Comparison of Automated Extraction Techniques for Volatile Analysis of Whole Milk Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Whole Milk Powder
2.2. Internal and External Standard Preparation
2.3. Extraction Procedures
2.3.1. Head-Space Solid Phase Microextraction (HS-SPME)
2.3.2. Thermal Desorption Extraction
2.3.3. Headspace and Direct Immersion Hi-Sorb Extraction
2.4. GC-MS Analysis
2.5. Data Analysis
3. Results and Discussion
3.1. Comparison of Volatile Compounds Extracted from Whole Milk Powder by Each Technique
3.2. The Percentage of Each Chemical Class Extracted from Whole Milk Powder by Each Technique
3.3. The Relationship between the Individual Volatile Compound Chemical Classes Extracted by Each Technique in the Whole Milk Powder
3.4. The Selectivity of Each Extraction Technique
3.5. The Abundance of Volatile Organic Compounds in Whole Milk Powder by Each Extraction Technique
3.6. The Reproducibility of Each Extraction Technique
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Milk Powder Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2019–2024. Available online: https://www.researchandmarkets.com/reports/4775812/milk-powder-market-global-industry-trends?utm_source=GNDIY&utm_medium=PressRelease&utm_code=7blvf3&utm_campaign=1297341+-+Global+Milk+Powder+Market+Volume+to+Reach+13.2+Million+Tons+by+2024%2c+Growing+from+10.4+Million+Tons+in+2018&utm_exec=joca220prd (accessed on 11 June 2021).
- Ireland Dairy Sector. Available online: https://www.clal.it/en/index.php?section=stat_irlanda (accessed on 11 June 2021).
- Park, C.; Drake, M. The distribution of fat in dried dairy particles determines flavor release and flavor stability. J. Food Sci. 2014, 79, R452–R459. [Google Scholar] [CrossRef]
- Clarke, H.J.; Mannion, D.T.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Development of a headspace solid-phase microextraction gas chromatography mass spectrometry method for the quantification of volatiles associated with lipid oxidation in whole milk powder using response surface methodology. Food Chem. 2019, 292, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzi, A.S.; McSweeney, P.L.H.; Rea, M.C.; Kilcawley, K.N. Detection of Volatile Compounds of Cheese and Their Contribution to the Flavor Profile of Surface-Ripened Cheese. Comp. Revs. Food Sci. Food Saf. 2018, 17, 371–390. [Google Scholar] [CrossRef] [Green Version]
- Tunick, M.H.; Iandola, S.K.; Van Hekken, D.L. Comparison of SPME methods for determining volatile compounds in milk, cheese and whey powder. Foods 2013, 2, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Jelen, H.H.; Majcher, M.; Dziadas, M. Review Microextraction techniques in the analysis of food flavor compounds: A Review. Anal. Chim. Acta 2012, 738, 13–16. [Google Scholar] [CrossRef]
- Heaven, M.W.; Nash, D. Review, recent analyses using solid phase microextraction in industries related to food made into or from liquids. Food. Control 2012, 27, 214–227. [Google Scholar] [CrossRef]
- Mondello, L.; Costa, R.; Tranchida, P.Q.; Chiofalo, B.; Zumbo, A.; Dugo, P.; Dugo, G. Determination of flavor components in Sicilian goat cheese by automated HS-SPME-GC. Flavour Frag. J. 2005, 20, 659–665. [Google Scholar] [CrossRef]
- Materić, D.; Bruhn, D.; Turner, C.; Morgan, G.; Mason, N.; Gauci, V. Methods in plant foliar volatile organic compounds research. Appl. Plant Sci. 2015, 3, 1500044. [Google Scholar] [CrossRef]
- Horn, A.F.; Green-Petersen, D.; Nielsen, N.S.; Andersen, U.; Søgaard Jensen, L.H.; Horsewell, A.; Jacobsen, C. Addition of fish oil to cream cheese affects lipid oxidation, sensory stability and microstructure. Agriculture 2012, 2, 359–375. [Google Scholar] [CrossRef] [Green Version]
- Jansson, T.; Jensen, S.; Eggers, N.; Clausen, M.; Larsen, L.; Ray, C.; Sundgren, A.; Andersen, H.J.; Bertram, H.C. Volatile component profiles of conventional and lactose-hydrolyzed UHT milk-a dynamic headspace gas chromatography-mass spectrometry study. Dairy Sci. Technol. 2014, 94, 311–325. [Google Scholar] [CrossRef] [Green Version]
- Valero, E.; Miranda, E.; Sanz, J.; Martinex-Castro, I. Automatic Thermal Desorption in GC Analysis of Dairy product volatiles. Chromatographia 1997, 44, 59–64. [Google Scholar] [CrossRef]
- Rabaud, N.E.; Ebeler, S.E.; Ashbaugh, L.L.; Flocchini, R.G. The application of thermal desorption GC/MS with simultaneous olfactory evaluation for the characterization and quantification of odor compounds from a dairy. J. Agric. Food Chem. 2002, 50, 5139–5145. [Google Scholar] [CrossRef] [PubMed]
- Lancas, F.M.; Eugênia, M.; Queiroz, C.; Grossi, P.; Olivares, I.R.B. Recent developments and applications of stir bar sorptive extraction. J. Sep. Sci. 2009, 32, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzi, A.S.; Kilcawley, K.N.; Sheehan, J.J.; O’Sullivan, M.G.; Kennedy, D.; McSweeney, P.L.H.; Rea, M.C. Use of smear bacteria and yeasts to modify flavour and appearance of Cheddar cheese. Intl. Dairy J. 2017, 72, 44–54. [Google Scholar] [CrossRef]
- Imhof, R.; Bosset, J.O. The performance of various capillary columns for the analysis of volatile flavor compounds in dairy products by dynamic headspace gas chromatography. J. High Resolut. Chromatogr. 1994, 17, 25–30. [Google Scholar] [CrossRef]
- Wehrens, R.; Weingart, G.; Mattivi, F. metaMS: An open-source pipeline for GC-MS-based untargeted metabolomics. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 966, 109–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- High, R.; Bremer, P.; Kebede, B.; Eyres, G.T. Comparison of four extraction techniques for the evaluation of volatile compounds in spray-dried New Zealand sheep milk. Molecules 2019, 24, 1917. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, M.A.; Drake, M.A.; Gerard, P.D. Flavor variability and flavor stability of U.S.-Produced whole milk powder. J. Food Sci. 2009, 74, S334–S343. [Google Scholar] [CrossRef]
- Park, C.W.; Drake, M.A. The effect of homogenization pressure on the flavor and flavor stability of whole milk powder. J. Dairy Sci. 2017, 100, 5195–5205. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Su, H.; Song, H.L. Comparison of four extraction methods, SPME, DHS, SAFE, versus SDE, for analysis of flavor compounds in Natto. Food Anal. Methods 2018, 11, 343–354. [Google Scholar] [CrossRef]
- Schiano, A.N.; Benoist, D.M.; Drake, M.A. Short Communication: Comparison of 3 rapid methods for analysis of vitamin degradation compounds in fluid skim milk. J. Dairy Sci. 2019, 102, 4906–4912. [Google Scholar] [CrossRef] [PubMed]
- Schieweck, A.; Gunschera, J.; Varol, D.; Salthammer, T. Analytical procedure for the determination of very volatile organic compounds (C3-C6) in indoor air. Anal. Bioanal. Chem. 2018, 410, 3171–3183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salum, P.; Erbay, E.; Kelebek, H.; Selli, S. Optimization of headspace solid-phase microextraction with different fibers for the anlaysis of volatile compounds of white-brined cheese using response surface methodology. Food Anal. Methods 2017, 10, 1956–1964. [Google Scholar] [CrossRef]
- Prieto, A.; Basauri, O.; Rodil, R.; Usbiaga, A.; Fernandez, L.A.; Etexbarria, N.; Zuloaga, O. Stir-bar sorptive extraction: A view on method optimization, novel applications, limitations and potential solutions. J. Chromatogr. A 2010, 1217, 2642–2666. [Google Scholar] [CrossRef]
- Merkle, S.; Kleeberg, K.K.; Jritsche, J. Recent developments and applications of solid phase microextraction (SPME) in food and environmental analysis—A review. Chromatography 2015, 2, 293–381. [Google Scholar] [CrossRef] [Green Version]
- Barba, C.; Thomas-Danguin, T.; Guichard, E. Comparison of stir bar sorptive extraction in the liquid and vapour phases, solvent-assisted flavour evaporation and headspace solid-phase microextraction for the (non)-targeted analysis of volatiles in fruit juice. LWT Food Sci. Technol. 2017, 85, 334–344. [Google Scholar] [CrossRef]
- Liberto, E.; Bicchi, C.; Cagliero, C.; Cordero, C.; Rubiolo, P.; Sgorbini, B. Headspace Sampling: An “Evergreen” method in constant evolution to characterize food flavors through their volatile fraction. In Advanced Gas Chromatography in Food Analysis, 1st ed.; Tranchida, P.Q., Ed.; The Royal Society of Chemistry: London, UK, 2020; pp. 3–37. [Google Scholar]
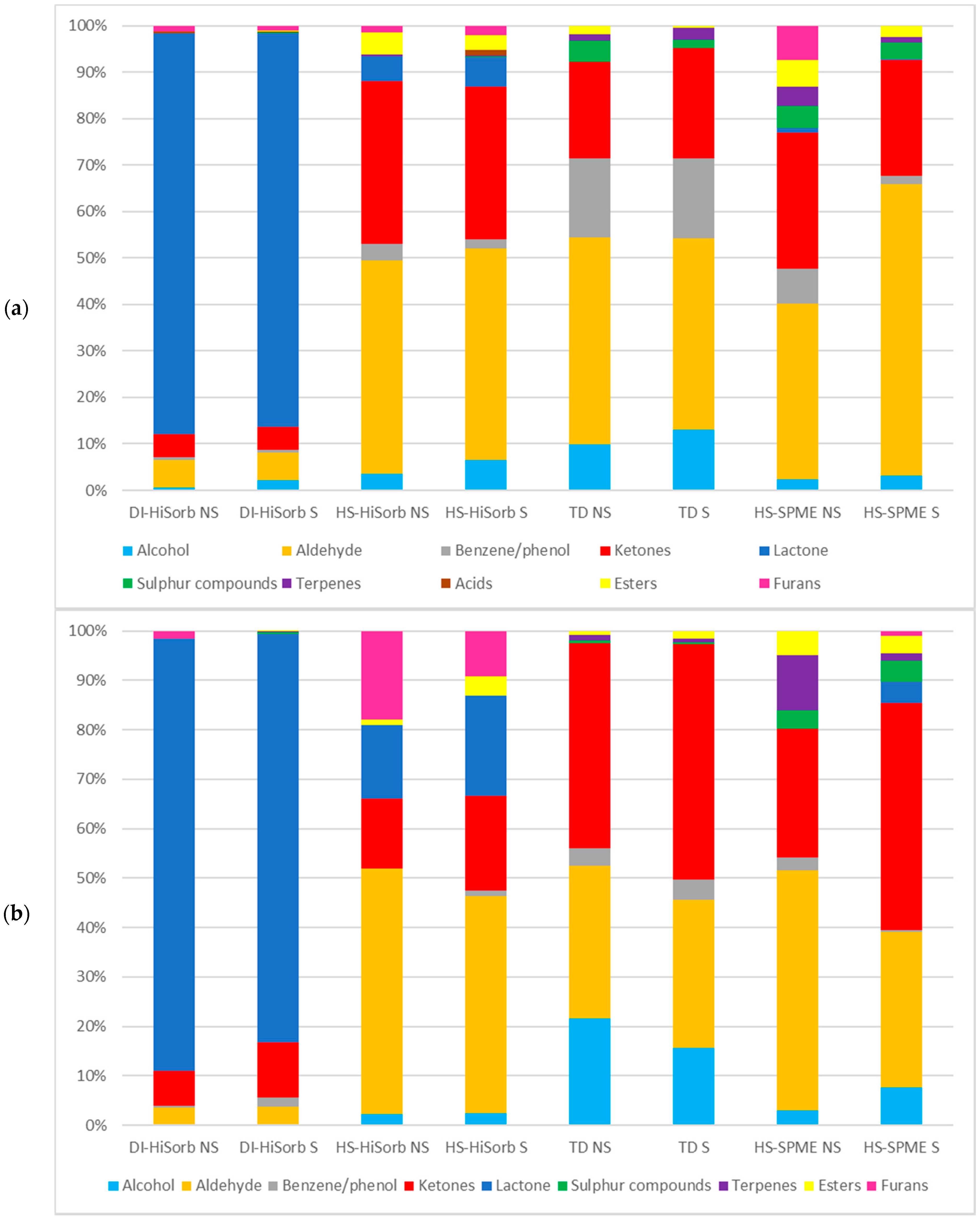
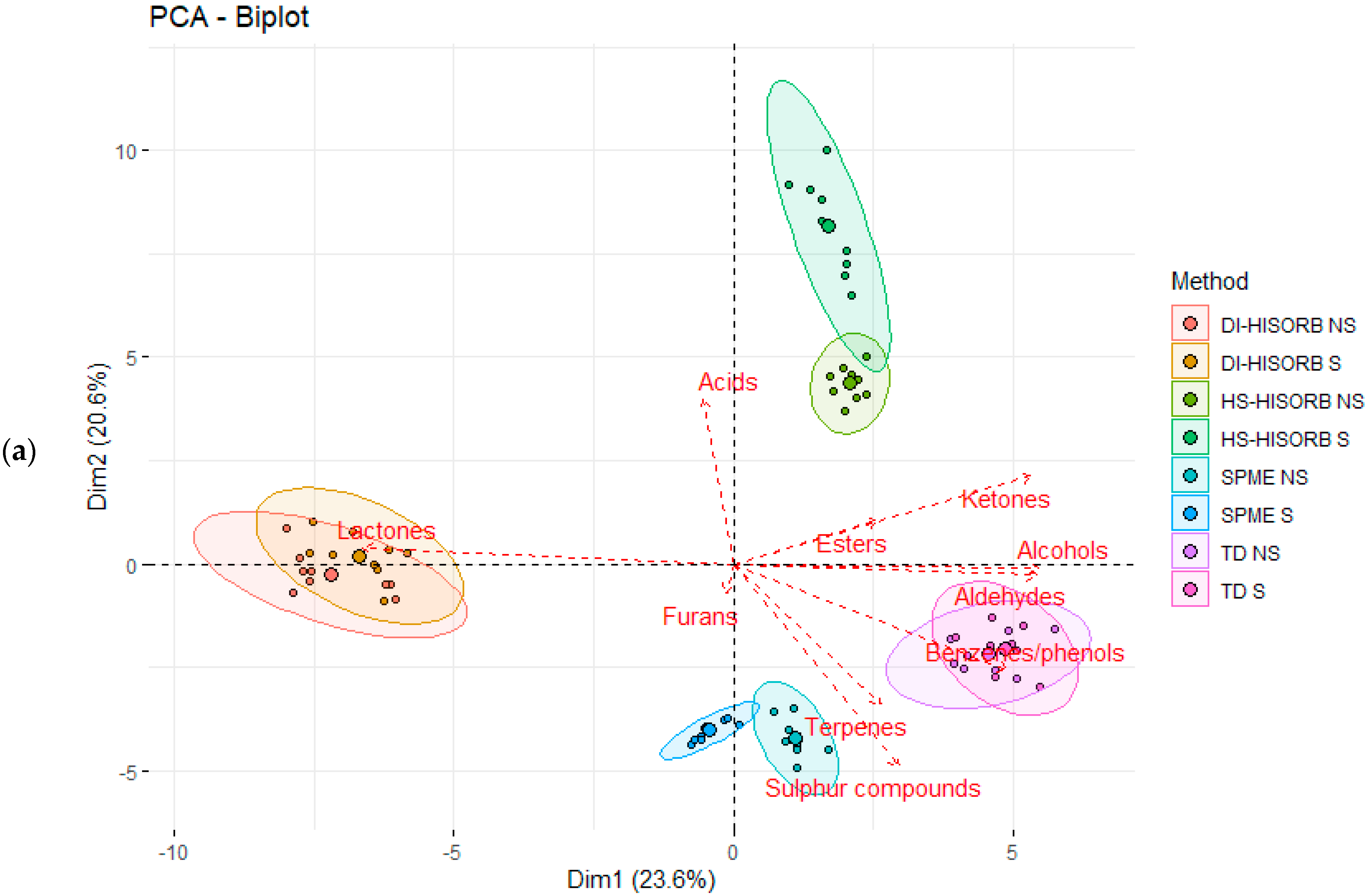
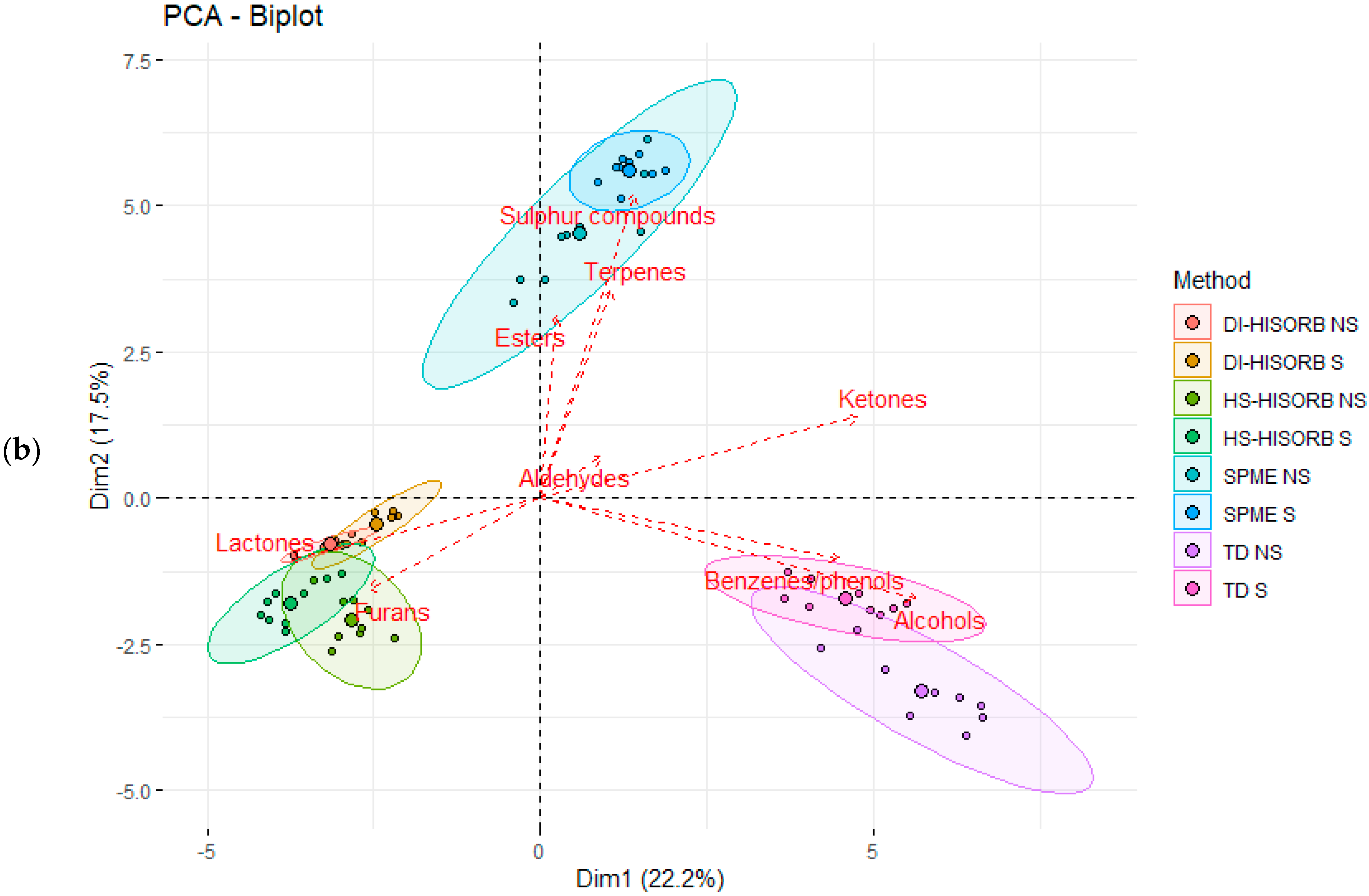
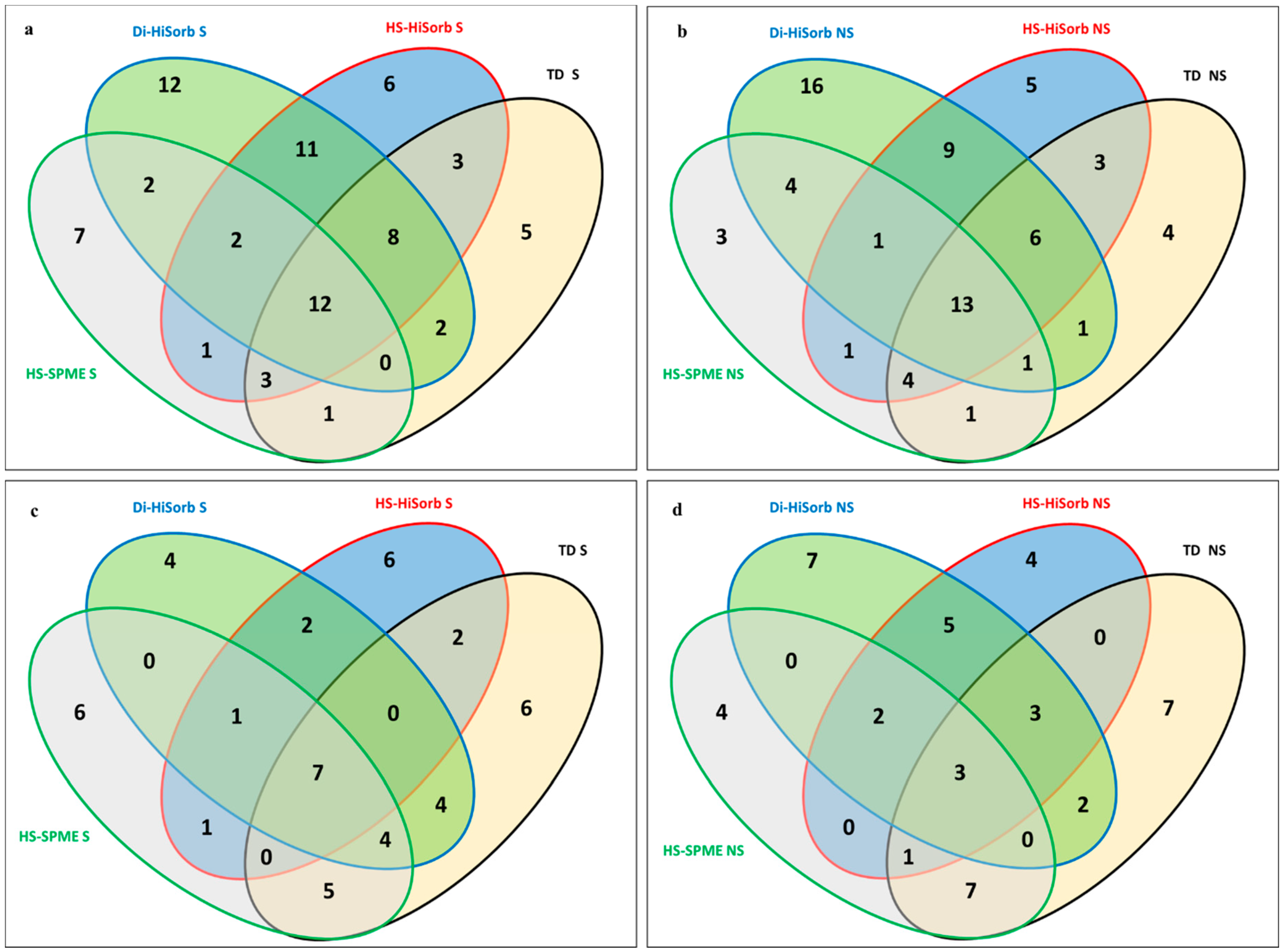
| Code | Description |
|---|---|
| HS-SPME S | Head space solid phase microextraction with salting out |
| HS-SPME NS | Head space solid phase microextraction without salting out |
| TD S | Thermal desorption with salting out |
| TD NS | Thermal desorption without salting out |
| DI-HiSorb S | Direct Immersion HiSorb with salting out |
| DI-HiSorb NS | Direct Immersion HiSorb without salting out |
| HS-HiSorb S | Head space HiSorb with salting out |
| HS-HiSorb NS | Head space HiSorb without salting out |
| (a) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RI | Identification | DI-HISorb S | DI-HISorb NS | HS-HiSorb S | HS-HiSorb NS | TD S | TD NS | HS-SPME S | HS-SPME NS | ||||||||||||
| No | Compound | CAS | ORI | REF | Methods | % Area | % Stdev | % Area | % Stdev | % Area | % Stdev | % Area | % Stdev | % Area | % Stdev | % Area | % Stdev | % Area | % Stdev | % Area | % Stdev |
| Alcohols | |||||||||||||||||||||
| 1 | Ethanol | 64-17-5 | 436 | 426 | MS, RI, STD | 0.73 | 0.16 | 0.27 | 0.26 | 0.72 | 0.31 | 0.53 | 0.38 | nd | nd | nd | nd | nd | nd | nd | nd |
| 2 | 1-Butanol | 71-36-3 | 655 | 675 | MS, RI, STD | nd | nd | nd | nd | 1.06 | 0.35 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 3 | 1-Pentanol | 71-41-0 | 762 | 768 | MS, RI, STD | 0.47 | 0.22 | 0.11 | 0.04 | 1.3 | 0.24 | 1.44 | 1.03 | 3.01 | 0.63 | 2.07 | 0.84 | 2.33 | 0.43 | 1.68 | 0.7 |
| 4 | 1-Hexanol | 111-27-3 | 865 | 868 | MS, RI, STD | 0.17 | 0.07 | nd | nd | 1.03 | 0.46 | 0.72 | 0.025 | 1.17 | 0.37 | 0.46 | 0.23 | 0.87 | 0.33 | 0.77 | 0.3 |
| 5 | 2-Butoxy-ethanol | 111-76-2 | 903 | 901 | MS, RI | 0.12 | 0.04 | nd | nd | 0.47 | 0.1 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 6 | 1-Octen-3-ol | 3391-86-4 | 977 | 981 | MS, RI | 0.07 | 0.04 | nd | nd | nd | nd | 0.39 | 0.31 | nd | nd | nd | nd | nd | nd | nd | nd |
| 7 | 2-Ethyl-1-hexanol | 104-76-7 | 1026 | 1030 | MS, RI, STD | 0.31 | 0.14 | 0.25 | 0.07 | 1.96 | 0.37 | nd | nd | 8.61 | 1.67 | 7.2 | 1.37 | nd | nd | nd | nd |
| 8 | 1-Octanol | 111-87-5 | 1067 | 1071 | MS, RI, STD | 0.22 | 0.15 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 9 | α-Terpineol | 10482-56-1 | 1198 | 1192 | MS, RI | nd | nd | nd | nd | nd | nd | 0.57 | 0.28 | 0.32 | 0.07 | 0.26 | 0.04 | nd | nd | nd | nd |
| Aldehydes | |||||||||||||||||||||
| 10 | Acrolein | 107-02-8 | 449 | 470 | MS, RI | 0.44 | 0.22 | 1.88 | 0.41 | nd | nd | 1.26 | 0.61 | nd | nd | nd | nd | nd | nd | nd | nd |
| 11 | Butanal | 123-72-8 | 578 | 596 | MS, RI, STD | 0.22 | 0.14 | 0.09 | 0.09 | nd | nd | nd | nd | nd | nd | nd | nd | 5 | 3.23 | nd | nd |
| 12 | 3-Methyl butanal | 590-86-3 | 647 | 654 | MS, RI, STD | nd | nd | nd | nd | 0.61 | 0.21 | 0.56 | 0.19 | 1.31 | 0.41 | 1.33 | 0.56 | 4.58 | 1.41 | 5.35 | 2.65 |
| 13 | Pentanal | 110-62-3 | 697 | 697 | MS, RI, STD | 0.9 | 0.84 | 0.34 | 0.11 | nd | nd | nd | nd | nd | nd | nd | nd | 32.05 | 12.67 | nd | nd |
| 14 | Hexanal | 66-25-1 | 799 | 801 | MS, RI, STD | 0.94 | 0.48 | 0.65 | 0.26 | 7.42 | 4.64 | 7.45 | 5.21 | 6.64 | 1.66 | 6.95 | 2.62 | 11.43 | 8.06 | 16.66 | 9.82 |
| 15 | 4-Heptenal,(Z)- | 6728-31-0 | 895 | 902 | MS, RI | nd | nd | nd | nd | 0.45 | 0.08 | nd | nd | nd | nd | nd | nd | 0.32 | 0.13 | nd | nd |
| 16 | Heptanal | 111-71-7 | 900 | 901 | MS, RI, STD | 0.69 | 0.15 | 0.59 | 0.08 | 7.11 | 1.68 | 7.73 | 2.42 | 8.48 | 1.38 | 9.38 | 2.32 | 5.87 | 1.93 | 9.89 | 2.19 |
| 17 | Benzaldehyde | 100-52-7 | 967 | 960 | MS, RI, STD | 0.15 | 0.04 | 0.2 | 0.05 | 1.3 | 0.6 | 1.86 | 0.4 | 1.13 | 0.37 | 1.81 | 1.18 | 0.34 | 0.14 | 1.26 | 0.12 |
| 18 | Octanal | 124-13-0 | 1002 | 1004 | MS, RI, STD | 0.31 | 0.08 | 0.29 | 0.06 | 3.21 | 0.94 | 3.08 | 1.09 | 3.49 | 0.99 | 3.78 | 2.13 | 0.71 | 0.12 | 1.77 | 0.46 |
| 19 | Benzeneacetaldehyde | 122-78-1 | 1048 | 1048 | MS, RI, STD | nd | nd | 0.04 | 0.01 | nd | nd | 1.43 | 0.64 | nd | nd | nd | nd | nd | nd | nd | nd |
| 20 | 2-Octenal,(E )- | 2548-87-0 | 1059 | 1057 | MS, RI | 0.07 | 0.01 | 0.1 | 0.04 | nd | nd | nd | nd | 0.22 | 0.09 | 0.3 | 0.2 | nd | nd | nd | nd |
| 21 | Nonanal | 124-19-6 | 1103 | 1106 | MS, RI, STD | 1.27 | 0.39 | 1.18 | 0.21 | 17.52 | 3.1 | 16.91 | 3.47 | 17.93 | 2.58 | 18.26 | 3.65 | 1.5 | 0.24 | 3.27 | 0.32 |
| 22 | 2-Nonenal,(E )- | 18829-56-6 | 1160 | 1160 | MS, RI | 0.17 | 0.11 | 0.11 | 0.04 | 0.41 | 0.13 | nd | nd | 0.22 | 0.15 | 0.2 | 0.06 | nd | nd | nd | nd |
| 23 | Decanal | 112-31-2 | 1204 | 1205 | MS, RI, STD | 0.36 | 0.1 | 0.28 | 0.1 | 5.27 | 1.96 | 4.57 | 1.4 | 1.24 | 0.46 | 1.76 | 0.79 | nd | nd | nd | nd |
| 24 | 2-Decenal,(E )- | 3913-81-3 | 1262 | 1266 | MS, RI | 0.12 | 0.09 | 0.05 | 0.01 | 1.32 | 0.29 | nd | nd | 0.11 | 0.09 | 0.22 | 0.21 | nd | nd | nd | nd |
| 25 | Undecanal | 112-44-7 | 1306 | 1309 | MS, RI, STD | 0.07 | 0.02 | 0.05 | 0.02 | 0.49 | 0.06 | 0.48 | 0.11 | 0.12 | 0.03 | 0.21 | 0.06 | nd | nd | nd | nd |
| 26 | 2-Undecenal | 2463-77-6 | 1364 | 1350 | MS, RI, STD | 0.2 | 0.09 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 27 | Dodecanal | 112-54-9 | 1407 | 1401 | MS, RI, STD | 0.12 | 0.03 | 0.11 | 0.03 | 0.41 | 0.03 | 0.53 | 0.1 | 0.25 | 0.07 | 0.3 | 0.17 | nd | nd | nd | nd |
| Benzene/Phenols | |||||||||||||||||||||
| 28 | Benzene | 71-43-2 | 658 | 669 | MS, RI, STD | 0.36 | 0.17 | 0.31 | 0.2 | nd | nd | 1.33 | 0.47 | 6.77 | 3.33 | 8.08 | 5.53 | nd | nd | 6.2 | 2.7 |
| 29 | Toluene | 108-88-3 | 766 | 763 | MS, RI, STD | 0.1 | 0.02 | 0.09 | 0.05 | 0.51 | 0.24 | 0.6 | 0.12 | 4.54 | 0.73 | 3.64 | 1.08 | 1.72 | 0.67 | 1.6 | 1.07 |
| 30 | p-Xylene | 106-42-3 | 870 | 867 | MS, RI, STD | nd | nd | nd | nd | nd | nd | 0.77 | 0.78 | 3.39 | 0.6 | 3.14 | 0.8 | nd | nd | nd | nd |
| 31 | o-xylene | 95-47-6 | 898 | 900 | MS, RI, STD | nd | nd | nd | nd | nd | nd | nd | nd | 1.74 | 0.75 | 1.23 | 0.34 | nd | nd | nd | nd |
| 32 | Benzyl alcohol | 108-95-2 | 974 | 995 | MS, RI, STD | nd | nd | nd | nd | 0.61 | 0.13 | nd | nd | nd | nd | 0.23 | 0.09 | nd | nd | nd | nd |
| 33 | Phenol | 100-51-6 | 1035 | 1037 | MS, RI, STD | 0.16 | 0.1 | 0.12 | 0.04 | 0.79 | 0.23 | 0.76 | 0.17 | 0.73 | 0.31 | 0.66 | 0.12 | nd | nd | nd | nd |
| Ketones | |||||||||||||||||||||
| 34 | Acetone | 67-64-1 | 451 | 496 | MS, RI, STD | 1.62 | 0.61 | nd | nd | 8.11 | 2.62 | 6.5 | 1.02 | nd | nd | nd | nd | 5.4 | 0.65 | nd | nd |
| 35 | Diacetyl | 431-03-8 | 548 | 596 | MS, RI, STD | nd | nd | nd | nd | nd | nd | 1.75 | 0.57 | 3.72 | 1.67 | 3.1 | 0.68 | nd | nd | nd | nd |
| 36 | Hydroxyacetone | 116-09-6 | 663 | 657 | MS, RI | 0.68 | 0.31 | 1.03 | 0.71 | 0.12 | 0.11 | 2.91 | 2.49 | nd | nd | nd | nd | nd | nd | nd | nd |
| 37 | 2-Pentanone | 107-87-9 | 684 | 687 | MS, RI, STD | nd | nd | 0.26 | 0.08 | 2.19 | 0.3 | 5.38 | 3.13 | 4.1 | 0.53 | 3.46 | 0.59 | 1.21 | 1.16 | 5.52 | 0.91 |
| 38 | 2-Butanone | 108-10-1 | 733 | 740 | MS, RI, STD | nd | nd | nd | nd | 1.82 | 0.39 | 1.25 | 0.53 | 10.04 | 1.65 | 7.3 | 0.83 | nd | nd | 4.41 | 1.19 |
| 39 | Methyl Isobutyl Ketone | 108-10-1 | 735 | 740 | MS, RI, STD | nd | nd | 0.09 | 0.02 | 0.58 | 0.11 | 0.5 | 0.23 | 1.48 | 0.8 | 0.93 | 0.23 | 0.67 | 0.13 | 0.76 | 0.46 |
| 40 | 2-Hexanone | 591-78-6 | 789 | 790 | MS, RI, STD | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.67 | 0.04 | nd | nd |
| 41 | 2-Heptanone | 110-43-0 | 887 | 891 | MS, RI, STD | 1.23 | 0.28 | 1.14 | 0.27 | 11.95 | 0.97 | 10.34 | 3.18 | 2.62 | 0.41 | 3.85 | 0.6 | 13.12 | 2.71 | 16.17 | 6.16 |
| 42 | 2,3-Octanedione | 585-25-1 | 981 | 967 | MS, RI, STD | 0.17 | 0.05 | 0.16 | 0.04 | 1.56 | 0.37 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 43 | 3-Octanone | 106-68-3 | 982 | 989 | MS, RI | 0.05 | 0.02 | 0.04 | 0.02 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 44 | 2-Octanone | 111-13-7 | 988 | 992 | MS, RI, STD | nd | nd | 0.07 | 0.02 | 0.39 | 0.16 | 0.45 | 0.07 | 0.56 | 0.13 | 0.55 | 0.28 | nd | nd | nd | nd |
| 45 | 3,5-Octadien-2-one,(E,E)- | 30086-02-3 | 1069 | 1072 | MS, RI | 0.46 | 0.06 | 0.37 | 0.06 | 1.44 | 0.38 | 1.33 | 0.22 | nd | nd | nd | nd | nd | nd | nd | nd |
| 46 | Acetophenone | 98-86-2 | 1070 | 1079 | MS, RI, STD | 0.09 | 0.02 | nd | nd | 0.65 | 0.13 | 0.52 | 0.07 | 0.68 | 0.25 | nd | nd | nd | nd | nd | nd |
| 47 | 3,5-Octadien-2-one | 38284-27-4 | 1076 | 1072 | MS, RI | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.69 | 0.48 | 0.92 | 0.23 | 0.45 | 0.13 |
| 48 | 2-Nonanone | 821-55-6 | 1088 | 1094 | MS, RI, STD | 0.3 | 0.09 | 0.51 | 0.12 | 3.19 | 0.19 | 3.43 | 0.18 | 0.54 | 0.12 | 0.69 | 0.05 | 0.93 | 0.1 | 2.33 | 0.21 |
| 49 | 2-Undecanone | 112-12-9 | 1295 | 1294 | MS, RI, STD | nd | nd | 0.08 | 0.02 | nd | nd | 0.77 | 0.08 | nd | nd | 0.15 | 0.02 | 1.64 | 0.26 | nd | nd |
| 50 | 2-Tridecanone | 593-08-8 | 1494 | 1480 | MS, RI, STD | nd | nd | 0.27 | 0.14 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 51 | 2-Pentadecanone | 2345-28-0 | 1695 | 1689 | MS, RI, STD | 0.41 | 0.4 | 0.4 | 0.26 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 52 | 2-Heptadecanone | 2922-51-2 | 1897 | 1878 | MS, RI, STD | nd | nd | 0.54 | 0.09 | 0.98 | 0.39 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Lactones | |||||||||||||||||||||
| 53 | γ-Crotonolactone | 497-23-4 | 912 | 916 | MS, RI | 0.21 | 0.11 | 0.19 | 0.11 | 0.89 | 0.3 | 0.92 | 0.21 | nd | nd | nd | nd | nd | nd | nd | nd |
| 54 | δ-Caprolactone | 823-22-3 | 1097 | 1084 | MS, RI | nd | nd | 0.1 | 0.03 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 55 | δ-Octalactone | 698-76-0 | 1288 | 1288 | MS, RI | nd | nd | 0.63 | 0.4 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 56 | δ-Nonalactone | 3301-94-8 | 1394 | 1404 | MS, RI | 0.22 | 0.1 | 0.14 | 0.03 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 57 | δ-Decalactone | 705-86-2 | 1502 | 1506 | MS, RI | 31.87 | 2.9 | 28.34 | 4.87 | 3.81 | 0.5 | 3.06 | 0.45 | nd | nd | nd | nd | 0.29 | 0.05 | 1.05 | 0.22 |
| 58 | δ-undecalactone | 710-04-3 | 1602 | 1627 | MS, RI | 0.08 | 0.02 | 1.68 | 0.63 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 59 | (Z) Dairy lactone | 18679-18-0 | 1664 | 1675 | MS, RI | 0.64 | 0.23 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 60 | γ-Dodecalactone | 2305-(05)-7 | 1685 | 1674 | MS, RI | 8.56 | 10.18 | 13.9 | 15.58 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 61 | δ-Dodecalactone | 713-95-1 | 1717 | 1719 | MS, RI | 29.02 | 3.99 | 40.05 | 7.86 | 1.52 | 0.25 | 1.37 | 0.21 | nd | nd | nd | nd | nd | nd | nd | nd |
| 62 | δ-Tridecalactone | 7370-92-5 | 1824 | 1778 | MS, RI | 0.55 | 0.18 | 1.21 | 0.37 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 63 | δ-Tetradecalactone | 2721-22-4 | 1930 | 1938 | MS, RI | 13.46 | 1.16 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Sulfurous Compounds | |||||||||||||||||||||
| 64 | Dimethyl sulfide | 75-18-3 | 519 | 510 | MS, RI, STD | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 1.68 | 0.65 | 3.93 | 1.68 |
| 65 | Dimethyl disulfide | 624-92-0 | 743 | 739 | MS, RI, STD | 0.09 | 0.02 | 0.1 | 0.02 | 0.19 | 0.09 | nd | nd | 1.74 | 0.32 | 4.62 | 1 | 1.7 | 0.86 | 0.85 | 0.15 |
| Terpenes | |||||||||||||||||||||
| 66 | α-Pinene | 80-56-8 | 939 | 930 | MS, RI, STD | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.58 | 0.47 | 1.93 | 1.24 |
| 67 | 3-Carene | 13466-78-9 | 1015 | 1009 | MS, RI, STD | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.49 | 0.17 | 1.85 | 1.02 |
| 68 | D-Limonene | 5989-27-5 | 1032 | 1022 | MS, RI, STD | 0.01 | 0.02 | 0.05 | 0.01 | 0.33 | 0.06 | 0.28 | 0.06 | 2.52 | 2.55 | 1.18 | 0.89 | 0.27 | 0.04 | 0.3 | 0.13 |
| 69 | Longifolene | 475-20-7 | 1439 | 1432 | MS, RI, STD | nd | nd | nd | nd | nd | nd | nd | nd | 0.14 | 0.03 | 0.17 | 0.08 | nd | nd | nd | nd |
| Acids | |||||||||||||||||||||
| 70 | Acetic acid | 64-19-7 | 535 | 629 | MS, RI, STD | 0.21 | 0.05 | 0.23 | 0.14 | 1.17 | 1.3 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Esters | |||||||||||||||||||||
| 71 | Methyl butanoate | 623-42-7 | 716 | 724 | MS, RI, STD | nd | nd | nd | nd | nd | nd | 1.05 | 0.3 | 0.22 | 0.04 | 1.16 | 0.4 | 1.5 | 0.55 | 2.44 | 1.14 |
| 72 | Methyl pyruvate | 108-10-1 | 735 | 740 | MS, RI | 0.29 | 0.19 | 0.16 | 0.08 | 0.54 | 0.36 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 73 | Ethylbenzene | 100-41-4 | 859 | 851 | MS, RI, STD | nd | nd | nd | nd | 0.53 | 0.11 | nd | nd | 0.22 | 0.15 | 0.68 | 0.41 | nd | nd | nd | nd |
| 74 | Methy hexanoate | 106-70-7 | 918 | 922 | MS, RI | nd | nd | nd | nd | nd | nd | 1.31 | 0.41 | nd | nd | nd | nd | 0.83 | 0.1 | 3.36 | 1.88 |
| 75 | Methyl octanoate | 111-11-5 | 1117 | 1126 | MS, RI | nd | nd | nd | nd | nd | nd | 0.53 | 0.43 | nd | nd | nd | nd | nd | nd | nd | nd |
| 76 | Methyl hexadecanoate | 112-39-0 | 1915 | 1909 | MS, RI | nd | nd | nd | nd | 2.16 | 0.77 | 2 | 0.32 | nd | nd | nd | nd | nd | nd | nd | nd |
| Furans | |||||||||||||||||||||
| 77 | 2-Methyl-furan | 534-22-5 | 602 | 604 | MS, RI, STD | nd | nd | 0.17 | 0.04 | 0.52 | 0.42 | nd | nd | nd | nd | nd | nd | nd | nd | 7.59 | 4.83 |
| 78 | 2-Pentyl-furan | 3777-69-3 | 989 | 991 | MS, RI, STD | nd | nd | 0.05 | 0.01 | 0.43 | 0.19 | 0.44 | 0.08 | nd | nd | nd | nd | nd | nd | nd | nd |
| 79 | 2-Furanmethanol | 98-0-0 | 851 | 850 | MS, RI, STD | 0.65 | 0.59 | 0.59 | 0.75 | 0.49 | 0.35 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 80 | Furfural | 98-01-1 | 833 | 852 | MS, RI, STD | 0.39 | 0.19 | 0.36 | 0.35 | 0.51 | 0.11 | 0.93 | 0.24 | nd | nd | nd | nd | nd | nd | nd | nd |
| (b) | |||||||||||||||||||||
| Alcohols | |||||||||||||||||||||
| 1 | α-Terpineol | 10482-56-1 | 1206 | 1192 | MS, RI | nd | nd | nd | nd | nd | nd | nd | nd | 0.73 | 0.29 | nd | nd | nd | nd | nd | nd |
| 2 | 1-Pentanol | 71-41-0 | 1262 | 1250 | MS, RI, STD | nd | nd | nd | nd | 2.34 | 1.86 | 2.17 | 3.35 | nd | nd | nd | nd | nd | nd | nd | nd |
| 3 | 1-Hexanol | 111-27-3 | 1365 | 1355 | MS, RI, STD | nd | nd | nd | nd | nd | nd | nd | nd | 1.11 | 0.4 | 0.88 | 0.38 | 1.44 | 0.86 | 0.87 | 0.91 |
| 4 | 1-Penten-3-ol | 111-27-3 | 1365 | 1355 | MS, RI | nd | nd | nd | nd | nd | nd | nd | nd | 1.89 | 1.02 | 1.25 | 0.95 | 2.06 | 1.76 | nd | nd |
| 5 | 2-Butoxy-ethanol | 111-76-2 | 1423 | 1405 | MS, RI | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 1.45 | 1.4 | nd | nd | nd | nd |
| 6 | 1-Octen-3-ol | 3391-86-4 | 1460 | 1450 | MS, RI | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.67 | 0.1 | nd | nd | nd | nd |
| 7 | 2-Ethyl-1-hexanol | 71-41-0 | 1502 | 1491 | MS, RI, STD | nd | nd | nd | nd | nd | nd | nd | nd | 12.02 | 1.89 | 15.69 | 3.3 | 4.18 | 0.51 | 2.18 | 0.81 |
| 8 | 1-Nonanol | 143-08-8 | 1673 | 1660 | MS, RI, STD | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 1.81 | 0.33 | nd | nd | nd | nd |
| 9 | Acrolein | 107-02-8 | 449 | 470 | MS, RI | 0.2 | 0.24 | 0.31 | 0.05 | 4.55 | 2.02 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 10 | Butanal | 123-72-8 | 578 | 596 | MS, RI, STD | nd | nd | 0.12 | 0.03 | nd | nd | nd | nd | 1.04 | 0.08 | 1.3 | 0.24 | nd | nd | nd | nd |
| 11 | 3-Methyl-butanal | 590-86-3 | 652 | 654 | MS, RI, STD | 0.28 | 0.05 | nd | nd | nd | nd | nd | nd | 0.59 | 0.48 | 1.08 | 0.65 | 2.2 | 1.13 | 17.4 | 17.85 |
| 12 | Pentanal | 110-62-3 | 994 | 979 | MS, RI, STD | 0.68 | 0.26 | 0.52 | 0.23 | 1.68 | 1.05 | 2.22 | 1.93 | 7.38 | 2.74 | nd | nd | 11.23 | 5.97 | nd | nd |
| 13 | Hexanal | 66-25-1 | 1098 | 1083 | MS, RI, STD | nd | nd | nd | nd | nd | nd | nd | nd | 2.6 | 0.64 | 3.87 | 0.58 | 5.9 | 0.33 | 4.55 | 6.02 |
| 14 | Heptanal | 111-71-7 | 1204 | 1184 | MS, RI, STD | 0.57 | 0.1 | 0.4 | 0.13 | 9.62 | 4.17 | 13.91 | 6.8 | 4.01 | 0.92 | 4.81 | 0.89 | 10.47 | 3.64 | 24.2 | 10.48 |
| 15 | Octanal | 124-13-0 | 1310 | 1289 | MS, RI, STD | nd | nd | 0.4 | 0.06 | 2.34 | 0.43 | 4.32 | 0.98 | 1.85 | 0.78 | 2.43 | 1.09 | nd | nd | nd | nd |
| 16 | Nonanal | 124-19-6 | 1416 | 1391 | MS, RI, STD | 1.02 | 0.24 | 0.89 | 0.31 | 1.27 | 0.96 | 8.41 | 6.43 | 10.12 | 3.05 | 12.09 | 2.97 | 0.62 | 0.37 | nd | nd |
| 17 | Decanal | 112-31-2 | 1523 | 1498 | MS, RI, STD | 0.48 | 0.12 | 0.61 | 0.31 | 16.88 | 9.22 | 8.54 | 12.98 | 1.44 | 0.24 | 2.99 | 1.03 | nd | nd | nd | nd |
| 18 | Benzaldehyde | 100-52-7 | 1570 | 1520 | MS, RI, STD | 0.52 | 0.1 | 0.33 | 0.07 | 5.63 | 4.25 | 4.56 | 4.43 | 1.21 | 0.31 | 2.55 | 0.45 | 0.9 | 0.11 | 2.28 | 1.06 |
| 19 | Dodecanal | 112-54-9 | 1733 | 1710 | MS, RI, STD | nd | nd | nd | nd | 0.31 | 0.47 | 6.08 | 1.32 | nd | nd | nd | nd | nd | nd | nd | nd |
| Benzene/Phenols | |||||||||||||||||||||
| 20 | Toluene | 108-88-3 | 766 | 763 | MS, RI, STD | 1.1 | 0.17 | nd | nd | nd | nd | nd | nd | 0.56 | 0.16 | 0.75 | 0.2 | 0.53 | 0.41 | 2.62 | 0.96 |
| 21 | Benzene | 71-43-2 | 955 | 957 | MS, RI, STD | 0.34 | 0.2 | 0.26 | 0.17 | nd | nd | nd | nd | 1.49 | 0.37 | 2 | 0.72 | nd | nd | nd | nd |
| 22 | p-Xylene | 106-42-3 | 1155 | 1138 | MS, RI, STD | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.86 | 0.17 | nd | nd | nd | nd |
| 23 | o-Xylene | 95-47-6 | 1156 | 1186 | MS, RI, STD | 0.35 | 0.04 | nd | nd | nd | nd | nd | nd | 2.02 | 1.21 | nd | nd | nd | nd | nd | nd |
| 24 | Phenol | 108-95-2 | 2042 | 2039 | MS, RI, STD | nd | nd | nd | nd | 1.14 | 1.37 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Ketones | |||||||||||||||||||||
| 25 | Hydroxyacetone | 116-09-6 | 663 | 657 | MS, RI | 2.68 | 1.68 | 2.49 | 1.14 | nd | nd | 0.51 | 0.79 | nd | nd | nd | nd | nd | nd | nd | nd |
| 26 | 2-Pentanone | 107-87-9 | 684 | 687 | MS, RI, STD | 0.74 | 0.43 | nd | nd | nd | nd | nd | nd | 5.18 | 3.58 | nd | nd | nd | nd | 8.65 | 2.87 |
| 27 | Methyl isopropyl ketone | 108-10-1 | 735 | 740 | MS, RI, STD | nd | nd | 0.45 | 0.18 | nd | nd | nd | nd | nd | nd | nd | nd | 8.97 | 1.96 | nd | nd |
| 28 | Acetone | 67-64-1 | 825 | 819 | MS, RI, STD | 1.6 | 0.62 | 1.17 | 0.33 | 2.89 | 1.09 | 3.46 | 1.67 | 26.04 | 7.92 | 29.76 | 8.27 | 9.61 | 2.67 | 6.07 | 5.05 |
| 29 | 2-Heptanone | 110-43-0 | 887 | 891 | MS, RI, STD | 3.04 | 1.15 | 0.36 | 0.1 | 1.44 | 0.51 | 1.84 | 0.79 | 6.33 | 1.89 | nd | nd | 17.94 | 4.91 | nd | nd |
| 30 | 2-Butanone | 108-10-1 | 913 | 907 | MS, RI, STD | 0.28 | 0.17 | nd | nd | nd | nd | nd | nd | 6.88 | 2.11 | 8.38 | 2.44 | 5.97 | 4.05 | 5.39 | 1.2 |
| 31 | Acetophenone | 98-86-2 | 1070 | 1079 | MS, RI, STD | 0.43 | 0.07 | nd | nd | nd | nd | nd | nd | 0.7 | 0.22 | nd | nd | nd | nd | nd | nd |
| 32 | 2,3-Pentanedione | 600-14-6 | 1073 | 1058 | MS, RI | nd | nd | nd | nd | nd | nd | nd | nd | 0.82 | 0.34 | 0.95 | 0.33 | nd | nd | nd | nd |
| 33 | 2-Nonanone | 821-55-6 | 1410 | 1390 | MS, RI, STD | 1.13 | 0.56 | 0.98 | 0.23 | nd | nd | nd | nd | 0.95 | 0.11 | 1.8 | 0.26 | 2.53 | 0.62 | 3.63 | 1.59 |
| 34 | 3,5-Octadien-2-one | 38284-27-4 | 1549 | 1522 | MS, RI | nd | nd | nd | nd | 11.26 | 11.15 | 7.02 | 5.4 | 0.95 | 0.67 | 0.83 | 0.28 | nd | nd | 2.29 | 0.94 |
| 35 | 3,5-Octadien-2-one,(E,E)- | 30086-02-3 | 1551 | 1570 | MS, RI | 1.38 | 0.83 | 1 | 0.47 | 2.84 | 2.36 | 0.66 | 1.32 | nd | nd | nd | nd | 0.92 | 0.74 | nd | nd |
| 36 | 2-Undecanone | 112-12-9 | 1622 | 1598 | MS, RI, STD | nd | nd | 0.68 | 0.17 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Lactones | |||||||||||||||||||||
| 37 | δ-Caprolactone | 823-22-3 | 1864 | 1791 | MS, RI | 0.25 | 0.24 | 0.33 | 0.09 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 38 | δ-Octalactone | 698-76-0 | 2037 | 1976 | MS, RI | nd | nd | nd | nd | 1.53 | 2.29 | nd | nd | nd | nd | nd | nd | 4.21 | 0.26 | nd | nd |
| 39 | δ-Decalactone | 705-86-2 | 2242 | 2190 | MS, RI | nd | nd | nd | nd | 12.46 | 3.67 | 14.39 | 3.58 | nd | nd | nd | nd | nd | nd | nd | nd |
| 40 | δ-Dodecalactone | 713-95-1 | 2570 | 2436 | MS, RI | 62.94 | 1.99 | 62.33 | 1.64 | 5.56 | 4.5 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 41 | δ-Tetradecalactone | 7370-92-5 | 2892 | 2688 | MS, RI | 19.61 | 1.57 | 24.59 | 2.66 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Sulfurous Compounds | |||||||||||||||||||||
| 42 | Dimethyl disulfide | 624-92-0 | 743 | 739 | MS, RI, STD | 0.38 | 0.04 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 43 | Dimethyl sulfide | 75-18-3 | 755 | 754 | MS, RI, STD | nd | nd | nd | nd | nd | nd | nd | nd | 0.36 | 0.45 | 0.69 | 0.34 | 4.24 | 2 | 3.72 | 0.91 |
| Terpenes | |||||||||||||||||||||
| 44 | α-Pinene | 80-56-8 | 1035 | 1028 | MS, RI, STD | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.9 | 0.22 | 2.09 | 1.66 |
| 45 | 3-Carene | 13466-78-9 | 1152 | 1147 | MS, RI, STD | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.64 | 0.19 | 1.45 | 1.94 |
| 46 | D-Limonene | 5989-27-5 | 1218 | 1200 | MS, RI, STD | nd | nd | 0.16 | 0.07 | nd | nd | nd | nd | 0.8 | 0.41 | 1.1 | 0.27 | nd | nd | 7.72 | 4.94 |
| Esters | |||||||||||||||||||||
| 47 | Methyl butanoate | 623-42-7 | 1001 | 982 | MS, RI, STD | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 2.46 | 0.87 | 4.9 | 2.72 |
| 48 | Butyl acetate | 123-86-4 | 1087 | 1074 | MS, RI, STD | nd | nd | nd | nd | nd | nd | nd | nd | 0.93 | 0.43 | nd | nd | nd | nd | nd | nd |
| 49 | Ethylbenzene | 100-41-4 | 1120 | 1129 | MS, RI, STD | 0.06 | 0.01 | nd | nd | nd | nd | nd | nd | 0.66 | 0.06 | 0.8 | 0.11 | nd | nd | nd | nd |
| 50 | Methyl hexadecanoate | 112-39-0 | 1915 | 1909 | MS, RI | nd | nd | nd | nd | 3.67 | 1.28 | 1.13 | 1.92 | nd | nd | nd | nd | nd | nd | nd | nd |
| 51 | Methyl octanoate | 111-11-5 | 1407 | 1385 | MS, RI, STD | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 1.05 | 0.07 | nd | nd |
| Furans | |||||||||||||||||||||
| 52 | 2-Ethyl-furan | 3208-16-0 | 968 | 950 | MS, RI, STD | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 1.01 | 0.41 | nd | nd |
| 53 | Furfural | 98-01-1 | 1497 | 1461 | MS, RI, STD | nd | nd | 0.8 | 0.36 | 8.91 | 7.71 | 17.33 | 10.71 | nd | nd | nd | nd | nd | nd | nd | nd |
| 54 | 2-Furanmethanol | 98-00-0 | 1681 | 1660 | MS, RI, STD | nd | nd | 0.81 | 0.73 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| No of VOCs Extracted | Non-Polar GC Column | Polar GC Column |
|---|---|---|
| With salting out | 75 | 48 |
| Without salting out | 72 | 45 |
| Total | 80 | 54 |
| Overall Total | 85 | |
| NON-POLAR GC COLUMN | ||||||||
|---|---|---|---|---|---|---|---|---|
| Extraction Technique | HS-SPME S | TD S | Di-HiSorb S | HS-HiSorb S | HS-SPME NS | TD NS | Di-HiSorb NS | HS-HiSorb NS |
| No of VOCs | 28 | 34 | 49 | 46 | 25 | 36 | 51 | 42 |
| Abundance % | 1.7 | 1.3 | 34.2 | 7.5 | 3.9 | 2.2 | 100 | 11.6 |
| Average RSD % | 39.3 | 33.7 | 45.3 | 33.5 | 38.3 | 38.1 | 40.5 | 35.2 |
| POLAR GC COLUMN | ||||||||
| Extraction Technique | HS-SPME S | TD S | Di-HiSorb S | HS-HiSorb S | HS-SPME NS | TD NS | Di-HiSorb NS | HS-HiSorb NS |
| No of VOCs | 23 | 28 | 23 | 19 | 17 | 25 | 22 | 16 |
| Abundance % | 1.2 | 2.5 | 39.7 | 4 | 1.3 | 3.1 | 100 | 7.7 |
| Average RSD % | 37.4 | 39.1 | 37.6 | 71.1 | 63.8 | 32.5 | 34.6 | 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Mannion, D.T.; O’Sullivan, M.G.; Miao, S.; Kerry, J.P.; Kilcawley, K.N. Comparison of Automated Extraction Techniques for Volatile Analysis of Whole Milk Powder. Foods 2021, 10, 2061. https://doi.org/10.3390/foods10092061
Cheng Z, Mannion DT, O’Sullivan MG, Miao S, Kerry JP, Kilcawley KN. Comparison of Automated Extraction Techniques for Volatile Analysis of Whole Milk Powder. Foods. 2021; 10(9):2061. https://doi.org/10.3390/foods10092061
Chicago/Turabian StyleCheng, Zeng, David T. Mannion, Maurice G. O’Sullivan, Song Miao, Joseph P. Kerry, and Kieran N. Kilcawley. 2021. "Comparison of Automated Extraction Techniques for Volatile Analysis of Whole Milk Powder" Foods 10, no. 9: 2061. https://doi.org/10.3390/foods10092061
APA StyleCheng, Z., Mannion, D. T., O’Sullivan, M. G., Miao, S., Kerry, J. P., & Kilcawley, K. N. (2021). Comparison of Automated Extraction Techniques for Volatile Analysis of Whole Milk Powder. Foods, 10(9), 2061. https://doi.org/10.3390/foods10092061










