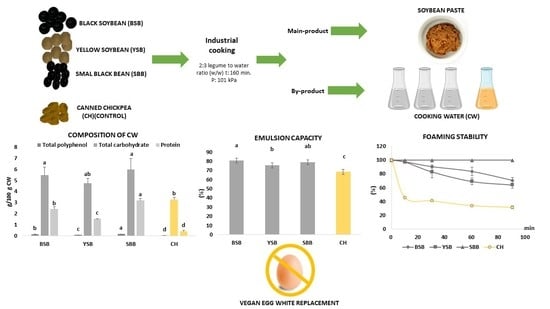
Revalorization of the Cooking Water (Aquafaba) from Soybean Varieties Generated as a By-Product of Food Manufacturing in Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Compositional Analysis
2.2.1. Total Polyphenol Content (TPC)
2.2.2. Total Carbohydrate Content
2.2.3. Protein Content
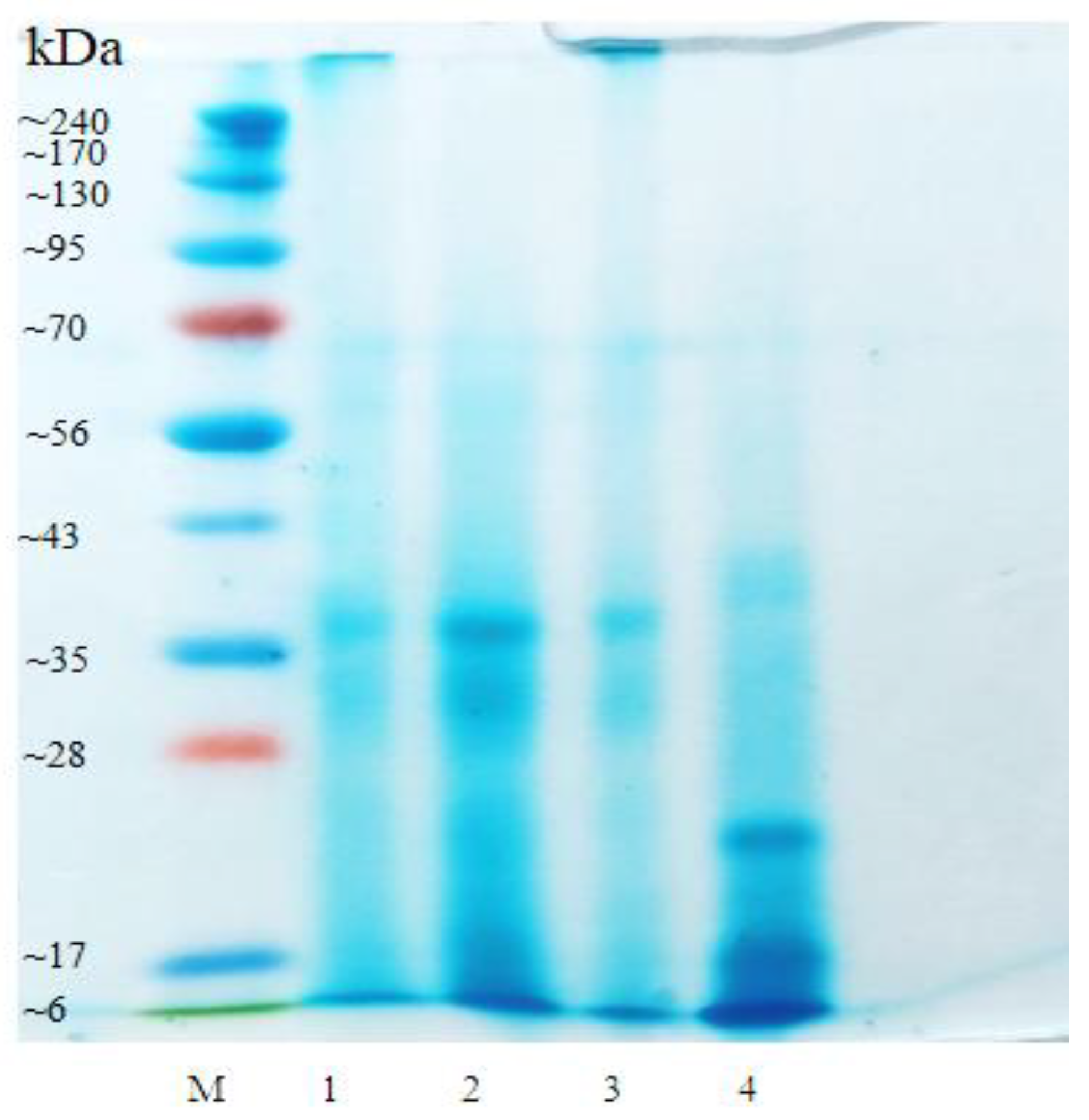
2.2.4. SDS-PAGE
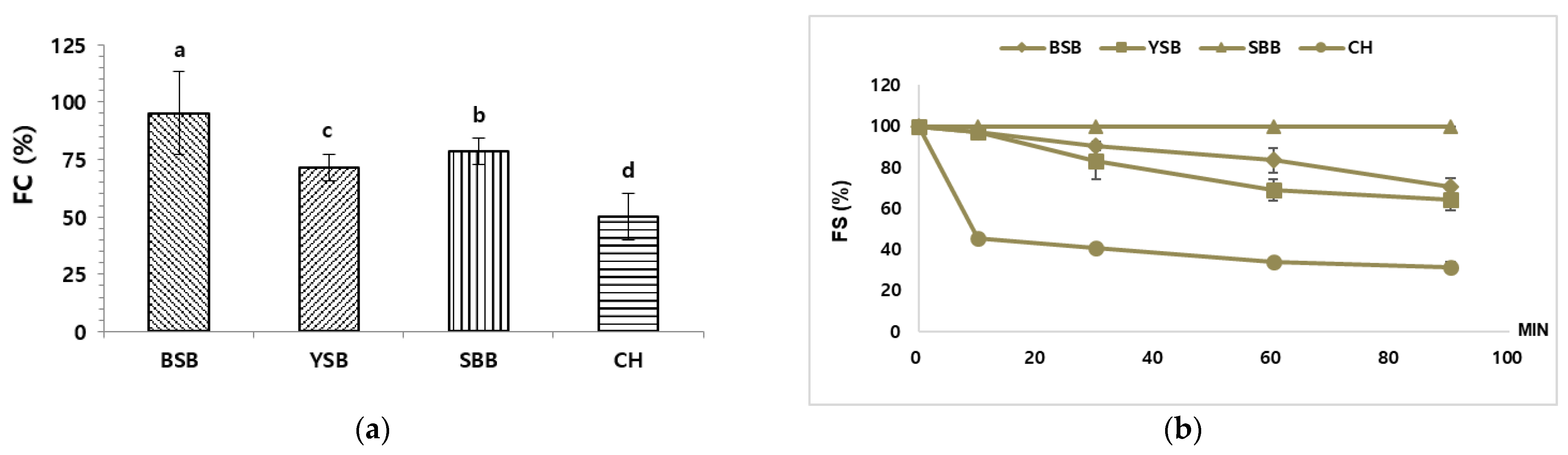
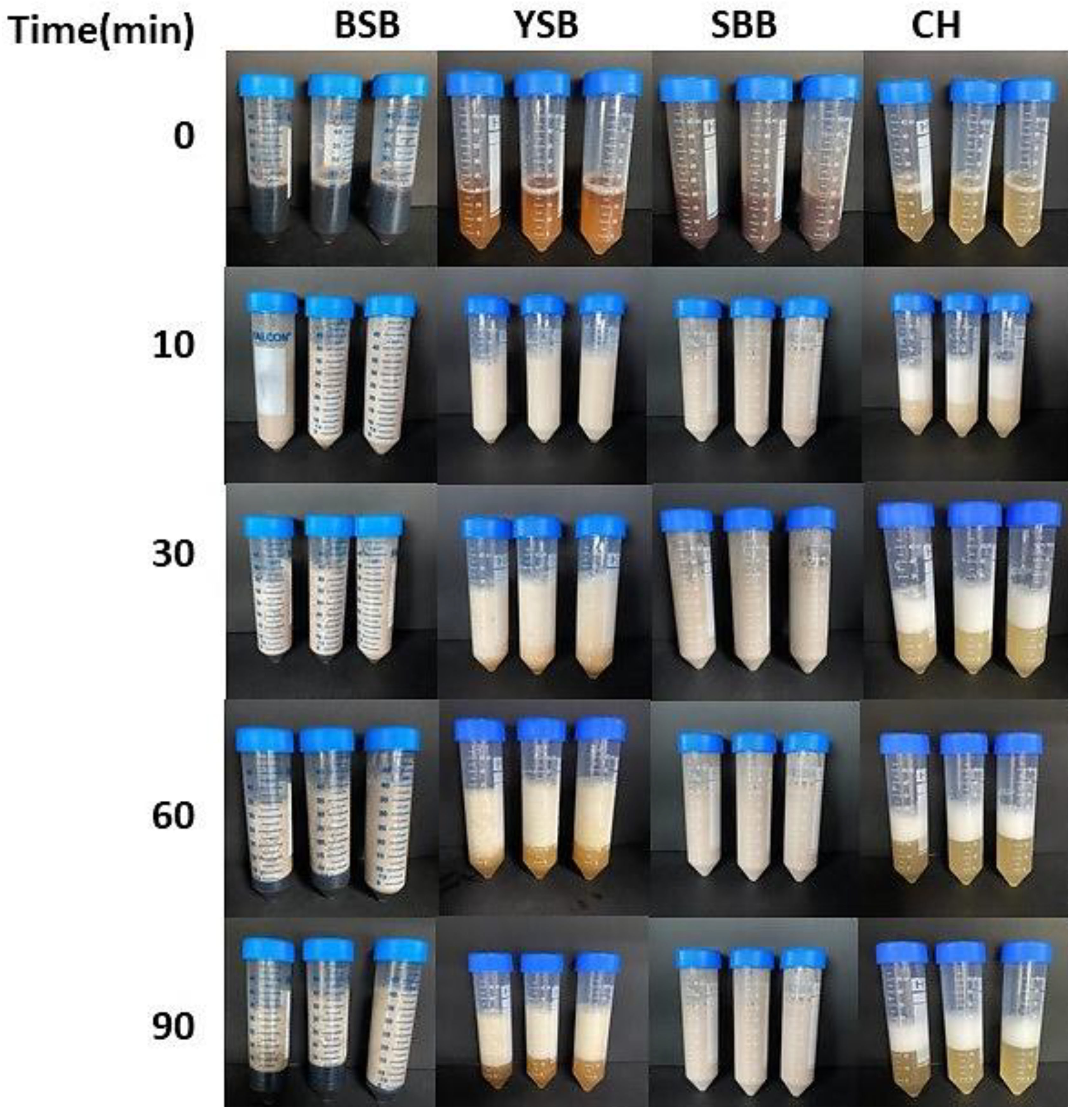
2.2.5. Foaming Capacity (FC) and Foam Stability (FS)
2.2.6. Emulsifying Capacity (EC) and Emulsion Stability (ES)
2.3. Statistical Analysis
3. Results and Discussion
3.1. Compositional Analysis
3.1.1. Total Polyphenol Content (TPC) and pH
3.1.2. Total Carbohydrates (CHO)
3.1.3. Protein Content
3.1.4. SDS-PAGE Analysis
3.2. Foaming Capacity (FC) and Foaming Stability (FS) Analysis
3.3. Emulsion Capacity (EC) and Emulsion Stability (ES) Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- de Boer, I.J.M.; van Ittersum, M.K. Circularity in Agricultural Production; Wageningen University & Research: Wageningen, The Netherlands, 2018; p. 9. [Google Scholar]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F.; et al. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Raikos, V.; Hayes, H.; Ni, H. Aquafaba from commercially canned chickpeas as potential egg replacer for the development of vegan mayonnaise: Recipe optimisation and storage stability. Int. J. Food Sci. Technol. 2019, 55, 1935–1942. [Google Scholar] [CrossRef]
- Mustafa, R.; He, Y.; Shim, Y.Y.; Reaney, M.J.T. Aquafaba, wastewater from chickpea canning, functions as an egg replacer in sponge cake. Int. J. Food Sci. Technol. 2018, 53, 2247–2255. [Google Scholar] [CrossRef]
- Admin, A. The Official Aquafaba Website. Available online: http://aquafaba.com/ (accessed on 23 January 2020).
- Yadav, S.S.; Chen, W. Chickpea Breeding and Management; Cromwell Press: Trowbridge, UK, 2007. [Google Scholar]
- Chakraborty, C.; Sharma, G. Development and Characterization of Herbal Custard. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 348–356. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, K.; Xu, B. A critical review on polyphenols and health benefits of black soybeans. Nutrients 2017, 9, 455. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.H.; Sim, M.J.; Kim, Y.C. Melanogenesis-promoting effects of rhynchosia nulubilis and rhynchosia volubilis ethanol extracts in melan-a cells. Toxicol. Res. 2016, 32, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.A.; Jung, K.G.; Kim, S.H.; Park, J.G.; Seo, M.H.; Cha, J.H.; Oh, H.J.; Lee, J.Y. Improved Osteoporotic Condition of Soybean and Yak-kong (Blackbean: Rhynchosia Molubilis) in Korean Postmenopausal Women. FASEB J. 2016, 30, 692. [Google Scholar]
- Sunchul Choi, T.M. Oilseeds and Products Annual; KS2021-0004; United States Department of Agriculture Foreign Agricultural Service: Washington, DC, USA, 2021; p. 32.
- Song, H.-N. Quality Analysis for Recycle of the Drained Soybean Boiling Water Discarded in the Mass Production of Fermented Soy Foods. Korean J. Food Cook. Sci. 2013, 29, 525–531. [Google Scholar] [CrossRef] [Green Version]
- Sharif, H.R.; Williams, P.A.; Sharif, M.K.; Abbas, S.; Majeed, H.; Masamba, K.G.; Safdar, W.; Zhong, F. Current progress in the utilization of native and modified legume proteins as emulsifiers and encapsulants—A review. Food Hydrocoll. 2018, 76, 2–16. [Google Scholar] [CrossRef]
- Union, I.V. IVU 2020 Accounts. Available online: https://ivu.org/ivu-bylaws/finances/107-ivu-2020-accounts.html (accessed on 18 January 2021).
- Papalamprou, E.M.; Doxastakis, G.I.; Kiosseoglou, V. Chickpea protein isolates obtained by wet extraction as emulsifying agents. J. Sci. Food Agric. 2010, 90, 304–313. [Google Scholar] [CrossRef]
- Lonnie, M.; Johnstone, A. The public health rationale for promoting plant protein as an important part of a sustainable and healthy diet. Nutr. Bull. 2020, 45, 281–293. [Google Scholar] [CrossRef]
- Hertzler, S.R.; Lieblein-Boff, J.C.; Weiler, M.; Allgeier, C. Plant proteins: Assessing their nutritional quality and effects on health and physical function. Nutrients 2020, 12, 3704. [Google Scholar] [CrossRef]
- Song, M.; Fung, T.T.; Hu, F.B.; Willett, W.C.; Longo, V.D.; Chan, A.T.; Giovannucci, E.L. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern. Med. 2016, 176, 1453–1463. [Google Scholar] [CrossRef]
- Aslan, M.; ErtaŞ, N. Possibility of using ‘chickpea aquafaba’ as egg replacer in traditional cake formulation. Harran Tarım Ve Gıda Bilimleri Derg. 2020, 24, 1–8. [Google Scholar] [CrossRef]
- Buhl, T.F.; Christensen, C.H.; Hammershøj, M. Aquafaba as an egg white substitute in food foams and emulsions: Protein composition and functional behavior. Food Hydrocoll. 2019, 96, 354–364. [Google Scholar] [CrossRef]
- Serventi, L.; Wang, S.; Zhu, J.; Liu, S.; Fei, F. Cooking water of yellow soybeans as emulsifier in gluten-free crackers. Eur. Food Res. Technol. 2018, 244, 2141–2148. [Google Scholar] [CrossRef]
- Nguyen, T.M.N.; Le Pham, T.Q.; Tran, G.B. Evaluation of Textural and Microstructural Properties of Vegan Aquafaba Whipped Cream from Chickpeas. Chem. Eng. Trans. 2021, 83, 421–426. [Google Scholar] [CrossRef]
- Nguyen, T.M.N.; Nguyen, T.P.; Tran, G.B.; Le, P.T.Q. Effect of processing methods on foam properties and application of lima bean (Phaseolus lunatus L.) aquafaba in eggless cupcakes. J. Food Process. Preserv. 2020, 44, e14886. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Laemmli, U.; Beguin, F.; Gujer-Kellenberger, G. A factor preventing the major head protein of bacteriophage T4 from random aggregation. J. Mol. Biol. 1970, 47, 69–85. [Google Scholar] [CrossRef]
- García-Vaquero, M.; López-Alonso, M.; Hayes, M. Assessment of the functional properties of protein extracted from the brown seaweed Himanthalia elongata (Linnaeus) SF Gray. Food Res. Int. 2017, 99, 971–978. [Google Scholar] [CrossRef] [Green Version]
- Stantiall, S.E.; Dale, K.J.; Calizo, F.S.; Serventi, L. Application of pulses cooking water as functional ingredients: The foaming and gelling abilities. Eur. Food Res. Technol. 2018, 244, 97–104. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Shin, S.-R.; Kong, H.-J.; Choi, E.-M.; Woo, S.-C.; Lee, M.-H.; Yang, K.-M. Antioxidant activity of extracts from soybean and small black bean. Korean J. Food Preserv. 2014, 21, 404–411. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Chang, S.K. Effect of soaking, boiling, and steaming on total phenolic contentand antioxidant activities of cool season food legumes. Food Chem. 2008, 110, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chang, S.K. Total phenolics, phenolic acids, isoflavones, and anthocyanins and antioxidant properties of yellow and black soybeans as affected by thermal processing. J. Agric. Food Chem. 2008, 56, 7165–7175. [Google Scholar] [CrossRef]
- Zhang, R.F.; Zhang, F.X.; Zhang, M.W.; Wei, Z.C.; Yang, C.Y.; Zhang, Y.; Tang, X.J.; Deng, Y.Y.; Chi, J.W. Phenolic composition and antioxidant activity in seed coats of 60 Chinese black soybean (Glycine max L. Merr.) varieties. J. Agric. Food Chem. 2011, 59, 5935–5944. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K. Antioxidant capacity of seed coat, dehulled bean, and whole black soybeans in relation to their distributions of total phenolics, phenolic acids, anthocyanins, and isoflavones. J. Agric. Food Chem. 2008, 56, 8365–8373. [Google Scholar] [CrossRef]
- Kim, J.-M.; Kim, J.-S.; Yoo, H.; Choung, M.-G.; Sung, M.-K. Effects of black soybean [Glycine max (L.) Merr.] seed coats and its anthocyanidins on colonic inflammation and cell proliferation in vitro and in vivo. J. Agric. Food Chem. 2008, 56, 8427–8433. [Google Scholar] [CrossRef]
- Damian, J.J.; Huo, S.; Serventi, L. Phytochemical content and emulsifying ability of pulses cooking water. Eur. Food Res. Technol. 2018, 244, 1647–1655. [Google Scholar] [CrossRef]
- National Institute of Agricultural Sciences. Korean Food Composition Table, 9th ed.; Rural Development Administration, National Academy of Agricultural Sciences, Ministry of Agriculture: Wanju, Jeollabuk-do, Korea, 2016.
- Guleria, S.; Sharma, S.; Gill, B.S.; Munshi, S.K. Distribution and biochemical composition of large and small seeds of soybean (Glycine max L.). J. Sci. Food Agric. 2008, 88, 269–272. [Google Scholar] [CrossRef]
- Hagely, K.B.; Palmquist, D.; Bilyeu, K.D. Classification of distinct seed carbohydrate profiles in soybean. J. Agric. Food Chem. 2013, 61, 1105–1111. [Google Scholar] [CrossRef]
- Carter, H. Aquafaba Products and Methods. US Patent 2020/0046001 A1, 13 February 2020. [Google Scholar]
- Ciabotti, S.; Silva, A.; Juhasz, A.; Mendonça, C.; Tavano, O.; Mandarino, J.; ConÇAlves, C. Chemical composition, protein profile, and isoflavones content in soybean genotypes with different seed coat colors. Int. Food Res. J. 2016, 23, 621–629. [Google Scholar]
- James, A.T.; Yang, A. Interactions of protein content and globulin subunit composition of soybean proteins in relation to tofu gel properties. Food Chem. 2016, 194, 284–289. [Google Scholar] [CrossRef]
- Perrechil, F.; Ramos, V.; Cunha, R. Synergistic functionality of soybean 7s and 11s fractions in oil-in-water emulsions: Effect of protein heat treatment. Int. J. Food Prop. 2015, 18, 2593–2602. [Google Scholar] [CrossRef]
- Raikos, V.; Hayes, H.E.; Agriopoulou, S.; Varzakas, T. Proteomic dataset of aquafaba from canned chickpea (Cicer arietinum L.) broth. Curr. Top. Pept. Protein Res. 2020, 21, 61–68. [Google Scholar]
- Foegeding, E.A.; Luck, P.; Davis, J.P. Factors determining the physical properties of protein foams. Food Hydrocoll. 2006, 20, 284–292. [Google Scholar] [CrossRef]
- Schmidt, I.; Novales, B.; Boué, F.; Axelos, M.A. Foaming properties of protein/pectin electrostatic complexes and foam structure at nanoscale. J. Colloid Interface Sci. 2010, 345, 316–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickinson, E. Proteins at interfaces and in emulsions stability, rheology and interactions. J. Chem. Soc. Faraday Trans. 1998, 94, 1657–1669. [Google Scholar] [CrossRef]
- Raikos, V.; Juskaite, L.; Vas, F.; Hayes, H.E. Physicochemical properties, texture, and probiotic survivability of oat-based yogurt using aquafaba as a gelling agent. Food Sci. Nutr. 2020, 8, 6426–6432. [Google Scholar] [CrossRef] [PubMed]
- Molina, E.; Papadopoulou, A.; Ledward, D. Emulsifying properties of high pressure treated soy protein isolate and 7S and 11S globulins. Food Hydrocoll. 2001, 15, 263–269. [Google Scholar] [CrossRef]
- He, Y.; Meda, V.; Reaney, M.J.T.; Mustafa, R. Aquafaba, a new plant-based rheological additive for food applications. Trends Food Sci. Technol. 2021, 111, 27–42. [Google Scholar] [CrossRef]
- Bouyer, E.; Mekhloufi, G.; Rosilio, V.; Grossiord, J.L.; Agnely, F. Proteins, polysaccharides, and their complexes used as stabilizers for emulsions: Alternatives to synthetic surfactants in the pharmaceutical field? Int. J. Pharm. 2012, 436, 359–378. [Google Scholar] [CrossRef]
- Serventi, L. Upcycling Legume Water: From Wastewater to Food Ingredients, 1st ed.; Springer Nature: Cham, Switzerland, 2020. [Google Scholar]
- Xu, W.; Zhao, X.-H. Structure and property changes of the soy protein isolate glycated with maltose in an ionic liquid through the Maillard reaction. Food Funct. 2019, 10, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]

| Composition (g/100 g CW) | Total Polyphenol | Total Carbohydrate | Protein | pH |
|---|---|---|---|---|
| BSB | 0.14 ± 0.00 b | 5.48 ± 0.72 a | 2.40 ± 0.23 b | 6.26 ± 0.03 a |
| YSB | 0.07 ± 0.00 c | 4.74 ± 0.47 ab | 1.51 ± 0.04 c | 6.22 ± 0.03 a |
| SBB | 0.16 ± 0.00 a | 5.97 ± 1.3 a | 3.19 ± 0.18 a | 6.25 ± 0.02 a |
| CH | 0.03 ± 0.00 d | 3.28 ± 0.19 b | 0.39 ± 0.11 d | 6.23 ± 0.03 a |
| Property (%) | BSB | YSB | SBB | CH |
|---|---|---|---|---|
| EC | 80.76 ± 2.59 a | 75.48 ± 3.07 b | 78.89 ± 1.11 ab | 68.47 ± 1.68 c |
| ES | 76.85 ± 1.61 a | 72.58 ± 1.67 b | 75.11 ± 0.77 ab | 74.50 ± 1.80 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echeverria-Jaramillo, E.; Kim, Y.-h.; Nam, Y.-r.; Zheng, Y.-f.; Cho, J.Y.; Hong, W.S.; Kang, S.J.; Kim, J.H.; Shim, Y.Y.; Shin, W.-S. Revalorization of the Cooking Water (Aquafaba) from Soybean Varieties Generated as a By-Product of Food Manufacturing in Korea. Foods 2021, 10, 2287. https://doi.org/10.3390/foods10102287
Echeverria-Jaramillo E, Kim Y-h, Nam Y-r, Zheng Y-f, Cho JY, Hong WS, Kang SJ, Kim JH, Shim YY, Shin W-S. Revalorization of the Cooking Water (Aquafaba) from Soybean Varieties Generated as a By-Product of Food Manufacturing in Korea. Foods. 2021; 10(10):2287. https://doi.org/10.3390/foods10102287
Chicago/Turabian StyleEcheverria-Jaramillo, Esteban, Yoon-ha Kim, Ye-rim Nam, Yi-fan Zheng, Jae Youl Cho, Wan Soo Hong, Sang Jin Kang, Ji Hye Kim, Youn Young Shim, and Weon-Sun Shin. 2021. "Revalorization of the Cooking Water (Aquafaba) from Soybean Varieties Generated as a By-Product of Food Manufacturing in Korea" Foods 10, no. 10: 2287. https://doi.org/10.3390/foods10102287
APA StyleEcheverria-Jaramillo, E., Kim, Y.-h., Nam, Y.-r., Zheng, Y.-f., Cho, J. Y., Hong, W. S., Kang, S. J., Kim, J. H., Shim, Y. Y., & Shin, W.-S. (2021). Revalorization of the Cooking Water (Aquafaba) from Soybean Varieties Generated as a By-Product of Food Manufacturing in Korea. Foods, 10(10), 2287. https://doi.org/10.3390/foods10102287