Nutrient-Dense Shelf-Stable Vegetable Powders and Extruded Snacks Made from Carrots and Broccoli
Abstract
:1. Introduction
2. Materials and Methods
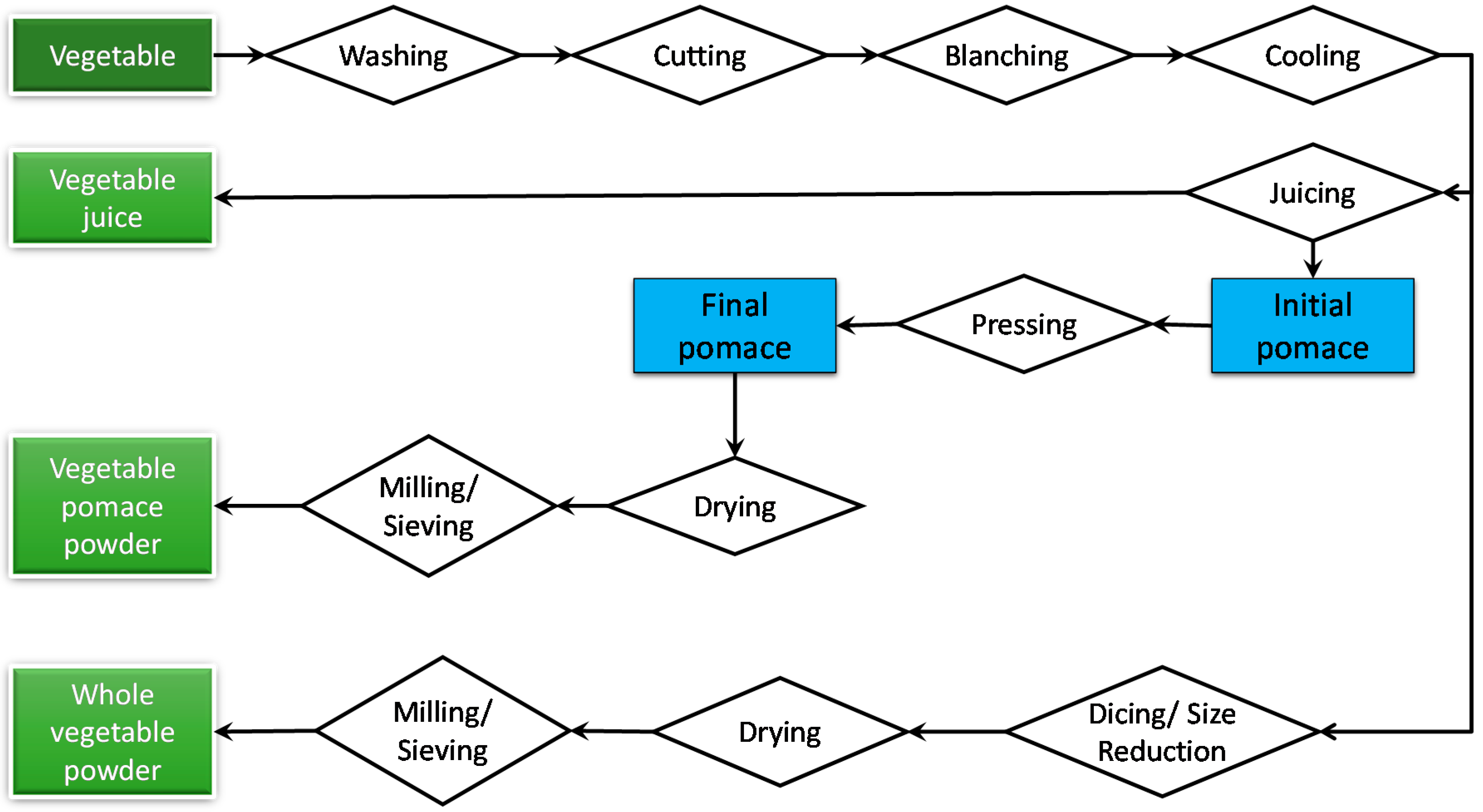
2.1. Processing of Vegetable Powders
2.2. Extrusion
2.3. Chemical Analyses of Raw Materials and Processed Vegetable Products
2.3.1. Compositional Analysis
2.3.2. Total Soluble Phenolics
2.3.3. Total Carotenoids Content
3. Results and Discussion
3.1. Composition of Vegetable and Pomace Powders after Manufacture
3.2. Effect of Processing on Measured Contents of Total Soluble Phenolics
3.3. Effect of Processing on Measured β-carotene Content
3.4. Properties of Extruded Snacks Containing Broccoli or Carrot
3.4.1. Physical Properties
3.4.2. Nutritional Properties
3.4.3. Analysis of Total Soluble Phenolics of Powders and Snacks during Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- FAO. The Future of Food and Agriculture: Trends and Challenges; FAO: Rome, Italy, 2017; ISBN 9789251095515. Available online: http://www.fao.org/3/a-i6583e.pdf (accessed on 12 January 2021).
- FAO. Global Food Losses and Food Waste—Extent, Causes and Prevention; FAO: Rome, Italy, 2011; pp. 1–37. [Google Scholar]
- FAO. Food Loss and Waste Must Be Reduced for Greater Food Security and Environmental Sustainability. 2020. Available online: www.fao.org/news/story/en/item/1310271/icode/ (accessed on 12 September 2021).
- Papargyropoulou, E.; Lozano, R.; Steinberger, J.K.; Wright, N.; Ujang, Z. The food waste hierarchy as a framework for the management of food surplus and food waste. J. Clean. Prod. 2014, 76, 106–115. [Google Scholar] [CrossRef]
- Li, Y.; Bahadur, R.; Ahuja, J.; Pehrsson, P.; Harnly, J. Macro-and micronutrients in raw plant foods: The similarities of foods and implication for dietary diversification. J. Food Compos. Anal. 2021, 102, 103993. [Google Scholar] [CrossRef]
- Augustin, M.A.; Sanguansri, L.; Fox, E.M.; Cobiac, L.; Cole, M.B. Recovery of wasted fruit and vegetables for improving sustainable diets. Trends Food Sci. Technol. 2020, 95, 75–85. [Google Scholar] [CrossRef]
- Kowalska, H.; Czajkowska, K.; Cichowska, J.; Lenart, A. What’s new in biopotential of fruit and vegetable by-products applied in the food processing industry. Trends Food Sci. Technol. 2017, 67, 150–159. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, M.; Adhikari, B. Fruit and vegetable powders. In Handbook of Food Powders; Bhandari, B., Bansal, N., Zhang, M., Schuck, P., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2013; pp. 532–552. ISBN 9780857095138. [Google Scholar]
- Bas-Bellver, C.; Barrera, C.; Betoret, N.; Seguí, L. Turning agri-food cooperative vegetable residues into functional powdered ingredients for the food industry. Sustainability 2020, 12, 1284. [Google Scholar] [CrossRef] [Green Version]
- Neacsu, M.; Vaughan, N.; Raikos, V.; Multari, S.; Duncan, G.J.; Duthie, G.G.; Russell, W.R. Phytochemical profile of commercially available food plant powders: Their potential role in healthier food reformulations. Food Chem. 2015, 179, 159–169. [Google Scholar] [CrossRef]
- Rana, S.; Gupta, S.; Rana, A.; Bhushan, A. Functional properties, phenolic constituents and potential of industrial apple pomace for utilization as active food ingredient. Food Sci. Hum. Wellness 2015, 4, 180–187. [Google Scholar] [CrossRef] [Green Version]
- Bochnak-Niedźwiecka, J.; Świeca, M. Quality of new functional powdered beverages enriched with lyophilized fruits—Potentially bioaccessible antioxidant properties, nutritional value, and consumer analysis. Appl. Sci. 2020, 10, 3668. [Google Scholar] [CrossRef]
- van Buren, L.; Grün, C.H.; Basendowski, S.; Spraul, M.; Newson, R.; Eilander, A. Nutritional quality of dry vegetable soups. Nutrients 2019, 11, 1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.H.; Cho, J.K.; Lee, S.J.; Koh, W.; Park, W.; Kim, C.H. Enhancing beta-carotene content in Asian noodles by adding pumpkin powder. Cereal Chem. 2002, 79, 593–595. [Google Scholar] [CrossRef]
- Salehi, F.; Aghajanzadeh, S. Effect of dried fruits and vegetables powder on cakes quality: A review. Trends Food Sci. Technol. 2020, 95, 162–172. [Google Scholar] [CrossRef]
- Potter, R.; Stojceska, V.; Plunkett, A. The use of fruit powders in extruded snacks suitable for Children’s diets. LWT Food Sci. Technol. 2013, 51, 537–544. [Google Scholar] [CrossRef]
- Wallace, T.C.; Bailey, R.L.; Blumberg, J.B.; Burton-Freeman, B.; Chen, C.O.; Crowe-White, K.M.; Drewnowski, A.; Hooshmand, S.; Johnson, E.; Lewis, R.; et al. Fruits, vegetables, and health: A comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit. Rev. Food Sci. Nutr. 2020, 60, 2174–2211. [Google Scholar] [CrossRef] [Green Version]
- Mahn, A.; Reyes, A. An overview of health-promoting compounds of broccoli (Brassica oleracea var. italica) and the effect of processing. Food Sci. Technol. Int. 2012, 18, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Arscott, S.A.; Tanumihardjo, S.A. Carrots of many colors provide basic nutrition and bioavailable phytochemicals acting as a functional food. Compr. Rev. Food Sci. Food Saf. 2010, 9, 223–239. [Google Scholar] [CrossRef]
- AOAC, 934.06, 964.22, AS2300.1.1. Available online: https://www.saiglobal.com/PDFTemp/Previews/OSH/AS/AS2000/2300/2300.1.3-2008.pdf (accessed on 27 September 2021).
- AOAC, 981.10, 920.152, 990.03, 920.87, AS2300.1.2.1. Available online: https://www.saiglobal.com/PDFTemp/Previews/OSH/AS/AS2000/2300/2300.1.3-2008.pdf (accessed on 27 September 2021).
- AOAC 954.02, 949.15, 922.08. Available online: https://www.aoac.org/official-methods-of-analysis-21st-edition-2019/ (accessed on 27 September 2021).
- AOAC. Ash of Flour (Direct Method), Method 923.03. In Official Methods of Analysis, 18th ed.; AOAC International Publisher: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC, 977.20, 980.13, 982.14, 984.17, 984.22. Available online: https://www.aoac.org/official-methods-of-analysis-21st-edition-2019/ (accessed on 27 September 2021).
- AUS and NZL 2013 Australia New Zealand Food Standards Code—Standard 1.2.8—Nutrition Information Requirements. Available online: https://extranet.who.int/nutrition/gina/sites/default/filesstore/AUS%20and%20NZL%202013%20Australia%20New%20Zealand%20Food%20Standards%20Code%20-%20Standard%201.2.8%20-%20Nutrition%20Information%20Requirements.pdf (accessed on 27 September 2021).
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Biswas, A.K.; Sahoo, J.; Chatli, M.K. A simple UV-Vis spectrophotometric method for determination of beta-carotene content in raw carrot, sweet potato and supplemented chicken meat nuggets. LWT Food Sci. Technol. 2011, 44, 1809–1813. [Google Scholar] [CrossRef]
- Shi, M.; Hlaing, M.M.; Ying, D.Y.; Ye, J.H.; Sanguansri, L.; Augustin, M.A. New food ingredients from broccoli by-products: Physical, chemical and technological properties. Int. J. Food Sci. Technol. 2019, 54, 1423–1432. [Google Scholar] [CrossRef]
- Pek, Z.; Daood, H.; Nagyne, M.G.; Nemenyi, A.; Kelys, L. Effect of environmental conditions and water status on the bioactive compounds of broccoli. Cent. Eur. J. Biol. 2013, 8, 777–787. [Google Scholar]
- U.S. Department of Agriculture; Agricultural Research Service. Food Data Central. Available online: https://fdc.nal.usda.gov/ndb/ (accessed on 13 January 2021).
- Nath, A.; Bagchi, B.; Misra, L.K.; Beka, B.C. Changes in oost-harvest phytochemical qualities of broccoli florests during ambient and refrigerated storage. Food Chem. 2011, 127, 1510–1514. [Google Scholar] [CrossRef]
- Palermo, M.; Pellegrini, N.; Fogliano, V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014, 94, 1057–1070. [Google Scholar] [CrossRef]
- Guan, Y.; Hu, W.; Jiang, A.; Xu, Y.; Yu, J.; Zhao, M.; Ji, Y.; Feng, K.; Sarengaowa Yang, X. Influence of cut type on quality, antioxidant substances and antioxidant activity of fresh-cut broccoli. Int. J. Food Sci. Technol. 2020, 55, 3019–3030. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Monteiro, F.; Passos, C.P.; Silva, A.M.S.; Wessel, D.F.; Coimbra, M.A.; Cardoso, S.M. Blanching impact on pigments, glucosinolates, and phenolics of dehydrated broccoli by-products. Food Res. Int. 2020, 132, 109055. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Li, J.; Jin, P.; Li, X.; Wang, L.; Zheng, Y. The effect of temperature on phenolic content in wounded carrots. Food Chem. 2017, 215, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, S.; Ratti, C.; Stevanovic, T. Impact of drying processes on properties of polyphenol-enriched maple sugar powders. J. Food Process. Eng. 2019, 42, e13239. [Google Scholar] [CrossRef]
- Kurilich, A.C.; Tsau, G.J.; Brown, A.F.; Howard, L.; Klein, B.P.; Jeffery, E.H.; Kushad, M.M.; Wallig, M.A.; Juvik, J.A. Carotene, tocopherol and ascorbate contents in subspecies of Brassica oleracea. J. Agric. Food Chem. 1999, 47, 1576–1581. [Google Scholar] [CrossRef]
- Baranski, R.; Allender, C.; Klimek-Chodacka, M. Towards better tasting and more nutritious carrots: Carotenoid and sugar content variation in carrot genetic resources. Food Res. Int. 2012, 47, 182–187. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N.; Raghavan, V. Plant carotenoids evolution during cultivation, postharvest storage, and food processing: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1561–1604. [Google Scholar] [CrossRef]
- Ainsworth, P.; Ibanoglu, S.; Plunkett, A.; Ibanoglu, E.; Stojceska, V. Effect of brewers spent grain addition and screw speed on the selected physical and nutritional properties of an extruded snack. J. Food Eng. 2007, 81, 702–709. [Google Scholar] [CrossRef]




| Sample Code | Wet Final Pomace (%, dry basis) | Freeze-Dried Whole-Vegetable Powder (%) | Rice Flour (%) |
|---|---|---|---|
| Extruded-3 | 3 | 0 | 97 |
| Extruded-20 | 0 | 20 | 80 |
| Extruded-40 | 0 | 40 | 60 |
| Extruded-60 | 0 | 60 | 40 |
| Extruded-80 | 0 | 80 | 20 |
| Extruded-100 | 0 | 100 | 0 |
| Parameter | Whole Broccoli | Whole Carrot | Pomace | |||||
|---|---|---|---|---|---|---|---|---|
| Trial 1 | Trial 2 | Trial 3 | Trial 1 | Trial 2 | Trial 3 | Broccoli | Carrot | |
| Energy (kJ/100 g) | 1110 | 1250 | 1150 | 1220 | 1280 | 1300 | 1120 | 1220 |
| Moisture (g/100 g) | 10.6 | 8.7 | 10.5 | 7.3 | 6.9 | 5.5 | 7.9 | 5.7 |
| Protein (g/100 g) | 30.4 | 31 | 29.3 | 6.7 | 6.3 | 6.5 | 29.2 | 6.0 |
| Fat (g/100 g) | 0.8 | 2.9 | 0.9 | 0.7 | 1.0 | 0.8 | 2.8 | 0.8 |
| Ash (g/100 g) | 9.3 | 7.8 | 8.0 | 7.4 | 6.8 | 7.3 | 7.4 | 5.8 |
| Carbohydrate (g/100 g) | 19.0 | 24 | 23 | 51.0 | 56 | 58 | 15.1 | 48.9 |
| - Sugars (g/100 g) | 18. 0 | 24 | 21 | 41.0 | 52 | 49 | 14.0 | 37 |
| Sodium (mg/100 g) | 370 | 39 | 230 | 980 | 1100 | 1100 | 280 | 640 |
| Dietary Fibre (g/100 g) | 29.7 | 26.1 | 28.2 | 27.1 | 23.1 | 21.8 | 37.6 | 32.4 |
| % Vegetable in Formulation | 100% Broccoli Snack | 20% Broccoli Snack | 100% Carrot Snack | 20% Carrot Snack |
|---|---|---|---|---|
| Energy (kJ/100 g) | 1230 | 1520 | 1260 | 1530 |
| Protein (g/100 g) | 29.9 | 13.2 | 4.3 | 7.6 |
| Fat (g/100 g) | 3.5 | 1.5 | 1.3 | 0.9 |
| Ash (g/100 g) | 7.4 | 3.3 | 6.5 | 3.1 |
| Carbohydrate (g/100 g) | 23.0 | 70.0 | 56.0 | 78.0 |
| - Sugars (g/100 g) | 12.0 | 4.1 | 47.0 | 11.0 |
| Sodium (mg/100 g) | 58 | 200 | 1000 | 420 |
| Dietary Fibre (g/100 g) | 24.8 | 6.8 | 23.7 | 4.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ying, D.; Sanguansri, L.; Cheng, L.; Augustin, M.A. Nutrient-Dense Shelf-Stable Vegetable Powders and Extruded Snacks Made from Carrots and Broccoli. Foods 2021, 10, 2298. https://doi.org/10.3390/foods10102298
Ying D, Sanguansri L, Cheng L, Augustin MA. Nutrient-Dense Shelf-Stable Vegetable Powders and Extruded Snacks Made from Carrots and Broccoli. Foods. 2021; 10(10):2298. https://doi.org/10.3390/foods10102298
Chicago/Turabian StyleYing, Danyang, Luz Sanguansri, Lijiang Cheng, and Mary Ann Augustin. 2021. "Nutrient-Dense Shelf-Stable Vegetable Powders and Extruded Snacks Made from Carrots and Broccoli" Foods 10, no. 10: 2298. https://doi.org/10.3390/foods10102298
APA StyleYing, D., Sanguansri, L., Cheng, L., & Augustin, M. A. (2021). Nutrient-Dense Shelf-Stable Vegetable Powders and Extruded Snacks Made from Carrots and Broccoli. Foods, 10(10), 2298. https://doi.org/10.3390/foods10102298





