Low-Protein Diet: History and Use of Processed Low-Protein Rice for the Treatment of Chronic Kidney Disease
Abstract
:1. Introduction
1.1. Increasing Trends of CKD in the World
1.2. Effects of a Low-Protein Diet on CKD
1.3. Adequate Amount of Protein Intake by CKD Patients
1.4. Effect of Prolonged Intake of Low-Protein Diet
2. Low-Protein Diet
2.1. How to Design a Low-Protein Diet?
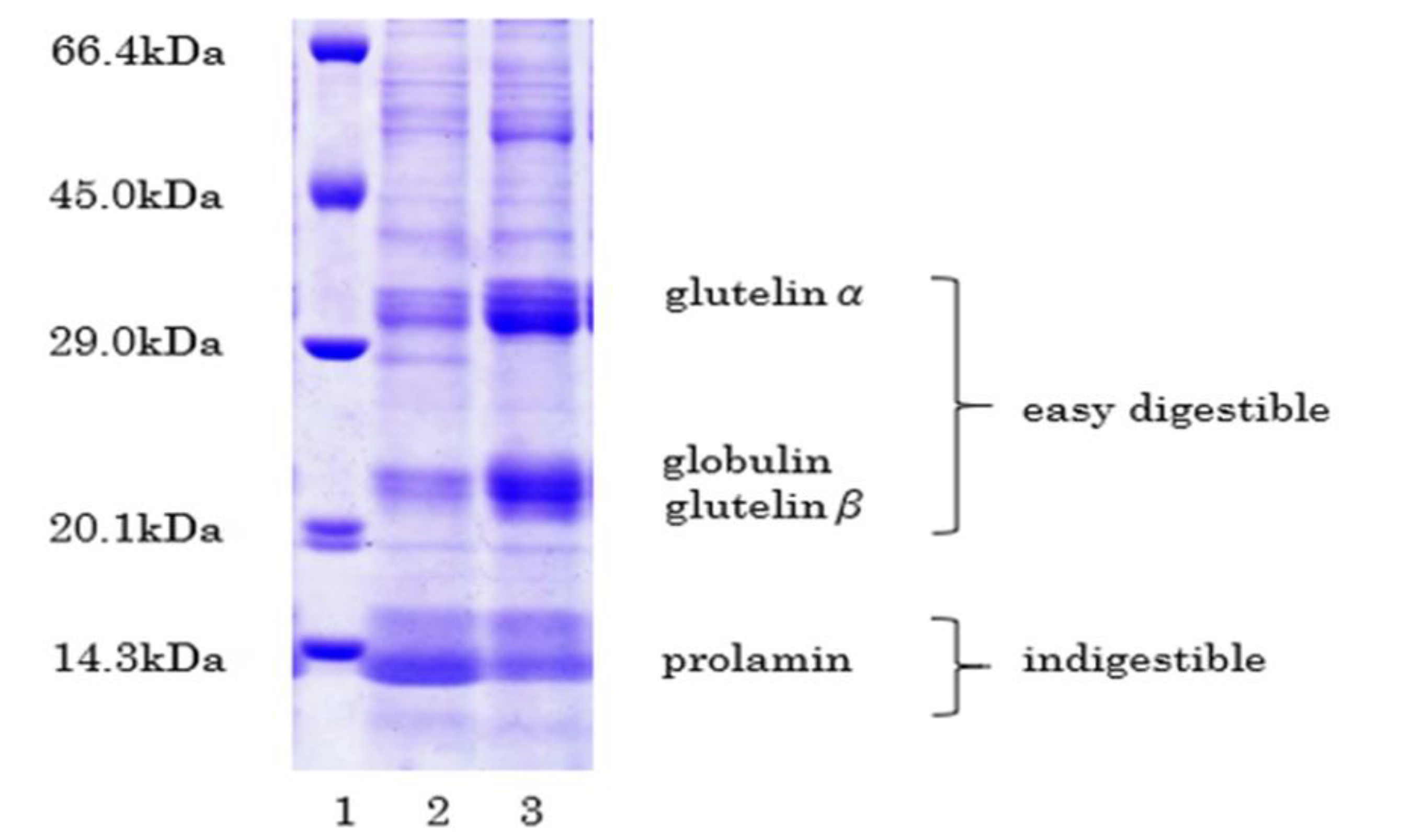
2.2. Structure of Low-Protein Rice
3. Methods to Manufacture PLC
3.1. Low-Protein White (Polished) Rice
3.2. Proteolytic Process
3.3. Cooking Process
4. Processed Low-Protein Brown Rice
4.1. Technical Challenges with Brown Rice
4.2. Selection of Rice
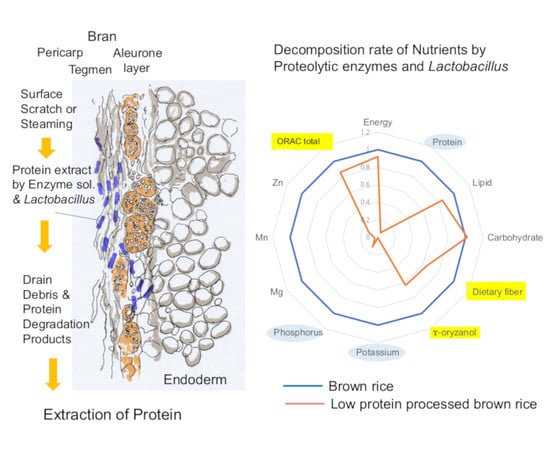
4.3. Pretreatment and Protein Extraction
4.4. Protein Extraction
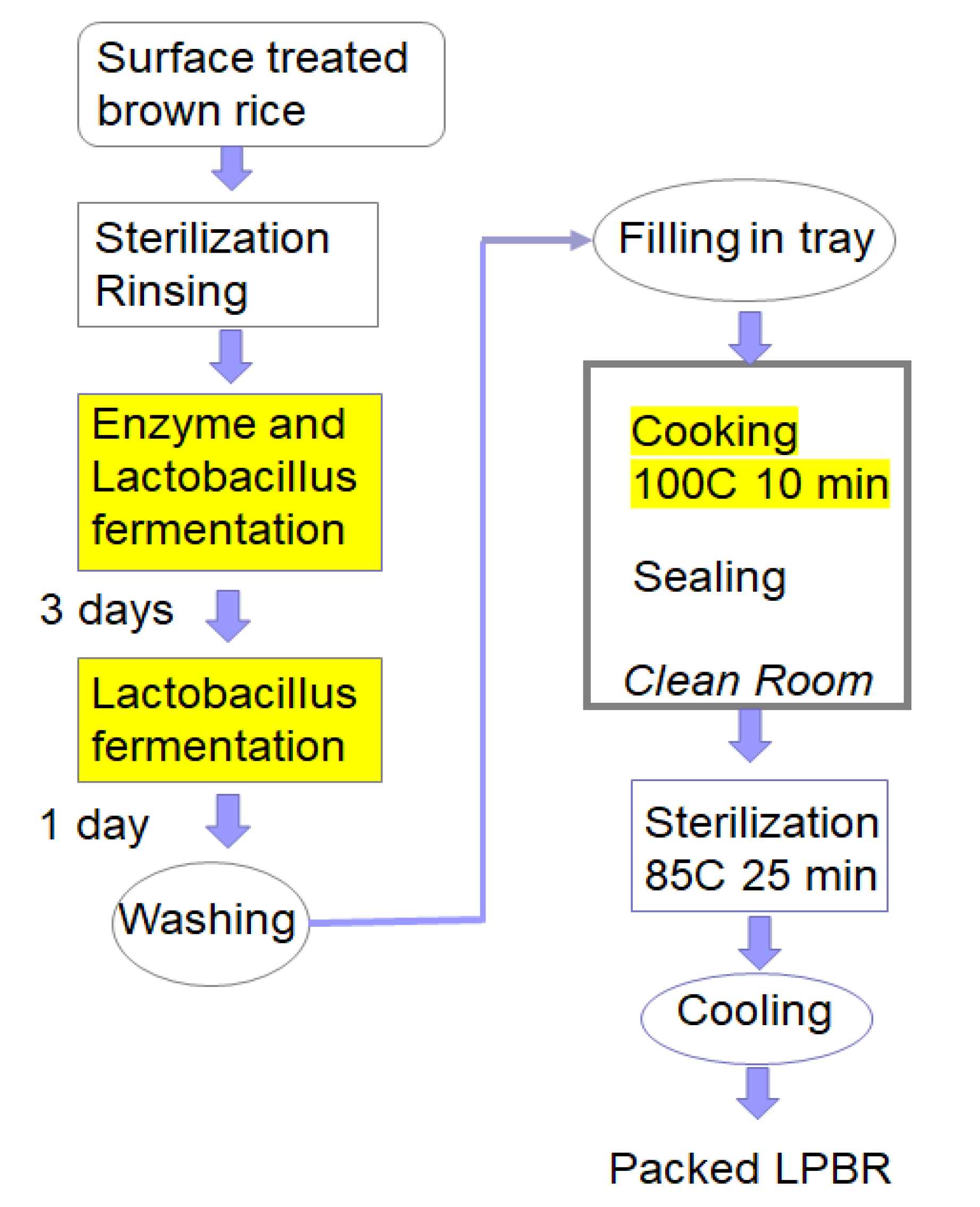
4.5. Importance of Lactobacillus on LPBR Production
4.6. Cooking and Packing
5. Nutrients and Functional Factors of New LPBR
6. Nutrient Evaluation of LPBR
7. Necessity to Expand the Market
8. Expansion of the Use of Processed Low-Protein Rice to the World
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Couser, W.G.; Remuzzi, G.; Mendis, S.; Tonelli, M. The contribution of chronic kidney disease to the global burden of major non-communicable diseases. Kidney Int. 2011, 80, 1258–1270. [Google Scholar] [CrossRef] [Green Version]
- Jha, V.; Garcia, G.G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.-M.; Yang, C.-W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Global Burden of Disease Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systemat ic analysis for the Global Burden of Disease study 2017. Lancet 2020, 395, 709–732. [Google Scholar] [CrossRef] [Green Version]
- Carney, E.F. The impact of chronic kidney disease on global health. Nat. Rev. Nephrol. 2020, 16, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.-L.; Rothenbacher, D. Prevalence of chronic kidney disease in population-based studies: Systematic review. BMC Public Health 2008, 8, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, E.; Horio, M.; Iseki, K.; Yamagata, K.; Watanabe, T.; Hara, S.; Ura, N.; Kiyohara, Y.; Hirakata, H.; Moriyama, T.; et al. Prevalence of chronic kidney disease (CKD) in the Japanese general population predicted by the MDRD equation modified by a Japanese coefficient. Clin. Exp. Nephrol. 2007, 11, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Shinkawa, K.; Yanagita, M.; Kawakami, K. Prevalence, recognition and management of chronic kidney disease in Japan: Population-based estimate using a healthcare database with routine health checkup data. Clin. Kidney J. 2021, sfab016. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef]
- Atkins, R.C.; Zimmet, P. teWorld kidney day 2010: Diabetic kidney disease—Act now or pay later. Am. J. Kidney Dis. 2010, 55, 205–208. [Google Scholar] [CrossRef] [PubMed]
- El Nahas, M. The global challenge of chronic kidney disease. Kidney Int. 2005, 68, 2918–2929. [Google Scholar] [CrossRef] [Green Version]
- Bilous, R. Microvascular disease: What does the UKPDS tell us about diabetic nephropathy? Diabet Med. 2003, 20, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Low-Protein Diets for Adults Without Diabetes Mellitus Who Have CKD. Available online: www.aafp.org/afp/2020/1201/p665.html (accessed on 5 June 2021).
- Kalantar-Zadeh, K.; Joshi, S.; Schlueter, R.; Cooke, J.; Brown-Tortorici, A.; Donnelly, M.; Schulman, S.; Lau, W.-L.; Rhee, C.M.; Streja, E.; et al. Plant-Dominant Low-Protein Diet for Conservative Management of Chronic Kidney Disease. Nutrients 2020, 12, 1931. [Google Scholar] [CrossRef] [PubMed]
- Medical Cost of Hemodialysis. Available online: http://www.mhlw.go.jp/toukei/saikin/hw/k-iryohi/J4/dl/kekka.pdf (accessed on 15 June 2021).
- CDC: Chronic Kidney Disease Basic. Available online: http://www.cdc.gov/kidneydisease/basics.html (accessed on 15 June 2021).
- The Japanese Society for Dialysis Therapy. Available online: https://www.jsdt.or.jp/dialysis/2227.html (accessed on 15 June 2021).
- Nomiyama, T.; Kiyohara, Y.; Tokuda, Y.; Doi, Y.; Arima, H.; Harada, A.; Ohashi, Y.; Ueshima, H. Japan arteriosclerosis longitudinal study group. Impact of kidney disease and blood pressure on the development of cardiovascular disease: An overview from the Japan Arteriosclerosis Longitudinal Study. Circulation 2008, 118, 2694–2701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Noorden, C. Clinical Treatises on the Pathology and Therapy of Disorders of Metabolism and Nutrition; EB Treat & Company: New York, NY, USA, 1906. [Google Scholar]
- Giovannetti, S.; Maggiore, Q. A low-nitrogen diet with proteins of high biological value for severe chronic uræmia. Lancet 1964, 283, 1000–1003. [Google Scholar] [CrossRef]
- Mariani, G.; Barsotti, S.; Bianchi, G. Nutritional aspects of plasma protein metabolic studies: Long-term treatment of chronic uraemia by a very-low-protein diet supplemented with essential amino acids and keto analogues. In Pathophysiology of Plasma Protein Metabolism; Mariane, G., Ed.; Springer Nature: Cham, Switzerland, 1984; pp. 325–331. [Google Scholar]
- Giordano, C. Use of exogenous and endogenous urea for protein synthesis in normal and uremic subjects. J. Lab. Clin. Med. 1963, 62, 231. [Google Scholar] [PubMed]
- Brenner, B.M.; Meyer, T.W.; Hostetter, T.H. Dietary protein intake and the progressive nature of kidney disease: The role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N. Engl. J. Med. 1982, 307, 652–659. [Google Scholar]
- National Research Council (US) Subcommittee on the Tenth Edition of the Recommended Dietary Allowances. Recommended Dietary Allowances: 6 Protein and Amino Acids, 10th ed.; National Academies Press: Washington, DC, USA, 1989.
- van der Velde, M.; Halbesma, N.; de Charro, F.T.; Bakker, S.J.; de Zeeuw, D.; de Jong, P.E.; Gansevoort, R.T. Screening for albuminuria identifies individuals at increased renal risk. J. Am. Soc. Nephrol. 2009, 20, 852–862. [Google Scholar] [CrossRef] [Green Version]
- Romundstad, S.; Holmen, J.; Kvenild, K.; Hallan, H.; Ellekjaer, H. Microalbuminuria and all-cause mortality in 2089 apparently healthy individuals: A 44-year follow-up study. The Nord-Trondelag Health Study (HUNT), Norway. Am. J. Kidney Dis. 2003, 42, 466–473. [Google Scholar] [CrossRef]
- Gansevoort, R.T.; de Jong, P.E. The case for using albuminuria in staging chronic kidney disease. J. Am. Soc. Nephrol. 2009, 20, 465–468. [Google Scholar] [CrossRef] [Green Version]
- Verhave, J.C.; Gansevoort, R.; Hillege, H.L.; Bakker, S.J.; De Zeeuw, D.; De Jong, P.E. An elevated urinary albumin excretion predicts de novo development of renal function impairment in the general population. Kidney Int. 2004, 66, S18–S21. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S. Low-protein diet for the prevention of renal failure. Proc. Jpn. Acad. Ser. B 2017, 93, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitch, W.E.; Remzzi, D. Diets for patients with chronic kidney disease, should we reconsider? BMC Nephrol. 2016, 17, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhee, C.M.; Ahmadi, S.-F.; Kovesdy, C.P.; Kalantar-Zadeh, K. Low-protein diet for conservative management of chronic kidney disease: A systematic review and meta-analysis of controlled trials. J. Cachex. Sarcopenia Muscle 2017, 9, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Alberti, D.; Graziani, G.; Buccianti, G.; Redaelli, B.; Giangrande, A. Prospective, randomised, multicentre trial of effect of protein restriction on progression of chronic renal insufficiency. Lancet 1991, 337, 1299–1304. [Google Scholar] [CrossRef]
- Williams, P.; Stevens, M.; Fass, G.; Irons, L.; Bone, J. Failure of dietary protein and phosphate restriction to retard the rate of progression of chronic renal failure: A prospective, randomized, controlled trial. Qjm Int. J. Med. 1991, 81, 837–855. [Google Scholar] [CrossRef]
- Cianciaruso, B.; Pota, A.; Pisani, A.; Torraca, S.; Annecchini, R.; Lombardi, P.; Capuano, A.; Nazzaro, P.; Bellizzi, V.; Sabbatini, M. Metabolic effects of two low protein diets in chronic kidney disease stage 4–5—A randomized controlled trial. Nephrol. Dial. Transplant. 2007, 23, 636–644. [Google Scholar] [CrossRef] [Green Version]
- Ihle, B.U.; Becker, G.J.; Whitworth, J.A.; Charlwood, R.A.; Kincaid-Smith, P.S. The effect of protein restriction on the progression of renal insufficiency. N. Engl. J. Med. 1989, 321, 1773–1777. [Google Scholar] [CrossRef]
- Rosman, J.B.; Langer, K.; Brandl, M.; Piers-Becht, T.P.; Van Der Hem, G.K.; Ter Wee, P.M.; Donker, A.J. Protein-restricted diets in chronic renal failure: A four year follow-up shows limited indications. Kidney Int. Suppl. 1989, 27, S96–S102. [Google Scholar]
- Harn, D.; Hodson, E.M.; Fouque, D. Low-protein diets for non-diabetic adults with chronic kidney disease. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef]
- Japan Nephrology Society. Japan Nephrology Society dietary recommendations for chronic kidney disease. Jpn. J. Renal Soc. 2014, 56, 553–599. [Google Scholar]
- Wilson, H.E.C. Studies on the physiology of protein retention. J. Physiol. 1931, 72, 327–343. [Google Scholar] [CrossRef] [Green Version]
- Vickery, H.B. Biographical Memoir of RUSSELL HENRY CHITTENDEN 1856-1943; National Academy of Sciences: Washington, DC, USA, 1944; pp. 59–104. [Google Scholar]
- Dietary Reference Intake of Japanese 2020. Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/eiyou/syokuji_kijyun.html (accessed on 15 June 2021).
- Watanabe, S. Evaluation of Modification of Diet in Renal Disease (MDRD) study. Clin. Funct. Nutr. 2009, 1, 238–241. [Google Scholar]
- Watanabe, S.; Noboru, M.; Yasunari, M.; Ideura, T. A cross-sectional study on the effects of long term very low protein diets in patients with chronic kidney disease. Anti-Aging Med. 2010, 7, 7–13. [Google Scholar] [CrossRef]
- Nakao, T.; Kanazawa, Y.; Takahashi, T. Once-weekly hemodialysis combined with low-protein and low-salt dietary treatment as a favorable therapeutic modality for selected patients with end-stage renal failure: A prospective observational study in Japanese patients. BMC Nephrol. 2018, 19, 151–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ideura, T.; Shimazui, M.; Morita, H.; Yoshimura, A. Protein intake of more than 0.5 g/kg BW/day is not effective in suppressing the progression of chronic renal failure. Contrib. Nephrol. 2007, 155, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, P.; Combe, C.; Fouque, D.; Aparicio, M. Vegetarianism: Advantages and drawbacks in patients with chronic kidney diseases. J. Ren. Nutr. 2013, 23, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Nazha, M.; Capizzi, I.; Vigotti, F.N.; Scognamiglio, S.; Consiglio, V.; Mongilardi, E.; Bilocati, M.; Avagnina, P.; Versino, E. Diet as a system: An observational study investigating a multi-choice system of moderately restricted low-protein diets. BMC Nephrol. 2016, 17, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccoli, G.B.; Cupisti, A. “Let food be thy medicine…”: Lessons from low-protein diets from around the world. BMC Nephrol. 2017, 18, 102. [Google Scholar] [CrossRef] [Green Version]
- Shirota, N.; Aoki, R.; Sato, Y.; Mineki, M. Characteristic of the smell of the low protein cooking rice, and relationship of taste. J. Integr. Study Diet. Habits 2017, 28, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Foods for Specified Health Use. Available online: https://www.e-healthnet.mhlw.go.jp/information/food/e-01-001.html (accessed on 15 June 2021).
- Standard Foods for Diseased. Ministry of Health, Labour and Welfare. Available online: www.mhlw.go.jp/shingi/2007/11/dl/s1121-13m.pdf (accessed on 15 June 2021).
- Watanabe, S.; Nobori, M. Low-Protein Diet Recipe to Preserve Renal Function; Shufu-No-Tomo: Tokyo, Japan, 2010; p. C2047. ISBN 978-4-67-269104-5. [Google Scholar]
- Nakayama, Y. Relationship between food properties and sensory evaluation of cooked low-protein rice. Kiryu Daigaku Kiyo 2012, 23, 31–36. [Google Scholar]
- Morita, Y. Proteins in rice seeds. Nippon Jozou Kyokai 1972, 67, 843–847. [Google Scholar]
- Takei, N.; Watanabe, N.; Nakajo, M. Low-Protein Rice (LPR) product: Processing method and product safety. Adv. Food Technol. Nutr. Sci. Open J. 2017, 3, 33–41. [Google Scholar] [CrossRef]
- Watanabe, S.; Mizuno, S.; Hirakawa, A. Effects of brown rice on obesity: GENKI Study I (Cross Sectional Epidemiological Study). J. Obes. Chronic Dis. 2018, 2, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S. (Ed.) Doctors’ Evidences of Genmai (Brown Rice); Kirajenne: Tokyo, Japan, 2015; ISBN 978-4-906913-32-9. (In Japanese) [Google Scholar]
- Watanabe, S.; Hirakawa, A.; Takei, N.; Beppu, S.; Hashimoto, H.; Saika, K. Medical rice: A new wax-free brown rice and its protein reduced rice. Adv. Food Technol. Nutr. Sci. Open J. 2018, 4, 10–16. [Google Scholar] [CrossRef]
- Watanabe, S.; Takahashi, M.; Hashimoto, H.; Kikuchi, H.; Matsuo, M.; Ohtsubo, K. Medical rice: A new whole grain functional food for health and diseases. In Functional Foods and Nutraceuticals in Metabolic and Non-Communicable Diseases; Singh, R.B., Watanabe, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Hirakawa, A.; Aoe, S.; Watanabe, S.; Hisada, T.; Mochizuki, J.; Mizuno, S.; Hoshi, T.; Kodama, S. The nested study on the intestinal microbiota in GENKI study with special reference to the effect of brown rice eating. J. Obes. Chronic Dis. 2019, 3, 1–13. [Google Scholar] [CrossRef]
- Kikuchi, K.; Watanabe, S.; Matsuo, M.; Ezaki, T.; Mizuno, S.; Hisada, T. Changes in microbiota and short-chain fatty acids following 3-month pilot intervention study feeding brown rice ball (Omusubi) to healthy volunteers. La Prensa Medica Argent. 2021, 107. [Google Scholar] [CrossRef]
- Crespo-Salgado, J.; Vehaskari, V.M.; Stewart, T.; Ferris, M.; Zhang, Q.; Wang, G.; Blanchard, E.E.; Taylor, C.M.; Kallash, M.; Greenbaum, L.A.; et al. Intestinal microbiota in pediatric patients with end stage renal disease: A Midwest Pediatric Nephrology Consortium study. Microbiome 2016, 4, 50. [Google Scholar] [CrossRef] [Green Version]
- Abe, T. Chronic renal disease and microbiota. Chonai Saikingaku Zasshi 2018, 32, 15–23. [Google Scholar]
- Wakino, S.; Watanabe, S. Renal dysfunction and heart disease: Elucidating the cardio-renal relationship. In Approaches to Diagnosing Cardiovascular Disease in Patients with Diabetic Nephropathy; Elsevier: Amsterdam, The Netherlands. (in press)
- Saika, K.; Watanabe, S. Producing rinse-free rice by the bran-grind method: A way to stop environmental pollution from rice industry waste water. Adv. Food Technol. Nutr. Sci. Open J. 2017, 3, 45–50. [Google Scholar] [CrossRef]
- Tanaka, K.; Masumura, T. Mechanisms of protein accumulation in rice crop. Kagaku to Seibutsu 1988, 26, 543–550. [Google Scholar] [CrossRef]
- Nishimura, M. Development of New Rice Cultivar, “LGC-1”; Beibakukairyo: Tokyo, Japan, 2002; pp. 38–46. (In Japanese) [Google Scholar]
- Mochizuki, T.; Hara, S. Usefulness of the low protein rice on the diet therapy in patients with chronic renal failure. Nihon Jinzo Gakkai Shi 2000, 42, 24–29. [Google Scholar]
- Morita, R.; Kusaba, M.; Iida, S.; Nishio, T.; Nishimura, M. Development of PCR markers to detect the glb1 and Lgc1 mutations for the production of low easy-to-digest protein rice varieties. Theor. Appl. Genet. 2009, 119, 125–130. [Google Scholar] [CrossRef]
- Matsui, T.; Ishizaki, K.; Nakamura, S.; Ohtsubo, K. Differences in physical properties of boiled rice and gelatinization properties of rice flour between pairs of near-isogenic lines for low glutelin gene (Lgc1) locus. Nippon Shokuhin Kagaku Kogaku Kaishi 2013, 60, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Minakuchi, S.; Nakaya, N. New rice varieties with low levels of easy-to-digest protein, ‘Himeiku 83′ Bull. Ehime Res. Inst. Agric. For. Fish. (in press).
- Hoshino, H.; Gochou, H. Change of the bacteria by the difference of washing times and preservation condition to cooled rice. Seitoku Eiyo Tanki Daigaku Kiyou 2014, 6, 11–17. (In Japanese) [Google Scholar]
- Sakuma, K.; Katona, H.; Fukaya, T.; Joh, T.; Ito, A.; Watanabe, A. Effect of cooking water pH on commercial sterility in the production of cooked rice packed under semi-asceptic condition. Nihon Shokuhin Kogyo Kaishi 2008, 9, 157–165. [Google Scholar] [CrossRef]
- Dumas Nitrogen Analyzer. Available online: https://www.actac.co.jp/products/food/nda701.html (accessed on 15 June 2021).
- Biotech Japan. Low-protein brown rice. Process. Methods 2004, 12, P4443398. [Google Scholar]
- Japan Food Research Laboratory. Available online: www.jfrl.or.jp/ (accessed on 15 June 2021).
- Food Analysis Technology Center SUNATEC, Yokkaichi-shi, Mie. Available online: www.jab.or.jp/system/service/upload/RTL02620/RTL02620-en.pdf (accessed on 15 June 2021).
- NIH National Center for Complementary and Integrative Health. Antioxidants: In-Depth. Available online: https://nccih.nih.gov/health/antioxidants/introduction.htm (accessed on 15 June 2021).
- Terashima, M.; Nakatani, I.; Harima, A.; Nakamura, S.; Shiiba, M. New method to evaluate water-soluble antioxidant activity based on protein structural change. J. Agric. Food Chem. 2007, 55, 165–169. [Google Scholar] [CrossRef]
- Watanabe, J.; Oki, T.; Takebayashi, J.; Yamasaki, K.; Takano-Ishikawa, Y.; Hino, A.; Yasui, A. Method validation by interlaboratory studies of improved hydrophilic oxygen radical absorbance capacity methods for the determination of antioxidant capacities of antioxidant solutions and food extracts. Anal. Sci. 2012, 28, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, S. Comprehensive food labeling for obesity control. Adv. Obes. Weight Manag. Control 2016, 4, 61–67. [Google Scholar] [CrossRef]
- Tatsumi, Y.; JPHC FFQ Validation Study Group; Ishihara, J.; Morimoto, A.; Ohno, Y.; Watanabe, S. Seasonal differences in total antioxidant capacity intake from foods consumed by a Japanese population. Eur. J. Clin. Nutr. 2014, 68, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Azadbakht, L.; Atabak, S.; Esmaillzadeh, A. Soy protein intake, cardiorenal indices, and C-reactive protein in type 2 diabetes with nephropathy: A longitudinal randomized clinical trial. Diabetes Care 2008, 31, 648–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.-Q.; Wang, Y. Effects of low-protein rice on nutrition status and renal function in patients with chronic kidney disease: A pilot study. In Proceedings of the East-Asia Conference on Standardization of Rice Function, Kyoto, Japan, 10–12 December 2014; pp. 49–50. [Google Scholar]
- Mafra, D.; Leal, V.O. A practical approach to a low protein diet in Brazil. BMC Nephrol. 2016, 17, 105. [Google Scholar] [CrossRef] [Green Version]
- Ashuntantang, G.E.; Fouda, H.; Kaze, F.F.; Halle, M.-P.; Tabi-Arrey, C.; Biwole-Sida, M. A practical approach to low protein diets for patients with chronic kidney disease in Cameroon. BMC Nephrol. 2016, 17, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]

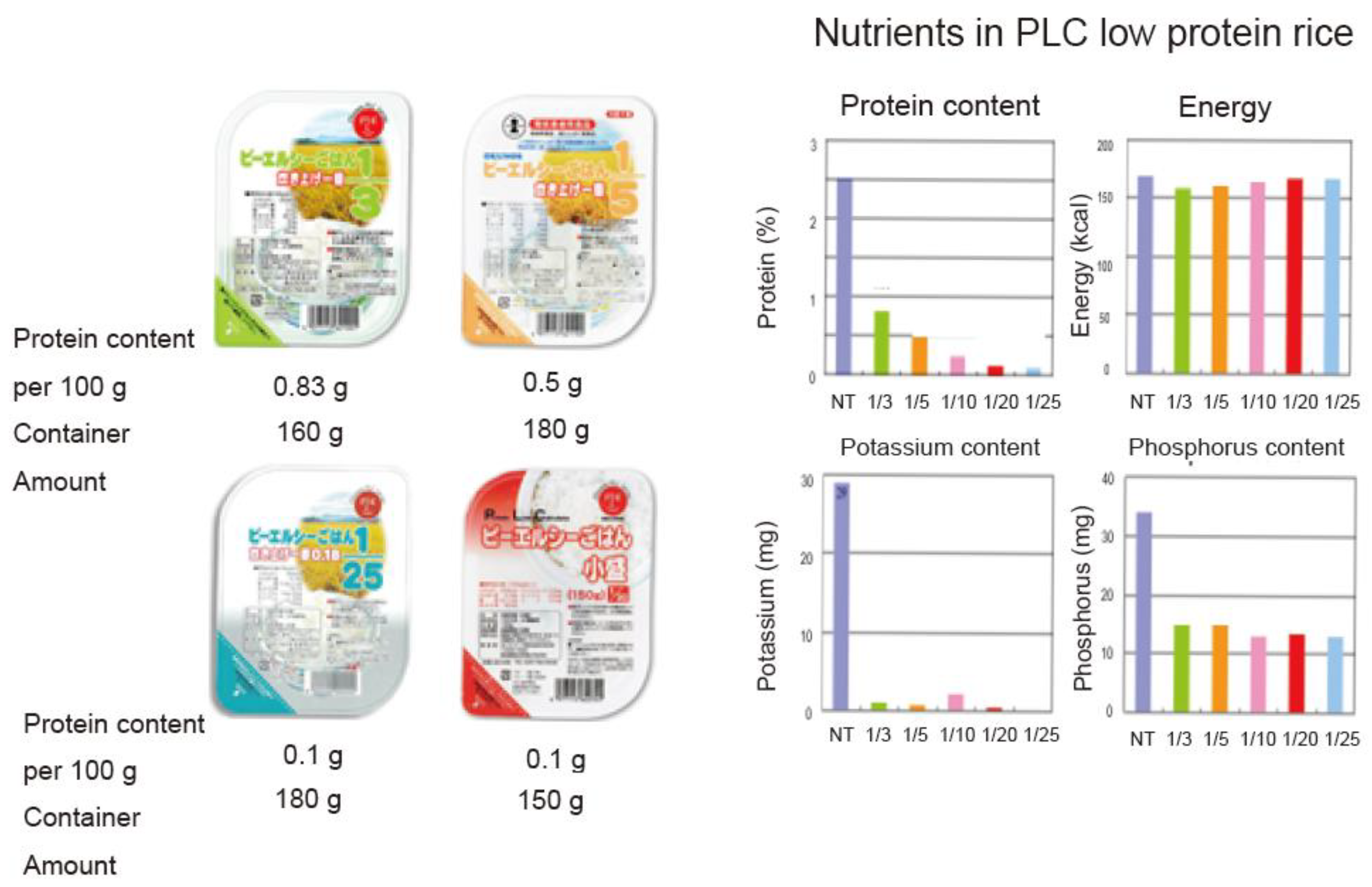
| White Rice | Protein Extracted Rice | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content/100 g Cooked Rice | Origin | 1/3 | 1/5 | 1/10 | 1/20 | 1/25 | |||||
| mean | sd | mean | sd | mean | sd | mean | sd | mean | sd | ||
| Energy (kcal) | 168 | 160 | 3.1 | 162 | 6.8 | 162 | 2.6 | 160 | 7.1 | 160 | 1.7 |
| Moisture (%) | 60.0 | 60.4 | 0.7 | 60.1 | 1.7 | 60.1 | 0.8 | 60.5 | 1.6 | 60.6 | 0.5 |
| Protein (%) | 2.5 | 0.87 | 0.1 | 0.44 | 0 | 0.24 | 0.1 | 0.13 | 0 | 0.1 | 0 |
| Fat (%) | 0.3 | 0.5 | 0.1 | 0.5 | 0.1 | 0.6 | 0.1 | 0.3 | 0.1 | 0.5 | 0.2 |
| Carbohydrate (%) | 37.1 | 38.1 | 0.7 | 38.9 | 1.7 | 39.0 | 0.7 | 39.1 | 1.4 | 38.8 | 0.7 |
| Ash (%) | 0.1 | 0.1 | 0 | 0.1 | 0.1 | 0.1 | 0.1 | 0 | 0 | 0 | 0.1 |
| Sodium (mg) | 1 | 2 | 0.3 | 2 | 0 | 2 | 0.2 | 2 | 0.1 | 2 | 0.1 |
| Potassium (mg) | 29 | 0.6 | 0.3 | 0.5 | 0.2 | 0.9 | 0.2 | 0.6 | 0.1 | 0.7 | 0.2 |
| Calcium (mg) | 3 | 5 | 0 | 5 | 0.3 | 5 | 1.2 | 5 | 0.7 | 5 | 0.6 |
| Phosphorus (mg) | 34 | 15 | 1.0 | 15 | 1.5 | 14 | 1.0 | 13 | 0.7 | 14 | 1.2 |
| Nutrients/100 g Cooked Rice | Brown Rice * | LPBR by Method | |
|---|---|---|---|
| Enzyme | Lactobacillus | ||
| Energy (kcal) | 244 | 142 | 156 |
| Water (g) | 40.7 | 65.5 | 62.2 |
| Protein (g) | 1.3 | 0.5 | 0.2 |
| Lipid (g) | 1.9 | 1.1 | 1.3 |
| Ash (g) | 0.1 | 0.1 | 0.1 |
| Carbohydrate (g) | 57.1 | 32.9 | 36.3 |
| Sugar (g) | 55.6 | 32.1 | 35.3 |
| Dietary fiber (g) | 1.5 | 0.8 | 1.0 |
| g-oryzanol (mg) | 10.4 | 7.9 | 6.3 |
| Sodium (mg) | 2.5 | 1.6 | 1.2 |
| NaCleq (g) | 0.0041 | 0.0041 | 0.003 |
| K (mg) | 85.3 | nd | 0.5 |
| P (mg) | 115 | nd | 14.8 |
| Ca (mg) | 6 | nd | 6 |
| Mg (mg) | 47.5 | nd | 2.2 |
| Mn (mg) | 0.83 | nd | 0.05 |
| Zn (mg) | 0.76 | nd | 0.12 |
| Fe (mg) | 0.4 | nd | <0.1 |
| Cu (mg) | 0.1 | nd | <0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, S.; Ohtsubo, K. Low-Protein Diet: History and Use of Processed Low-Protein Rice for the Treatment of Chronic Kidney Disease. Foods 2021, 10, 2255. https://doi.org/10.3390/foods10102255
Watanabe S, Ohtsubo K. Low-Protein Diet: History and Use of Processed Low-Protein Rice for the Treatment of Chronic Kidney Disease. Foods. 2021; 10(10):2255. https://doi.org/10.3390/foods10102255
Chicago/Turabian StyleWatanabe, Shaw, and Ken’ichi Ohtsubo. 2021. "Low-Protein Diet: History and Use of Processed Low-Protein Rice for the Treatment of Chronic Kidney Disease" Foods 10, no. 10: 2255. https://doi.org/10.3390/foods10102255