Lawless, H.T.; Heymann, H. Descriptive analysis. In Sensory Evaluation of Food; Springer: Berlin/Heidelberg, Germany, 2010; pp. 227–257. [Google Scholar]
- Brattoli, M.; Cisternino, E.; Dambruoso, P.R.; De Gennaro, G.; Giungato, P.; Mazzone, A.; Palmisani, J.; Tutino, M. Gas chromatography analysis with olfactometric detection (GC-O) as a useful methodology for chemical characterization of odorous compounds. Sensors 2013, 13, 16759–16800. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, J.E.; Acree, T.E. Gas chromatography olfactometry (GC/O) of dairy products. Int. Dairy J. 1998, 8, 235–241. [Google Scholar] [CrossRef]
- Rychlik, M.; Bosset, J.O. Flavour and off-flavour compounds of Swiss Gruyere cheese. Evaluation of potent odorants. Int. Dairy J. 2001, 11, 895–901. [Google Scholar] [CrossRef]
- Kobayashi, N.; Nishimura, O. Availability of detection frequency method using three-port gas chromatography-olfactometry for rapid comparison of whole milk powders. Food Sci. Technol. Res. 2014, 20, 809–814. [Google Scholar] [CrossRef] [Green Version]
- Ballance, P. Production of volatile compounds related to the flavour of foods from the Strecker degradation of DL-methionine. J. Sci. Food Agric. 1961, 12, 532–536. [Google Scholar] [CrossRef]
- Tressl, R.; Helak, B.; Martin, N.; Kersten, E. Formation of Amino Acid Specific Maillard Products and Their Contribution to Thermally Generated Aromas; ACS Publications: Washington, DC, USA, 1989. [Google Scholar]
- Adhikari, K.; Dooley, L.M.; Chambers, E.; Bhumiratana, N. Sensory characteristics of commercial lactose-free milks manufactured in the United States. LWT—Food Sci. Technol. 2010, 43, 113–118. [Google Scholar] [CrossRef] [Green Version]
- DSM. Global Insight Series. Lactose-Free Dairy: What Is Driving Global Consumer Preference? 2020. Available online: https://www.futuremarketinsights.com/press-release/lactose-free-dairy-products-market (accessed on 28 September 2021).
- Gandy, A.L.; Schilling, M.; Coggins, P.; White, C.; Yoon, Y.; Kamadia, V. The effect of pasteurization temperature on consumer acceptability, sensory characteristics, volatile compound composition, and shelf-life of fluid milk. J. Dairy Sci. 2008, 91, 1769–1777. [Google Scholar] [CrossRef] [Green Version]
- Santos, M.; Ma, Y.; Caplan, Z.; Barbano, D. Sensory threshold of off-flavors caused by proteolysis and lipolysis in milk. J. Dairy Sci. 2003, 86, 1601–1607. [Google Scholar] [CrossRef] [Green Version]
- Potts, H.; Amin, K.; Duncan, S. Retail lighting and packaging influence consumer acceptance of fluid milk. J. Dairy Sci. 2017, 100, 146–156. [Google Scholar] [CrossRef]
- Ferdenzi, C.; Joussain, P.; Digard, B.; Luneau, L.; Djordjevic, J.; Bensafi, M. Individual differences in verbal and non-verbal affective responses to smells: Influence of odor label across cultures. Chem. Senses 2017, 42, 37–46. [Google Scholar] [CrossRef]
- Murray, J.; Delahunty, C.; Baxter, I. Descriptive sensory analysis: Past, present and future. Food Res. Int. 2001, 34, 461–471. [Google Scholar] [CrossRef]
- Forss, D. Fishy flavor in dairy products. J. Dairy Sci. 1964, 47, 245–250. [Google Scholar] [CrossRef]
- Brown, W.C.; Thurston, L. A review of oxidation in milk and milk products as related to flavor. J. Dairy Sci. 1940, 23, 629–685. [Google Scholar] [CrossRef]
- Zabbia, A.; Buys, E.M.; De Kock, H.L. Undesirable sulphur and carbonyl flavor compounds in UHT milk: A review. Crit. Rev. Food Sci. Nutr. 2012, 52, 21–30. [Google Scholar] [CrossRef]
- Jo, Y.; Carter, B.; Barbano, D.; Drake, M. Identification of the source of volatile sulfur compounds produced in milk during thermal processing. J. Dairy Sci. 2019, 102, 8658–8669. [Google Scholar] [CrossRef]
- Lloyd, M.; Hess, S.; Drake, M. Effect of nitrogen flushing and storage temperature on flavor and shelf-life of whole milk powder. J. Dairy Sci. 2009, 92, 2409–2422. [Google Scholar] [CrossRef]
- Su, X.; Tortorice, M.; Ryo, S.; Li, X.; Waterman, K.; Hagen, A.; Yin, Y. Sensory Lexicons and Formation Pathways of Off-Aromas in Dairy Ingredients: A Review. Molecules 2020, 25, 569. [Google Scholar] [CrossRef]
| Method | Advantages | Limitations | Applications | Reference |
|---|---|---|---|---|
| Extraction Methodology | ||||
| Headspace solid-phase microextraction (HS-SPME) |
|
|
| [137,138,139,141,142,144] |
| In-tube extraction (ITEX) |
|
|
| [162] |
| Thermal desorption (TD) |
|
|
| [85,147,148,150,151,152] |
| Solvent-assisted flavour evaporation (SAFE) |
|
|
| [154] |
| Stir bar sorptive extraction (SBSE) |
|
|
| [151,159,160,163] |
| HiSorb extraction |
|
|
| [164] |
| Identification Methodology | ||||
| Mass Spectrometry (MS) |
|
|
| [51,71,86] |
| Flame Ionised Detector (FID) |
|
|
| [165] |
| Gas chromatography olfactometry (GC-O) |
|
|
| [166] |
| GCxCG-ToF-MS (Time of Flight-MS) |
|
|
| [167] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clarke, H.J.; McCarthy, W.P.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Oxidative Quality of Dairy Powders: Influencing Factors and Analysis. Foods 2021, 10, 2315. https://doi.org/10.3390/foods10102315
Clarke HJ, McCarthy WP, O’Sullivan MG, Kerry JP, Kilcawley KN. Oxidative Quality of Dairy Powders: Influencing Factors and Analysis. Foods. 2021; 10(10):2315. https://doi.org/10.3390/foods10102315
Chicago/Turabian StyleClarke, Holly J., William P. McCarthy, Maurice G. O’Sullivan, Joseph P. Kerry, and Kieran N. Kilcawley. 2021. "Oxidative Quality of Dairy Powders: Influencing Factors and Analysis" Foods 10, no. 10: 2315. https://doi.org/10.3390/foods10102315








