Abstract
Grifola frondosa (G. frondosa), generally known as hen-of-the-woods or maitake in Japanese and hui-shu-hua in Chinese, is an edible mushroom with both nutritional and medicinal properties. This review provides an up-to-date and comprehensive summary of research findings on its bioactive constituents, potential health benefits and major structural characteristics. Since the discovery of the D-fraction more than three decades ago, many other polysaccharides, including β-glucans and heteroglycans, have been extracted from the G. frondosa fruiting body and fungal mycelium, which have shown significant antitumor and immunomodulatory activities. Another class of bioactive macromolecules in G. frondosa is composed of proteins and glycoproteins, which have shown antitumor, immunomodulation, antioxidant and other activities. A number of small organic molecules such as sterols and phenolic compounds have also been isolated from the fungus and have shown various bioactivities. It can be concluded that the G. frondosa mushroom provides a diverse array of bioactive molecules that are potentially valuable for nutraceutical and pharmaceutical applications. More investigation is needed to establish the structure–bioactivity relationship of G. frondosa and to elucidate the mechanisms of action behind its various bioactive and pharmacological effects.
1. Introduction
Grifola frondosa (G. frondosa) is a Basidiomycetes fungus that belongs to the family of Grifolaceae and the order of Polyporales. In Japan its edible fruiting body is known as maitake. In Japanese, mai means dance and take means mushroom. G. frondosa is known as “hui-shu-hua” (grey tree flower) in Chinese, possibly due to its appearance. G. frondosa grows around the stumps of broadleaf trees or trunks and is edible when young. The environment of the northeastern part of Japan is suitable for the growth of G. frondosa. The temperate forests in eastern North America, Europe and Asia are also ideal for its growth. Meanwhile, it is a common mushroom in the Unites States and Canada, known as sheep’s head, king of mushrooms, hen-of-the-woods, and cloud mushroom [1].
Japan was one of the countries that first started the artificial cultivation of G. frondosa in the mid-1980s. There are in general three methods for the artificial cultivation of the G. frondosa fruiting body, they are bottle culture, bag culture and outdoor bed culture. Bag culture is the most popular cultivation method in Japan [2] because of its advantages such as the low cost of plastic bags, small space requirements and easily-controlled indoor environment. Bag culture can achieve higher yields of mature G. frondosa mushrooms than bottle culture and requires a shorter cultivation time than outdoor bed cultures. As shown in Figure 1 [2], the major steps of bag cultivation include substrate preparation, substrate sterilization, mycelium inoculation and incubation. In addition to the fruiting body, there is also an increasing demand for G. frondosa’s mycelium and its bioactive metabolites. Solid-state fermentation (SSF) [3] and submerged fermentation [4] are two common methods of mycelium cultivation. A common substrate for SSF is sawdust supplemented with rice bran or wheat bran [5]. Submerged or liquid fermentation is usually more efficient, providing a higher mycelial productivity in a shorter time, requiring smaller plant space and allowing for more effective product quality control [6]. A typical submerged fermentation process is presented in Figure 2.
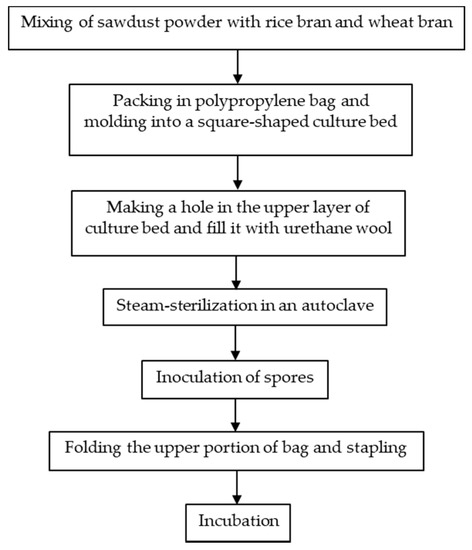
Figure 1.
A typical bag culture procedure for the G. frondosa fruiting body.

Figure 2.
Submerged culture fermentation of the G. frondosa mycelium adapted from [4].
G. frondosa is edible and is regarded as a healthy food because it is a good source of protein, carbohydrates, dietary fiber [7,8,9,10,11,12,13], vitamin D2 (ergocalciferol) [13,14,15] and minerals (K, P, Na, Ca, Mg) [7,9,12,15,16], with low fat content and caloric value [15]. G. frondosa is delicious, with a sweet and umami taste, which is mainly attributed to its high trehalose, glutamic and aspartic amino acid and 5′-nucleotide content [10,11,13,17]. Due to its delicious and special taste, G. frondosa is not only used as a food ingredient, but also as a food-flavoring substance in dried powder form. Apart from its high nutraceutical value, G. fondosa is reported to possess a wide range of pharmacological effects. G. frondosa was first discovered to have antitumor activity in the 1980s from hot water extracts of the G. frondosa fruiting body [16,17]. The major bioactive components were found to be β-glucans [17,18,19,20]. The D-fraction, a β-glucan complex with about 30% protein, was first discovered by Nanba’s group in the late 1980s [21]. Since then, the D-fraction has been widely studied and gradually developed into commercially available complementary medicines and healthcare products. In addition to the D-fraction, there are many other bioactive polysaccharide fractions that are obtained from G. frondosa, such as the MD-fraction [22], X-fraction [23], Grifolan [24], MZ-fraction [25] and MT-α-glucan [26]. The different polysaccharide fractions isolated from G. frondosa possess various bioactive effects such as immunomodulation [24], antitumor [25], antivirus [27], antidiabetic [26] and anti-inflammation [28]. In recent years, an increasing number of studies have attributed or linked the health and therapeutic effects of G. frondosa polysaccharides to their capacity for modifying gut microbiota, microorganisms that play an important role in human health and diseases. In particular, gut microbiota play a role in maintaining immune homeostasis, which may have a connection to the antitumor effects of polysaccharides [29]. The regulation of gut microbiota composition by G. frondosa polysaccharides has also been suggested to contribute to the treatment of metabolic disorders such as non-alcoholic fatty liver disease (NAFLD) [30] and diabetes [31], indicating their potential for preventing or treating hyperglycemia and hyperlipidemia. Apart from polysaccharides, other molecular fractions isolated from G. frondosa fruiting bodies or mycelial biomass have shown promising medicinal values as well. For instance, the protein components of G. frondosa, including glycoprotein, have shown anti-tumor [32], immune-enhancing [33], anti-diabetic, anti-hypertensive, anti-hyperlipidemic [34] and anti-viral effects [35]. Moreover, other small biomolecules in G. frondosa have been found to possess health benefits such as anti-inflammation [36], hypoglycemia [37], antitumor [38] and antioxidation [39].
This review gives an up-to-date and comprehensive summary and assessment of the basic composition and bioactive components of G. frondosa, with an overview of their structural characteristics and bioactivities. It has two major parts. The first part covers the composition and nutritional effects of the G. frondosa fungus and the second part focuses on its medicinal properties, involving major bioactive molecules, their structural characteristics and bioactivities. Given that there are relatively few reviews in the literature which provide an overall picture in terms of both the nutritional and medicinal values of G. frondosa, this review may provide a useful and up-to-date reference for further research on the constituents, properties and functions of G. frondosa and for development and commercial applications in the form of new functional foods and therapeutic products.
2. Chemical and Nutritional Compositions
2.1. Proximate Composition
Generally, proximate composition is determined by the methods suggested by the Association of Official Analytical Chemists (AOAC). The total carbohydrate content can be calculated by subtracting the percentages of ash, crude fat and protein [7,40]. For the determination of crude protein, the nitrogen conversion factor is 4.38 instead of the usual 6.25, due to the large amount of chitin that is usually contained within the fungus, a component that may interfere with the correct calculation of the result of total nitrogen [41].
As shown in Table 1, G. frondosa is made up of around 83–96% moisture and 4–17% dry matter in its fresh fruiting body [7,8,9,10,11,12,13] and mycelium [11,17,42], indicating the watery texture of G. frondosa. Carbohydrates and protein are the major constituents contributing to the dry weight of G. frondosa, taking up around 70–80% and 13–21%, respectively, of the fruiting body. Based on the average values of component percentage, it could be found that the mycelium of G. frondosa has a similar moisture content, a lower content of carbohydrate and crude ash and a higher content of crude fat and protein, compared with the fruiting body of G. frondosa.

Table 1.
Proximate composition of G. frondosa’s fruiting body and mycelium.
According to the research findings of Kurasawa and coworkers [8], the composition of the G. frondosa fruiting body resembles that of normal cultivated mushrooms. It is worth mentioning that the crude fat content of the G. frondosa fruiting body is generally lower than the average crude fat content in cultivated mushrooms (4.3%), and the amounts of protein and carbohydrates are slightly higher than the average of other mushrooms (17.2% and 70.3%), indicating the excellent nutritional values of G. frondosa.
2.2. Soluble Sugar Content
The content of soluble sugar within G. frondosa is mostly determined by the method described in the research work of Ajlouni and coworkers [42]. As shown in Table 2, the total sugar content in G. frondosa is higher in the fruiting body (90–190 mg/g) than in the mycelium (70–90 mg/g). The content of total sugar in the fruiting body of G. frondosa is also superior to some edible mushrooms such as Lactarius glaucescens and Craterellus odoratus [45], which may be one of the reasons for the good taste of G. frondosa. Table 2 also shows variations in both total soluble sugar content and individual sugar content among different G. frondosa samples, which may be attributed to factors such as cultivation period and cultivation environment. Trehalose, a disaccharide that comprises two molecules of glucose, is the major sugar component of both the fruiting body and mycelia of G. frondosa [9,10,11,43,44]. Compared with the amount in the mycelium (40–60 mg/g), the fruiting body contains more trehalose than the mycelium, around 50–160 mg/g in dry weight. In addition to trehalose, the fruiting body also contains glucose and mannitol, whereas the mycelium has glucose and mannitol, together with arabitol and fructose.

Table 2.
Soluble sugar content of G. frondosa fruiting body and mycelium in dry weight.
2.3. Free Amino Acid Content
The content of free amino acids in G. frondosa was quantitatively measured by the method described in the research work of Mau and coworkers using HPLC [10,44]. Results regarding the free amino acid content of G. frondosa are exhibited in Table 3. The total free amino acid content in the fruiting body of G. frondosa is around 15–60 mg/g in dry weight, which is higher than that in many other edible mushrooms, such as Dictyophora indusiata and Tricholoma giganteum [10]. The mycelium of G. frondosa contains a relatively higher total free amino acid content in comparison with the fruiting body. There is also a great variety of amino acids in G. frondosa. There are around eighteen kinds of free amino acids, including essential amino acids such as L-histidine and L-methionine, in both the fruiting body and the mycelium of G. frondosa, indicating that G. frondosa is an excellent source of amino acids.

Table 3.
Free amino acid assay of G. frondosa’s fruiting body and mycelium in dry weight.
However, large variations exist in the amount of each amino acid in G. frondosa. For instance, Mau et al. (2001) and Tsai et al. (2006) found that threonine was the major free amino acid in G. frondosa [10,44], whereas Huang and coworkers found that histidine and glutamic acid were the major free amino acids in the fruiting body (>12 mg/g) and lysine, aspartic acid and tyrosine were the major free amino acids in the mycelium (>17 mg/g) [11]. Huang and coworkers also obtained a larger amount of free amino acid from G. frondosa than other groups in both the fruiting body and the mycelium, a result that might be due to the different sources of the fruiting body and the preparation methods of the mycelium by the different research groups. The choice in cultivation substrate was also found to affect the variety and amount of amino acids in G. frondosa. As shown in Table 3, G. frondosa fruiting bodies cultivated in sawdust and log substrates have different amounts of each amino acid [13], although the total amino acid content was similar. Moreover, GABA (γ-aminobutyric acid), a biologically active compound which is related to the therapeutic effect of G. frondosa, is mainly detected in the mycelium but not in the fruiting body [11] (Table 3).
3. Bioactive Ingredients
3.1. Polysaccharides
In the past 30 years, over 47 bioactive polysaccharide fractions have been isolated and purified from the fruiting body, mycelium and cultured medium of G. frondosa using different extraction methods. As previously reported, G. frondosa contains 3.8% water-soluble polysaccharides on a dry weight basis, of which 13.2% was (1→3, 1→6)-β-D-glucan [46], and others include heteroglycan or the heteroglycan/protein complex [47]. Among these bioactive polysaccharide fractions, the D-fraction and the MD-fraction (purified D-fraction) [22], which are regarded as the most important bioactive polysaccharides, have been officially used as antitumor, anticancer and immunomodulatory agents [48]. Through structural characterization, Nanba and Kubo discovered the complex structure of the β-D-glucan in the D-fraction. Unlike other mushroom-derived β-glucans that contained a (1→3) main chain with (1→6) branches only, the β-D-glucan in D-fraction possessed both a (1→6) main chain with (1→3) branches and a (1→3) main chain with (1→6) branches [22]. The high molecular weight of the D-fraction was considered a factor contributing to its strong immunomodulatory effects [22]. Figure 3 shows a typical structure of the D-fraction. The D-fraction and the MD-fraction could be extracted and fractionated from both the mycelium and fruiting body of G. frondosa. Figure 4 is a general flowchart for the extraction and purification of the MD-fraction as reported by Nanba and coworkers. The MD fraction is typically extracted from the dried G. frondosa powder with boiling water and then isolated by ethanol precipitation. The precipitate (crude MD fraction) is further fractionated through column chromatography, including ion exchange and gel permeation chromatography, to obtain the purified MD fraction.
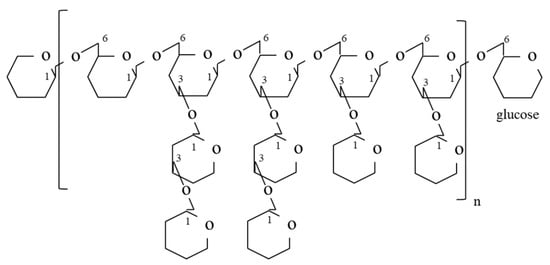
Figure 3.
Typical structure of the D-fraction with (1→6)-glucan having a (1→3)-branched chain [49].

Figure 4.
A typical extraction method of the MD fraction from G. frondosa adapted from [50].
Apart from the D-fraction and the MD-fraction, other polysaccharide fractions have also been derived from G. frondosa, with hot water being the most commonly used extraction solvent. Ultrasound [51] and other solvents such as hot sodium hydroxide [18] and citrate buffer [24] have also been utilized. Table 4 summarizes the chemical properties, sources and extraction solvents of representative bioactive polysaccharide fractions isolated from G. frondosa. The chemical properties listed include molecular weight, structure information, as well as monosaccharide compositions. For instance, Kubo et al. extracted the X-fraction from the fruiting body of G. frondosa, which was a (1→6)-β-glucan with (1→4)-α branches. The X-fraction showed anti-diabetic activity, which was found to be directly associated with insulin receptors [23]. Masuda et al. separated the MZ-fraction from G. frondosa, which had similar structure to the MD-fraction but with a much smaller molecular weight, of around 20,000 Da (the molecular weight of MD-fraction was around 1–2 million Da). The MZ-fraction showed immunomodulatory effects in vitro and antitumor activity in vivo [25]. Moreover, Grifolan (GRN), a gel-forming (1→6)-branched (1→3)-β-D-glucan, was found in G. frondosa with immunomodulatory effects [24]. α-D-glucan could also be extracted by hot water from G. frondosa. Instead of antitumor activity, hypoglycemic, hypolipidemic, antioxidative and immunomodulatory effects were discovered [26,52,53,54]. In recent years, Liu’s group from China has isolated various polysaccharides from G. frondosa with the names of GFP-A [55], LMw-GFP [51] and GFAP [56], all of which exhibited promising anti-tumor activities. Due to variations in external factors such as the fungal source and extraction temperature, the properties of polysaccharide fractions from different sources may vary significantly.

Table 4.
Bioactive polysaccharide fractions isolated from G. frondosa with significant medicinal values.
3.2. Proteins and Peptides
Several kinds of bioactive proteins and peptides have been isolated from G. frondosa with notable health benefits and medicinal values, although studies on these aspects are fewer than those on the bioactive polysaccharide fractions. Table 5 summarizes some typical bioactive proteins and peptides isolated from G. frondosa. As shown in the table, these bioactive proteins/peptides were extracted mainly from the G. frondosa fruiting body, with average molecular weights around 20–88 kDa.

Table 5.
Bioactive proteins and peptides isolated from G. frondosa with medicinal values.
The N-acetylgalactosamine-specific lectin GFL isolated from the G. frondosa fruiting body exhibited cytotoxicity against HeLa cells [71]. The protein designated GFAHP showed a significant anti-herpes simplex virus (HSV) effect, as reported by Gu and coworkers [35]. Glycoprotein is another type of bioactive protein in G. frondosa. Cui and coworkers isolated the glycoprotein (containing 6.2% carbohydrates) from cultured mycelia of G. frondosa and demonstrated its anti-tumor activity [32]. Zhuang and coworkers patented a bioactive glycoprotein from G. frondosa, which showed obvious anti-hypertensive, anti-obesity, anti-hyperlipidemic and anti-diabetic effects [34]. In addition to the glycoprotein, Chan et al. found that chemical phosphorylation of G. frondosa polysaccharide-peptides could remarkably enhance both tumor inhibition in vitro and adjuvant effects in vivo. Meanwhile, modified and unmodified MPSP both showed an insignificant effect on normal cells, indicating their potential application for anticancer therapy without significant side effects [72].
3.3. Other Bioactive Molecules
Apart from the macromolecular components, such as polysaccharides and proteins/peptides, bioactive small molecules have also been discovered in G. frondosa. Table 6 lists some bioactive small molecules in G. frondosa from representative research studies. The major small molecules discovered with bioactivities mainly include fatty acids, ergosterols, flavonoids, alkaloids, ascorbic acid and tocopherol.

Table 6.
Bioactive small molecules isolated from G. frondosa.
As reported by Zhang and coworkers, fatty acids and three compounds, namely ergosterol (1), ergostra-4,6,8(14),22-tetraen-3-one (2) and 1-oleoyl-2-linoleoyl-3-palmitoylglycerol (3), were extracted from the cultured mycelia of G. frondosa by hexane. The fatty acid fraction, together with all three compounds, exhibited cyclooxygenase (COX) enzyme inhibitory and anti-oxidant activities [73]. He and coworkers extracted a furanone named grifolaone A from G. frondosa, which showed specific antifungal activity against the opportunistic human pathogen of Pseudallescheria boydii and some plant pathogens [74]. Han and Cui isolated agaricoglycerides (AGF) from the fermented mycelium of G. frondosa. Their study suggested a promising possibility of using AGF as medicine for inflammatory pain with 500 mg/kg as the optimal dosage [36]. Chen et al. extracted three fractions, GF-1 to GF-3, from G. frondosa and discovered the inhibitory effect of GF-3 against the proliferation of human tumor cells and α-glucosidase. The major bioactive compounds in GF-3 were detected to be alkaloids (first found in G. frondosa), ergosterols and a new compound named pyrrolefronine. Since α-glucosidase was involved in the hydrolysis of starch into disaccharide sugars, inhibition of its activity indicated possible reduction of blood glucose level [37]. o-orsellinaldehyde, which showed obvious tumoricidal activity, especially selective cytotoxic effect against Hep 3B cells through apoptosis, was also extracted from a submerged culture of G. frondosa by Lin and Liu [38]. In addition, other bioactive molecules in G. frondosa, such as polyphenolics, α-tocopherol, ascorbic acid and flavonoids, were reported to have anti-oxidant properties [39].
4. Biological Activities and Medicinal Properties
4.1. Antitumor Effects
Previous studies over the past 30 years have strongly suggested that there are three possible ways by which G. frondosa exerts its anticancer effect—they are protection of healthy cells, prevention of tumor metastasis and inhibition of tumor growth. In other words, G. frondosa can fight against tumors both directly and indirectly via enhancement of the immune system. This section will mainly focus on the direct antitumor function of G. frondosa, whereas the immunomodulatory effect will be discussed in the next section.
The antitumor activity of G. frondosa was first reported by Miyazaki et al. in 1982 [16], followed by their further study on the chemical structure of glucans extracted from the G. frondosa fruiting body and their antitumor activity against Sarcoma 180 tumors in Institute of Cancer Research (ICR) mice [17]. Nanba’s group obtained different polysaccharide fractions and reported the D-fraction for the first time in 1988 [21]. Unlike many other antitumor polysaccharides derived from Basidiomycetes, which may become ineffective if administered orally, the D-fraction exhibited promising prospects, because it could be administered orally, intravenously and intraperitoneally [21]. Nanba and Kubo conducted a nonrandomized clinical study to assess the effects of the D-fraction from G. frondosa on 165 advanced cancer patients who received the D-fraction as crude powder tablets alone or in addition to chemotherapy. Results showed that G. frondosa was effective against breast, liver and lung cancers, but less effective against leukemia, stomach and bone cancers [22]. Further studies conducted by Alonso et al. demonstrated that the D-fraction was able to function on mammary tumor cells directly through the modulation of different cellular processes during cancer development [48]. Zhao and coworkers found that a combination of the D-fraction (0.2 mg/mL) and vitamin C (0.3 mmol/L) resulted in a 70% reduction in the viability of human hepatocarcinoma SMMC-7721 cells [76]. Further purification of the D-fraction yielded the MD-fraction, which, as described in the patent of Nanba and Kubo, showed even better results than the D-fraction in terms of the inhibitory effect on mouse tumor growth [22]. In addition to intraperitoneal injection in Nanba and Kubo’s test, the MD-fraction has also been demonstrated to inhibit tumor growth in mice via oral administration [77]. Both the D-fraction and the MD-fraction were proven safe, with low or no toxicity.
Apart from the D and MD-fractions, other polysaccharide fractions have also exhibited anti-tumor activity. As reported by Bie et al. [65], the polysaccharide GFP-A, isolated from G. frondosa, was able to inhibit the proliferation of human colon cancer HT-29 cells in vitro, with 180 μg/mL as the most effective concentration. Li and Liu reported that the polysaccharide fraction GFP-4, extracted from G. frondosa, showed an inhibitory effect on human lung cancer cells at 4 °C. The inhibitory effect became lower after heat treatment at over 30 °C due to structural changes [78]. Alonso and coworkers explained that the polysaccharides in G. frondosa could regulate gene expression involved in the apoptosis of breast cancer cells so that cell proliferation was inhibited and the cell cycle was blocked [79].
In addition to polysaccharide fractions, the ergosterol derivatives from non-polar extracts of G. frondosa were also found to have anti-proliferative effects on human tumor cells [37]. Moreover, the ο-orsellinaldehyde component of submerged cultures of G. frondosa exhibited tumoricidal activity against Hep 3B cells via apoptosis [38]. Some glycoproteins isolated from G. frondosa, such as GFL and GFG-3a, exhibited antitumor effects as well due to their anti-proliferative activity against cancer cells [32,71]. Table 7 summarizes the testing methods and potency of antitumor activity based on reported studies on G. frondosa. The testing methods for in vivo studies mainly include microscopy observation and assessment of the inhibition rate by measuring tumor weight, whereas for in vitro studies, the MTT assay is commonly used to determine cells’ viability. It is worth mentioning that G. frondosa was able to achieve an inhibition ratio of over 90% for the treatment of MM46 liver carcinoma, BEL 7402 cells and TMK-1 gastric cancer cells.

Table 7.
Bioactivity potency and testing methods of major bioactive molecules isolated from G. frondosa.
4.2. Immunomodulation
Immunomodulation is the most well-known effect of G. frondosa components and has been confirmed by many studies. These immunomodulatory components have been shown to enhance the actions of macrophages and many other immune-related cells, such as cytotoxic T-cells and natural killer (NK) cells [99]. Furthermore, G. frondosa components could increase the secretion of cytokines, which are signaling molecules, including interferons (IFN), interleukins (IL), tumor necrosis factors (TNF) and lymphokines with antiproliferative activity, causing apoptosis and differentiation in tumor cells, thus further increasing the efficiency of immune-related cells.
Polysaccharides have been recognized as the major immunomodulating components of G. frondosa. The D-fraction, in addition to its direct antitumor effect as mentioned previously, is also a major polysaccharide fraction of G. frondosa with significant immunomodulatory activity. Kodama and coworkers suggested that the D-fraction could activate NK cells through upregulating their expression of TNF-α and interferon-gamma (IFN-γ) proteins. Meanwhile, the D-fraction were also able increase macrophage-derived IL-12, which further activated NK cells, implying that the D-fraction could provide long-term tumor-suppressive effects [49,82]. Further investigation by Kodama et al. found that the application of the D-fraction could reduce the effective dosage of the chemotherapeutic agent, mitomycin-C (MMC) by increasing the proliferation, differentiation and activation of immunocompetent cells. It could also reduce the immunosuppressive activity caused by MMC [80].
Apart from the D-fraction, other polysaccharide fractions with immunomodulatory activity have also been isolated from G. frondosa. Ishibashi and coworkers isolated insoluble and a high-molecular-weight soluble forms of Grifolan (GRN) from G. frondosa, both of which can activate macrophages through triggering cytokine secretion to produce TNF [61,100]. Similarly, Mao et al. observed increased levels of TNF-α, IL-2, IL-1β and nitric oxide (NO) in the serum with the dosage of polysaccharide GP11 from G. frondosa, suggesting the activation of macrophages and the stimulation of tumoricidal activity [64]. Masuda and coworkers found that the anti-cancer activity of the polysaccharide fraction MZF from G. frondosa was associated with the activation of cell-mediated immunity resulting from the induction of macrophage proliferation, increasing levels of IL12, IL2, IFN-γ and TNF-α, as well as enhancement of NK cells and cytotoxic T lymphocytes [25,59]. The GFP fraction isolated by Meng et al. promoted the production of cytokines and chemokines such as IL-6, IFN-γ and TNF-α, and also effectively enhanced the proliferative activity of fibroblasts, contributing to strong immune-stimulating activity [68]. Table 7 presents the common testing methods and potency indices of immunomodulatory effects. In vitro testing is usually performed with cytokine production evaluated based on ELISA and macrophage activity by MTT assay.
4.3. Antiviral and Antibacterial Effects
There have been a number of studies reporting the beneficial effects of G. frondosa in the treatment of viral infections, including those caused by hepatitis B virus (HBV), enterovirus 71 (EV71), herpes simplex virus type 1 (HSV-1) and human immunodeficiency virus (HIV). Mayell and coworkers reported a study on patients with chronic hepatitis B. The results showed that patients who took G. frondosa fruiting body polysaccharides showed positive signs, specifically a higher recovery rate compared with the control group [1]. Nanba et al. reported that the MD-fraction from G. frondosa could fight against HIV through several pathways, including direct inhibition of HIV, stimulation of the natural defense system against HIV and a reduction in vulnerability to opportunistic infections [50]. The GFP1 fraction extracted by Zhao and coworkers was found to fight against EV71, the causative pathogen of hand-foot-and-mouth disease. The researchers found that G. frondosa could hinder EV71 viral replication, suppressing genomic RNA synthesis and protein expression, and thus could be used as a promising therapeutic compound for EV71 treatment [27]. In addition to polysaccharide fractions, the protein fraction GFAHP purified from G. frondosa by Gu et al., has also shown anti-viral effects. It significantly inhibited HSV-1 replication in vitro and reduced HSV-1 induced symptoms such as blepharitis in a murine model [35].
In addition to antiviral effect, the D-fraction isolated from G. frondosa has also shown antibacterial effects. Kodama and coworkers found that the mechanism of antibacterial action of D-fraction was related to the immune-stimulating activity. The D-fraction could activate immuno-competent cells and induce the production of cytokines, which further lead to the activity enhancement of splenic T cells to kill Listeria monocytogenes [84]. Unlike the antibacterial mechanism, the antiviral action of the D-fraction is not directly related to the immune system. According to Gu et al., the D-fraction interfered with HBV replication through the inhibition of HBV polymerase [85].
4.4. Antidiabetic Activity
The hypoglycemic effects of G. frondosa extracts have been demonstrated in multiple animal studies. To test the antidiabetic activity of active ingredients in G. frondosa, in vivo fasting serum glucose (FSG) or fasting blood glucose (FBG) measurements are generally performed after feeding the bioactive ingredients to animal models for 2 to 4 weeks (Table 7). A high FSG level is one of the characteristics of diabetes mellitus sufferers and the influence on FSG level could directly indicate the antidiabetic effect of active ingredients in G. frondosa.
The hypoglycemic mechanisms of these polysaccharide fractions are most likely to be linked to insulin activity. For instance, F2 and F3 polysaccharides and SX glycoprotein fractions have been suggested to exert hypoglycemic effect through insulin signal pathway [63,87]. Konno et al. reported that the SX-fraction could facilitate glucose uptake, leading to activation of the insulin receptor (IR) and insulin receptor substrate 1 (IRS-1), and eventually resulting in increased insulin secretion. In a normal situation, a high glucose level would lead to low glucose uptake, but the SX-fraction overcame this suppressive effect and impaired the insulin signaling pathway [87]. The hypoglycemic mechanisms of F2 and F3 polysaccharides were also related to IR and IRS-1. Xiao et al. reported that they could improve insulin sensitivity and decrease FSG levels by increasing protein levels of IR and decreasing protein levels of IRS-1 [63]. The anti-diabetic effects of MT-α-glucan, such as ameliorating insulin resistance of peripheral target tissue and improving insulin sensitivity, were also reported to be associated with IR [26].
In addition to the enhancement of insulin activity, the hypoglycemic effects of G. frondosa may be generated through the inhibition of α-glucosidase activity, because an anti-α-glucosidase effect could prevent starch hydrolysis into disaccharides and decrease the blood glucose level. Shen and coworkers examined the hyperglycemic effects of non-polar fractions in G. frondosa both in vivo and in vitro. Research findings showed that G. frondosa exhibited strong anti-α-glucosidase activity in vitro, and could significantly lower the blood glucose level in high-fat-diet-fed and streptozotocin-induced hyperglycemic animals [86]. Chen et al. attributed the anti-α-glucosidase effect to the pyrrole alkaloids and ergosterols extracted from G. frondosa [37], whereas Wu et al. suggested that the ergosterol peroxide isolated from G. frondosa contributed to its anti-diabetic effect [75]. However, Su and coworkers concluded that the strong anti-α-glucosidase activity was mainly attributed to the oleic acid and linoleic acid, rather than ergosterol and ergosterol peroxide in G. frondosa [101]. Some previous studies suggested that the anti-diabetic activity of G. frondosa originated from its regulatory effect on gut microbiota, which shall be discussed later in this paper.
4.5. Lipid Metabolism Regulation and Anti-Hypertension Effects
The effects of G. frondosa on lipid metabolism regulation and anti-hypertension have been shown in many reports. Kubo and Nanba found that with the G. frondosa fruiting body as the feed, the triglyceride, cholesterol and phospholipid levels in the serum of rats were suppressed by 30–80% compared with those of the control group of animals. Meanwhile, the weight of the extirpated liver was also 60–70% lower than that of the control group, and the corresponding cholesterol excretion ratio in feces increased by 1.8 times with G. frondosa treatment, further demonstrating that G. frondosa treatment helped improve lipid metabolism and inhibit increases in liver lipid and serum lipid after the ingestion of high-fat feed [88]. Similar results were obtained by Fukushima and coworkers, who showed that serum total cholesterol concentrations and very-low-density lipoprotein (VLDL) levels in rats fed with 50 g/kg G. frondosa were lowered compared with those of the control group (50 g/kg cellulose powder), and the fecal cholesterol excretion was significantly higher compared with the control group [89].
The antihypertensive effects of the active ingredients of G. frondosa have been determined mainly through the measurement of systolic blood pressure (SBP) in animal models, as summarized in Table 7. Kabir et al. conducted an experiment on hypertensive rats with a diet containing 5% G. frondosa or Lentinus edodes (L. edodes) mushroom powder. The results showed that both G. frondosa and L. edodes treatment could result in a significant decrease in the SBP of spontaneously hypertensive rats. The reduction in SBP level was similar for G. frodosa and L. edodes, which was around 15 mmHg after 63 days of a mushroom diet compared with the control group [102]. Their further research showed that G. frondosa could not only suppress the development of hypertension (preventive effect), but also lower elevated blood pressure (treatment effect) [103]. Preuss and coworkers compared rats fed with two commercially-available fractions of SX and D with a control group fed on a baseline diet and found that G. frondosa fractions could lessen age-related hypertension partly via their effects on the renin-angiotensin system [90].
4.6. Antioxidant Activities
Several components in G. frondosa, including polysaccharides, proteins, fatty acids and some other constituents, have shown notable antioxidant activities. The common activity assay methods and anti-oxidant potencies are summarized in Table 7. As shown in this table, the common antioxidant activity assays include the scavenging abilities of hydroxyl radicals, DPPH radicals, superoxide radicals and hydrogen peroxide; as well as the reducing power and Fe2+ chelating activity. Lee and coworkers suggested that polysaccharides from G. frondosa could be potential ingredients for cosmetic applications due to their antioxidant activity, radical scavenging activity after UV irradiation, proliferation of fibroblasts and collagen biosynthesis [92]. Similar findings were obtained by Chen and coworkers, who showed that the crude polysaccharide GFP, extracted from G. frondosa fruiting bodies, possessed significant inhibitory effects on hydroxyl, superoxide and DPPH radicals [93].
The antioxidant activity of G. frondosa polysaccharides could be further enhanced by incorporation of zinc or selenium. Zhang and coworkers used the strain of G. frondosa as a vector of zinc biotransformation to produce zinc-incorporated intracellular polysaccharides, which showed notable antioxidant and anti-aging activities compared with the corresponding non-zinc-incorporated intracellular polysaccharides [91]. Li and coworkers purified crude Se-polysaccharides (Se-GFP) from the fruiting bodies of Se-enriched G. frondosa and obtained a heteropolysaccaride of Se-GFP-22 with more remarkable antioxidant effects than that of non-Se-incorporated GFP-22. The antioxidant activity might be affected by the degree of branching, molecular weight and configuration, as well as the synergistic effect of polysaccharide and Se [67].
Other than polysaccharides, proteins, fatty acids and other molecules from G. frondosa such as phenols and flavonoids also showed antioxidant activities. Dong and coworkers hydrolyzed protein from the G. frondosa fruiting body using different proteases and found that trypsin hydrolysate had the strongest antioxidant potential, especially the GFHT-4 fraction, with a molecular weight lower than 3 kDa [94]. Moreover, according to Zhang et al., the inhibition levels of cyclooxygenase (COX)-1 enzyme and COX-2 enzyme activities by a fatty acid of G. frondosa were 98% and 99%, respectively. Moreover, the inhibition of liposome peroxidation by the fatty acid was also as high as 79% [73]. Yeh and coworkers obtained several antioxidant components including flavonoids, phenols, α-tocopherol and ascorbic acid from the ethanol, cold-water and hot-water extracts of G. frondosa. All of these extracts exhibited various antioxidant activities, including reducing power, chelating ferrous ions and scavenging DPPH and superoxide anions [39].
4.7. Gut Microbiota Regulation
In recent years, there has been growing evidence of the important role of gut microbiota in the mediation/action of the various health benefits of mushrooms, especially their polysaccharide components [104]. Many studies have investigated the regulation of gut microbiota by the bioactive polysaccharides from edible and medicinal mushrooms such as G. frondosa because the biological macromolecules of polysaccharides, which cannot be directly absorbed, can be utilized by intestinal flora [105]. Friedman reviewed mushroom polysaccharides and their anti-obesity, anti-diabetes, anti-cancer and antibiotic properties, and suggested that the regulation of gut microbiota by polysaccharides was the major mechanism behind these properties [106]. Specifically, the maintaining of gut microbiota homeostasis has been found to be related with improved treatment of type 2 diabetes mellitus (T2DM) [107] and non-alcoholic fatty liver disease (NAFLD) [108]. To evaluate the microbiota regulation activity of bioactive components, in vivo measurement of gut microbiota is usually preferred by high throughput sequencing, as shown in Table 7.
Very recently, Chen and coworkers reported the regulatory efficacy of a novel G. frondosa polysaccharide GFP-N on the intestinal microflora of diabetic groups in vivo using single-molecule real-time sequencing technology (SMRT) [31]. There were significant differences exhibited in the composition of microbial populations in gut microbiota between the GFP-N-treated group and the diabetic control group. The relative abundance of some bacterial species such as Lactobacillus acidophilus (L. acidophilus) and Bacteroides acidifaciens (B. acidifaciens) was increased with GFP-N treatment. L. acidophilus has been shown to delay the progression of high fructose-induced diabetes in rats [96], and B. acidifaciens has shown the potential for treatment of metabolic diseases such as obesity and diabetes [109]. Guo et al. obtained similar results, showing that GFP could regulate intestinal microflora by significantly elevating the relative abundance of Alistipes and Bacteroides and reducing Enterococcus, which was associated with the improved hyperlipidemia and hyperglycemia in T2DM induced by streptozotocin and a high-fat diet (HFD) [107]. The same research group also developed G. frondosa polysaccharide-chromium (III) (GFP-Cr(III)) through chelation because chromium (III) was the most important trace mineral for T2DM treatment. Compared with inorganic chromium, organic chromium (III) has been found to have much better effects, with lower toxicity and genotoxicity. The researchers found that GFP-Cr(III) not only had the effects of GFP as shown in their previous work but also significantly increased the relative abundance of Enterorhabdus and Coriobacteriaceae due to the presence of Cr(III) [110].
Additionally, GFP has also been found to regulate the gut microbiota of rats with non-alcoholic fatty liver disease (NAFLD). Li and coworkers found that GFP could partly recover the HFD-induced alteration of cecal microbiota structure [30]. GFP treatment could decrease the Firmicutes to Bacteroidetes ratio, indicating a lower possibility of lipid production from undigested carbohydrates [108]. Friedman also suggested in his review that the ratio decrease of the two major classes of gut bacteria, namely, Firmicutes and Bacteroides, could have fat-lowering effects in obesity treatment [106]. In addition, GFP supplementation significantly increased the proportion of Allobaculum, Bacteroides, Bifidobacterium and some other microbial groups in the cecal microbiota, which might boost the immune system of the host and the defense against NAFLD [30]. The boosting of the immune system may contribute to the anti-tumor and anti-inflammatory effect of GFP as well [29,111]. Nevertheless, the functions of gut microbiota on the various bioactivities of G. frondosa polysaccharides and other components still require further exploration.
5. Conclusions
Edible and medicinal fungi or mushrooms are among the most common sources of health foods and nutraceutical products. G. frondosa is one of the most widely explored fungal species for nutraceutical and therapeutic compounds. The fungal biomass of G. frondosa displays a high content of proteins and carbohydrates and a relatively low content of fat compared with other commonly cultivated mushrooms. The crude water extracts, isolated fractions and purified components have shown a number of bioactivities, including antitumor, immunomodulatory, antiviral, antibacterial, antidiabetic, lipid metabolism regulation, hypertension control and antioxidation. Some of these health effects may be associated with the regulation of the human gut microbiota. Polysaccharides, which represent the most significant bioactive components of G. frondosa, contribute to many of its bioactivities and health benefits. The most successful and valuable health products from this fungal species are represented by the polysaccharide fractions and polysaccharide protein complexes, including the D-fraction or the MD-fraction and Grifolan, which have been approved for human use in immunotherapy and complimentary treatment of cancers with chemotherapy and radiotherapy.
Although some of the constituents of G. frondosa have been widely used in health foods or dietary supplements, very few have been used in prescribed medication, which requires more rigorous assessment and clinical trials. For a wider and more reliable application of the various components in nutraceutical and therapeutic products, it is fundamental to gain a better understanding of the structure–bioactivity relationship and the underlying mechanisms of action in the human body. Structural modification of the polysaccharides is another feasible strategy to attain enhanced bioactivity and novel bioactive molecules. As for many food and medicinal products, good manufacturing practice (GMP) should be implemented in the production process and systems, and standardized protocols should be established and followed for the preparation and quality control of the useful components. With increasing public concern about health threats from food contamination, environmental pollution and new infectious organisms such as the COVID-19 virus, the protection of human health through the immunomodulatory and health-promoting functions of G. frondosa constituents is even more attractive and promising. Therefore, it is worthwhile to put more effort into the research and development of this edible fungal species.
Author Contributions
Original draft preparation, P.G.; literature search, table summary and editing, P.G. and K.-C.S.; review and editing, P.G., K.-C.S. and J.-Y.W.; funding acquisition, J.-Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
This review study used published data in the reference literatures.
Acknowledgments
This work was financially supported by the Hong Kong Polytechnic University.
Conflicts of Interest
No potential conflict of interest was reported by the authors.
Abbreviations
| B. acidifaciens: | Bacteroides acidifaciens |
| EV71: | enterovirus 71 |
| FBG: | fasting blood glucose |
| FSG: | fasting serum glucose |
| G. frondosa: | Grifola frondosa |
| GFP: | Grifola frondosa polysaccharide |
| GRN: | Grifolan |
| HBV: | hepatitis B virus |
| HFD: | high-fat diet |
| HIV: | human immunodeficiency virus |
| IFN: | interferons |
| IL: | interleukins |
| IR: | insulin receptor |
| IRS-1: | insulin receptor substrate 1 |
| HSV: | herpes simplex virus |
| L. acidophilus: | Lactobacillus acidophilus |
| L. edodes: | Lentinus edodes |
| NAFLD: | non-alcoholic fatty liver disease |
| NK cell: | natural killer cell |
| SBP: | systolic blood pressure |
| SSF: | solid-state fermentation |
| T2DM: | type 2 diabetes mellitus |
| TNF: | tumor necrosis factors |
References
- Mayell, M. Maitake extracts and their therapeutic potential—A review. Altern. Med. Rev. 2001, 6, 48–60. [Google Scholar] [PubMed]
- Mayuzumi, Y.; Mizuno, T., III. Cultivation methods of maitake (Grifola frondosa). Food Rev. Int. 1997, 13, 357–364. [Google Scholar] [CrossRef]
- Montoya Barreto, S.; Orrego Alzate, C.E.; Levin, L. Modeling Grifola frondosa fungal growth during solid-state fermentation. Eng. Life Sci. 2011, 11, 316–321. [Google Scholar] [CrossRef]
- Lee, B.C.; Bae, J.T.; Pyo, H.B.; Choe, T.B.; Kim, S.W.; Hwang, H.J.; Yun, J.W. Submerged culture conditions for the production of mycelial biomass and exopolysaccharides by the edible Basidiomycete Grifola frondosa. Enzym. Microb. Technol. 2004, 35, 369–376. [Google Scholar] [CrossRef]
- Takama, F.; Minomiya, S.; Yoda, R.; Ishii, H.; Muraki, S. Parenchyma cells, chemical components of maitake mushroom (Grifola frondosa SF Gray) cultured artificially, and their changes by storage and boiling. In Proceedings of the Eleventh International Scientific Congress on the Cultivation of Edible Fungi, Sydney, Australia, 14–19 August 1981. [Google Scholar]
- Shih, L.; Chou, B.-W.; Chen, C.-C.; Wu, J.-Y.; Hsieh, C. Study of mycelial growth and bioactive polysaccharide production in batch and fed-batch culture of Grifola frondosa. Bioresour. Technol. 2008, 99, 785–793. [Google Scholar] [CrossRef]
- Cohen, N.; Cohen, J.; Asatiani, M.D.; Varshney, V.K.; Yu, H.-T.; Yang, Y.-C.; Li, Y.-H.; Mau, J.-L.; Wasser, S.P. Chemical composition and nutritional and medicinal value of fruit bodies and submerged cultured mycelia of culinary-medicinal higher Basidiomycetes mushrooms. Int. J. Med. Mushrooms 2014, 16, 273–291. [Google Scholar] [CrossRef]
- Kurasawa, S.-I.; Sugahara, T.; Hayashi, J. Proximate and dietary fibre analysis of mushrooms. Nippon Shokuhin Kogyo Gakkaishi 1982, 29, 400–406. [Google Scholar] [CrossRef]
- Muratsubaki, T.; Sayama, K.; Sato, K. Change of constituents in fruit body formation of Grifola frondosa. Nippon Shokuhin Kogyo Gakkaishi 1986, 33, 181–185. [Google Scholar] [CrossRef][Green Version]
- Mau, J.-L.; Lin, H.-C.; Ma, J.-T.; Song, S.-F. Non-volatile taste components of several speciality mushrooms. Food Chem. 2001, 73, 461–466. [Google Scholar] [CrossRef]
- Huang, S.-J.; Tsai, S.-Y.; Lin, S.-Y.; Liang, C.-H.; Mau, J.-L. Nonvolatile taste components of culinary-medicinal Maitake mushroom, Grifola frondosa (Dicks.:Fr.) S.F. Gray. Int. J. Med. Mushrooms 2011, 13, 265–272. [Google Scholar] [CrossRef]
- Kawai, H.; Matsuzawa, M.; Tsutagawa, Y.; Sasaki, H.; Kasuga, A.; Aoyagi, Y. Relationship between fruiting bodies compositions and substrate in Hiratake and Maitake mushrooms cultivated on sawdust substrate beds. Nippon Shokuhin Kogyo Gakkaishi 1994, 41, 419–424. [Google Scholar] [CrossRef]
- Tabata, T.; Yamasaki, Y.; Ogura, T. Comparison of chemical compositions of Maitake (Grifola frondosa (Fr.) SF Gray) cultivated on logs and sawdust substrate. Food Sci. Technol. Res. 2004, 10, 21–24. [Google Scholar] [CrossRef][Green Version]
- Phillips, K.M.; Ruggio, D.M.; Horst, R.L.; Minor, B.; Simon, R.R.; Feeney, M.J.; Byrdwell, W.C.; Haytowitz, D.B. Vitamin D and sterol composition of 10 types of mushrooms from retail suppliers in the United States. J. Agric. Food Chem. 2011, 59, 7841–7853. [Google Scholar] [CrossRef] [PubMed]
- USDA. FoodData Central Search Results. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169403/nutrients (accessed on 4 January 2019).
- Miyazaki, T.; Yadomae, T.; Suzuki, I.; Nishijima, M.; Yui, S.; Oikawa, S.; Sato, K. Antitumor activity of fruiting bodies of cultured Grifola frondosa. Jpn. J. Med Mycol. 1982, 23, 261–263. [Google Scholar] [CrossRef]
- Ohno, N.; Suzuki, I.; Oikawa, S.; Sato, K.; Miyazaki, T.; Yadomae, T. Antitumor activity and structural characterization of glucans extracted from cultured fruit bodies of Grifola frondosa. Chem. Pharm. Bull. 1984, 32, 1142–1151. [Google Scholar] [CrossRef]
- Iino, K.; Ohno, N.; Suzuki, I.; Miyazaki, T.; Yadomae, T.; Oikawa, S.; Sato, K. Structural characterisation of a neutral antitumour β-d-glucan extracted with hot sodium hydroxide from cultured fruit bodies of Grifola frondosa. Carbohydr. Res. 1985, 141, 111–119. [Google Scholar] [CrossRef]
- Ohno, N.; Adachi, Y.; Suzuki, I.; Sato, K.; Oikawa, S.; Yadomae, T. Characterization of the antitumor glucan obtained from liquid-cultured Grifola frondosa. Chem. Pharm. Bull. 1986, 34, 1709–1715. [Google Scholar] [CrossRef]
- Nanba, H.; Hamaguchi, A.; Kuroda, H. The chemical structure of an antitumor polysaccharide in fruit bodies of Grifola frondosa (Maitake). Chem. Pharm. Bull. 1987, 35, 1162–1168. [Google Scholar] [CrossRef]
- Hishida, I.; Nanba, H.; Kuroda, H. Antitumor activity exhibited by orally administered extract from fruit body of Grifola frondosa (Maitake). Chem. Pharm. Bull. 1988, 36, 1819–1827. [Google Scholar] [CrossRef]
- Nanba, H.; Kubo, K. Antitumor Substance Extracted from Grifola. U.S. Patent 5,854,404, 29 December 1998. [Google Scholar]
- Kubo, K.; Aoki, H.; Nanba, H. Anti-diabetic activity present in the fruit body of Grifola frondosa (Maitake). I. Biol. Pharm. Bull. 1994, 17, 1106–1110. [Google Scholar] [CrossRef]
- Adachi, Y.; Okazaki, M.; Ohno, N.; Yadomae, T. Enhancement of cytokine production by macrophages stimulated with (1→3)-β-D-glucan, grifolan (GRN), isolated from Grifola frondosa. Biol. Pharm. Bull. 1994, 17, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Kodama, N.; Nanba, H. Macrophage J774. 1 cell is activated by MZ-Fraction (Klasma-MZ) polysaccharide in Grifola frondosa. Mycoscience 2006, 47, 360–366. [Google Scholar] [CrossRef]
- Lei, H.; Ma, X.; Wu, W. Anti-diabetic effect of an α-glucan from fruit body of maitake (Grifola frondosa) on KK-Ay mice. J. Pharm. Pharmacol. 2007, 59, 575–582. [Google Scholar] [CrossRef]
- Zhao, C.; Gao, L.; Wang, C.; Liu, B.; Jin, Y.; Xing, Z. Structural characterization and antiviral activity of a novel heteropolysaccharide isolated from Grifola frondosa against enterovirus 71. Carbohydr. Polym. 2016, 144, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-H.; Lu, M.-K.; Lu, T.-J.; Lai, M.-N.; Ng, L.-T. A (1→6)-Branched (1→4)-β-d-Glucan from Grifola frondosa Inhibits Lipopolysaccharide-Induced Cytokine Production in RAW264. 7 Macrophages by Binding to TLR2 Rather than Dectin-1 or CR3 Receptors. J. Nat. Prod. 2020, 83, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, M.; Yu, M.; Shen, M.; Wang, Q.; Yu, Y.; Xie, J. Natural polysaccharides exhibit anti-tumor activity by targeting gut microbiota. Int. J. Biol. Macromol. 2019, 121, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zeng, F.; Huang, Y.; Liu, B. The positive effects of Grifola frondosa heteropolysaccharide on NAFLD and regulation of the gut microbiota. Int. J. Mol. Sci. 2019, 20, 5302. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, D.; Wang, D.; Lai, S.; Zhong, R.; Liu, Y.; Yang, C.; Liu, B.; Sarker, M.R.; Zhao, C. Hypoglycemic activity and gut microbiota regulation of a novel polysaccharide from Grifola frondosa in type 2 diabetic mice. Food Chem. Toxicol. 2019, 126, 295–302. [Google Scholar] [CrossRef]
- Cui, F.; Zan, X.; Li, Y.; Yang, Y.; Sun, W.; Zhou, Q.; Yu, S.; Dong, Y. Purification and partial characterization of a novel anti-tumor glycoprotein from cultured mycelia of Grifola frondosa. Int. J. Biol. Macromol. 2013, 62, 684–690. [Google Scholar] [CrossRef]
- Tsao, Y.-W.; Kuan, Y.-C.; Wang, J.-L.; Sheu, F. Characterization of a novel maitake (Grifola frondosa) protein that activates natural killer and dendritic cells and enhances antitumor immunity in mice. J. Agric. Food Chem. 2013, 61, 9828–9838. [Google Scholar] [CrossRef]
- Zhuang, C.; Kawagishi, H.; Preuss, H.G. Glycoprotein with Antidiabetic, Antihypertensive, Antiobesity and Antihyperlipidemic Effects from Grifola frondosa, and a Method for Preparing Same. U.S. Patent 7,214,778, 8 May 2007. [Google Scholar]
- Gu, C.-Q.; Li, J.-W.; Chao, F.; Jin, M.; Wang, X.-W.; Shen, Z.-Q. Isolation, identification and function of a novel anti-HSV-1 protein from Grifola frondosa. Antivir. Res. 2007, 75, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Cui, B. Pharmacological and pharmacokinetic studies with agaricoglycerides, extracted from Grifola frondosa, in animal models of pain and inflammation. Inflammation 2012, 35, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yong, T.; Xiao, C.; Su, J.; Zhang, Y.; Jiao, C.; Xie, Y. Pyrrole alkaloids and ergosterols from Grifola frondosa exert anti-α-glucosidase and anti-proliferative activities. J. Funct. Foods 2018, 43, 196–205. [Google Scholar] [CrossRef]
- Lin, J.-T.; Liu, W.-H. ο-Orsellinaldehyde from the submerged culture of the edible mushroom Grifola frondosa exhibits selective cytotoxic effect against Hep 3B cells through apoptosis. J. Agric. Food Chem. 2006, 54, 7564–7569. [Google Scholar] [CrossRef]
- Yeh, J.-Y.; Hsieh, L.-H.; Wu, K.-T.; Tsai, C.-F. Antioxidant properties and antioxidant compounds of various extracts from the edible basidiomycete Grifola frondosa (Maitake). Molecules 2011, 16, 3197–3211. [Google Scholar] [CrossRef]
- Sim, K.Y.; Liew, J.Y.; Ding, X.Y.; Choong, W.S.; Intan, S. Effect of vacuum and oven drying on the radical scavenging activity and nutritional contents of submerged fermented Maitake (Grifola frondosa) mycelia. Food Sci. Technol. 2017, 37, 131–135. [Google Scholar] [CrossRef]
- Chang, S.-T.; Hayes, W.A. The Biology and Cultivation of Edible Mushrooms; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Ajlouni, S.O.; Beelman, R.B.; Thompson, D.B.; Mau, J.-L. Changes in soluble sugars in various tissues of cultivated mushrooms, Agaricus bisporus, during postharvest storage. In Developments in Food Science; Charalambous, G., Ed.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 37, pp. 1865–1880. [Google Scholar]
- Yoshida, H.; Sasaki, H.; Fujimoto, S.; Sugahara, T. The chemical components of the vegetative mycelia of Basidiomycetes. Nippon Shokuhin Kagaku Kogaku Kaishi 1996, 43, 748–755. [Google Scholar] [CrossRef]
- Tsai, S.-Y.; Weng, C.-C.; Huang, S.-J.; Chen, C.-C.; Mau, J.-L. Nonvolatile taste components of Grifola frondosa, Morchella esculenta and Termitomyces albuminosus mycelia. LWT Food Sci. Technol. 2006, 39, 1066–1071. [Google Scholar] [CrossRef]
- Sanmee, R.; Dell, B.; Lumyong, P.; Izumori, K.; Lumyong, S. Nutritive value of popular wild edible mushrooms from northern Thailand. Food Chem. 2003, 82, 527–532. [Google Scholar] [CrossRef]
- Su, C.-H.; Lai, M.-N.; Lin, C.-C.; Ng, L.-T. Comparative characterization of physicochemical properties and bioactivities of polysaccharides from selected medicinal mushrooms. Appl. Microbiol. Biotechnol. 2016, 100, 4385–4393. [Google Scholar] [CrossRef]
- Mizuno, T.; Zhuang, C. Maitake, Grifola frondosa: Pharmacological effects. Food Rev. Int. 1995, 11, 135–149. [Google Scholar] [CrossRef]
- Alonso, E.N.; Ferronato, M.J.; Gandini, N.A.; Fermento, M.E.; Obiol, D.J.; López Romero, A.; Arévalo, J.; Villegas, M.E.; Facchinetti, M.M.; Curino, A.C. Antitumoral effects of D-fraction from Grifola frondosa (maitake) mushroom in breast cancer. Nutr. Cancer 2017, 69, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Kodama, N.; Komuta, K.; Sakai, N.; Nanba, H. Effects of D-Fraction, a polysaccharide from Grifola frondosa on tumor growth involve activation of NK cells. Biol. Pharm. Bull. 2002, 25, 1647–1650. [Google Scholar] [CrossRef] [PubMed]
- Nanba, H.; Kodama, N.; Schar, D.; Turner, D. Effects of Maitake (Grifola frondosa) glucan in HIV-infected patients. Mycoscience 2000, 41, 293–295. [Google Scholar] [CrossRef]
- Ji, H.-Y.; Yu, J.; Liu, A.-J. Structural characterization of a low molecular weight polysaccharide from Grifola frondosa and its antitumor activity in H22 tumor-bearing mice. J. Funct. Foods 2019, 61, 103472. [Google Scholar] [CrossRef]
- Lei, H.; Wang, W.; Wang, Q.; Guo, S.; Wu, L. Antioxidant and immunomodulatory effects of a α-glucan from fruit body of maitake (Grifola frondosa). Food Agric. Immunol. 2013, 24, 409–418. [Google Scholar]
- Lei, H.; Guo, S.; Han, J.; Wang, Q.; Zhang, X.; Wu, W. Hypoglycemic and hypolipidemic activities of MT-α-glucan and its effect on immune function of diabetic mice. Carbohydr. Polym. 2012, 89, 245–250. [Google Scholar] [CrossRef]
- Ma, X.; Meng, M.; Han, L.; Cheng, D.; Cao, X.; Wang, C. Structural characterization and immunomodulatory activity of Grifola frondosa polysaccharide via toll-like receptor 4–mitogen-activated protein kinases–nuclear factor κB pathways. Food Funct. 2016, 7, 2763–2772. [Google Scholar] [CrossRef]
- Chen, X.; Ji, H.; Zhang, C.; Yu, J.; Liu, A. Structural characterization and antitumor activity of a novel polysaccharide from Grifola frondosa. J. Food Meas. Charact. 2020, 14, 272–282. [Google Scholar] [CrossRef]
- Yu, J.; Ji, H.-Y.; Liu, C.; Liu, A.-J. The structural characteristics of an acid-soluble polysaccharide from Grifola frondosa and its antitumor effects on H22-bearing mice. Int. J. Biol. Macromol. 2020, 158, 1288–1298. [Google Scholar] [CrossRef]
- Cui, F.; Tao, W.; Xu, Z.; Guo, W.; Xu, H.; Ao, Z.; Jin, J.; Wei, Y. Structural analysis of anti-tumor heteropolysaccharide GFPS1b from the cultured mycelia of Grifola frondosa GF9801. Bioresour. Technol. 2007, 98, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.-K.; Gu, Y.-A.; Jeong, Y.-T.; Jeong, H.; Song, C.-H. Chemical characteristics and immuno-modulating activities of exo-biopolymers produced by Grifola frondosa during submerged fermentation process. Int. J. Biol. Macromol. 2007, 41, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Matsumoto, A.; Toida, T.; Oikawa, T.; Ito, K.; Nanba, H. Characterization and antitumor effect of a novel polysaccharide from Grifola frondosa. J. Agric. Food Chem. 2009, 57, 10143–10149. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wang, Y.; Lv, X.; Shen, X.; Ni, X.; Ding, K. Structure of a β-glucan from Grifola frondosa and its antitumor effect by activating Dectin-1/Syk/NF-κB signaling. Glycoconj. J. 2012, 29, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, J.; Ni, X.; Li, J.; Liu, Q.; Dong, Q.; Duan, J.; Ding, K. Inducement of cytokine release by GFPBW2, a novel polysaccharide from fruit bodies of Grifola frondosa, through dectin-1 in macrophages. J. Agric. Food Chem. 2013, 61, 11400–11409. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, X.; Liao, W.; Fang, J.; Chen, X.; Dong, Q.; Ding, K. A heteropolysaccharide, l-fuco-d-manno-1, 6-α-d-galactan extracted from Grifola frondosa and antiangiogenic activity of its sulfated derivative. Carbohydr. Polym. 2014, 101, 631–641. [Google Scholar] [CrossRef]
- Xiao, C.; Wu, Q.; Xie, Y.; Zhang, J.; Tan, J. Hypoglycemic effects of Grifola frondosa (Maitake) polysaccharides F2 and F3 through improvement of insulin resistance in diabetic rats. Food Funct. 2015, 6, 3567–3575. [Google Scholar] [CrossRef]
- Mao, G.-H.; Ren, Y.; Feng, W.-W.; Li, Q.; Wu, H.-Y.; Jin, D.; Zhao, T.; Xu, C.-Q.; Yang, L.-Q.; Wu, X.-Y. Antitumor and immunomodulatory activity of a water-soluble polysaccharide from Grifola frondosa. Carbohydr. Polym. 2015, 134, 406–412. [Google Scholar] [CrossRef]
- Bie, N.; Han, L.; Wang, Y.; Wang, X.; Wang, C. A polysaccharide from Grifola frondosa fruit body induces HT-29 cells apoptosis by PI3K/AKT-MAPKs and NF-κB-pathway. Int. J. Biol. Macromol. 2020, 147, 79–88. [Google Scholar] [CrossRef]
- Mao, G.-H.; Ren, Y.; Li, Q.; Wu, H.-Y.; Jin, D.; Zhao, T.; Xu, C.-Q.; Zhang, D.-H.; Jia, Q.-D.; Bai, Y.-P. Anti-tumor and immunomodulatory activity of selenium (Se)-polysaccharide from Se-enriched Grifola frondosa. Int. J. Biol. Macromol. 2016, 82, 607–613. [Google Scholar] [CrossRef]
- Li, Q.; Wang, W.; Zhu, Y.; Chen, Y.; Zhang, W.; Yu, P.; Mao, G.; Zhao, T.; Feng, W.; Yang, L. Structural elucidation and antioxidant activity a novel Se-polysaccharide from Se-enriched Grifola frondosa. Carbohydr. Polym. 2017, 161, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Cheng, D.; Han, L.; Chen, Y.; Wang, C. Isolation, purification, structural analysis and immunostimulatory activity of water-soluble polysaccharides from Grifola Frondosa fruiting body. Carbohydr. Polym. 2017, 157, 1134–1143. [Google Scholar] [CrossRef]
- Zhang, A.; Deng, J.; Yu, S.; Zhang, F.; Linhardt, R.J.; Sun, P. Purification and structural elucidation of a water-soluble polysaccharide from the fruiting bodies of the Grifola frondosa. Int. J. Biol. Macromol. 2018, 115, 221–226. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, F.; Chen, G.; Chen, Y.; Zhang, W.; Mao, G.; Zhao, T.; Zhang, M.; Yang, L.; Wu, X. Purification, characterization and immunomodulatory activity of a novel polysaccharide from Grifola frondosa. Int. J. Biol. Macromol. 2018, 111, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, H.; Nomura, A.; Mizuno, T.; Kimura, A.; Chiba, S. Isolation and characterization of a lectin from Grifola frondosa fruiting bodies. BBA Gen. Subj. 1990, 1034, 247–252. [Google Scholar] [CrossRef]
- Chan, J.Y.-Y.; Chan, E.; Chan, S.-W.; Sze, S.-Y.; Chan, M.-F.; Tsui, S.-H.; Leung, K.-Y.; Chan, R.Y.-K.; Chung, I.Y.-M. Enhancement of in vitro and in vivo anticancer activities of polysaccharide peptide from Grifola frondosa by chemical modifications. Pharm. Biol. 2011, 49, 1114–1120. [Google Scholar] [CrossRef]
- Zhang, Y.; Mills, G.L.; Nair, M.G. Cyclooxygenase inhibitory and antioxidant compounds from the mycelia of the edible mushroom Grifola frondosa. J. Agric. Food Chem. 2002, 50, 7581–7585. [Google Scholar] [CrossRef]
- He, X.; Du, X.; Zang, X.; Dong, L.; Gu, Z.; Cao, L.; Chen, D.; Keyhani, N.O.; Yao, L.; Qiu, J. Extraction, identification and antimicrobial activity of a new furanone, grifolaone A, from Grifola frondosa. Nat. Prod. Res. 2016, 30, 941–947. [Google Scholar] [CrossRef]
- Wu, S.-J.; Tung, Y.-J.; Ng, L.-T. Anti-diabetic effects of Grifola frondosa bioactive compound and its related molecular signaling pathways in palmitate-induced C2C12 cells. J. Ethnopharmacol. 2020, 260, 112962. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, Y.-F.; Song, L.; Jin, J.-X.; Zhang, Y.-Q.; Gan, H.-Y.; Yang, K.-H. Synergistic apoptotic effect of d-fraction from Grifola frondosa and vitamin C on hepatocellular carcinoma SMMC-7721 cells. Integr. Cancer Ther. 2017, 16, 205–214. [Google Scholar] [CrossRef]
- Masuda, Y.; Inoue, H.; Ohta, H.; Miyake, A.; Konishi, M.; Nanba, H. Oral administration of soluble β-glucans extracted from Grifola frondosa induces systemic antitumor immune response and decreases immunosuppression in tumor-bearing mice. Int. J. Cancer 2013, 133, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Q.; Liu, A.-J. Relationship between heat treatment on structural properties and antitumor activity of the cold-water soluble polysaccharides from Grifola frondosa. Glycoconj. J. 2020, 37, 107–117. [Google Scholar] [CrossRef]
- Alonso, E.N.; Orozco, M.; Nieto, A.E.; Balogh, G.A. Genes related to suppression of malignant phenotype induced by Maitake D-Fraction in breast cancer cells. J. Med. Food 2013, 16, 602–617. [Google Scholar] [CrossRef] [PubMed]
- Kodama, N.; Murata, Y.; Asakawa, A.; Inui, A.; Hayashi, M.; Sakai, N.; Nanba, H. Maitake D-Fraction enhances antitumor effects and reduces immunosuppression by mitomycin-C in tumor-bearing mice. Nutrition 2005, 21, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Shomori, K.; Yamamoto, M.; Arifuku, I.; Teramachi, K.; Ito, H. Antitumor effects of a water-soluble extract from Maitake (Grifola frondosa) on human gastric cancer cell lines. Oncol. Rep. 2009, 22, 615–620. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kodama, N.; Kakuno, T.; Nanba, H. Stimulation of the natural immune system in normal mice by polysaccharide from maitake mushroom. Mycoscience 2003, 44, 257–261. [Google Scholar] [CrossRef]
- Wu, M.-J.; Cheng, T.-L.; Cheng, S.-Y.; Lian, T.-W.; Wang, L.; Chiou, S.-Y. Immunomodulatory properties of Grifola frondosa in submerged culture. J. Agric. Food Chem. 2006, 54, 2906–2914. [Google Scholar] [CrossRef]
- Kodama, N.; Yamada, M.; Nanba, H. Addition of Maitake D-fraction reduces the effective dosage of Vancomycin for the treatment of Listeria-infected mice. Jpn. J. Pharmacol. 2001, 87, 327–332. [Google Scholar] [CrossRef]
- Gu, C.-Q.; Li, J.-W.; Chao, F.-H. Inhibition of hepatitis B virus by D-fraction from Grifola frondosa: Synergistic effect of combination with interferon-α in HepG2 2.2.15. Antivir. Res. 2006, 72, 162–165. [Google Scholar] [CrossRef]
- Shen, K.-P.; Su, C.-H.; Lu, T.-M.; Lai, M.-N.; Ng, L.-T. Effects of Grifola frondosa non-polar bioactive components on high-fat diet fed and streptozotocin-induced hyperglycemic mice. Pharm. Biol. 2015, 53, 705–709. [Google Scholar] [CrossRef]
- Konno, S.; Alexander, B.; Zade, J.; Choudhury, M. Possible hypoglycemic action of SX-fraction targeting insulin signal transduction pathway. Int. J. Gen. Med. 2013, 6, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.; Nanba, H. Anti-hyperliposis effect of Maitake fruit body (Grifola frondosa). I. Biol. Pharm. Bull. 1997, 20, 781–785. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fukushima, M.; Ohashi, T.; Fujiwara, Y.; Sonoyama, K.; Nakano, M. Cholesterol-lowering effects of maitake (Grifola frondosa) fiber, shiitake (Lentinus edodes) fiber, and enokitake (Flammulina velutipes) fiber in rats. Exp. Biol. Med. 2001, 226, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Preuss, H.G.; Echard, B.; Bagchi, D.; Perricone, N.V. Maitake mushroom extracts ameliorate progressive hypertension and other chronic metabolic perturbations in aging female rats. Int. J. Med Sci. 2010, 7, 169. [Google Scholar] [CrossRef][Green Version]
- Zhang, C.; Gao, Z.; Hu, C.; Zhang, J.; Sun, X.; Rong, C.; Jia, L. Antioxidant, antibacterial and anti-aging activities of intracellular zinc polysaccharides from Grifola frondosa SH-05. Int. J. Biol. Macromol. 2017, 95, 778–787. [Google Scholar] [CrossRef]
- Lee, B.C.; Bae, J.T.; Pyo, H.B.; Choe, T.B.; Kim, S.W.; Hwang, H.J.; Yun, J.W. Biological activities of the polysaccharides produced from submerged culture of the edible Basidiomycete Grifola frondosa. Enzym. Microb. Technol. 2003, 32, 574–581. [Google Scholar] [CrossRef]
- Chen, G.-t.; Ma, X.-m.; Liu, S.-t.; Liao, Y.-l.; Zhao, G.-q. Isolation, purification and antioxidant activities of polysaccharides from Grifola frondosa. Carbohydr. Polym. 2012, 89, 61–66. [Google Scholar] [CrossRef]
- DonG, Y.; Qi, G.; YanG, Z.; WanG, H.; WanG, S.; CHen, G. Preparation, separation and antioxidant properties of hydrolysates derived from Grifola frondosa protein. Czech J. Food Sci. 2015, 33, 500–506. [Google Scholar] [CrossRef]
- Li, L.; Guo, W.-L.; Zhang, W.; Xu, J.-X.; Qian, M.; Bai, W.-D.; Zhang, Y.-Y.; Rao, P.-F.; Ni, L.; Lv, X.-C. Grifola frondosa polysaccharides ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet fed rats. Food Funct. 2019, 10, 2560–2572. [Google Scholar] [CrossRef]
- Yadav, H.; Jain, S.; Sinha, P. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition 2007, 23, 62–68. [Google Scholar] [CrossRef]
- Pan, Y.; Wan, X.; Zeng, F.; Zhong, R.; Guo, W.; Lv, X.-C.; Zhao, C.; Liu, B. Regulatory effect of Grifola frondosa extract rich in polysaccharides and organic acids on glycolipid metabolism and gut microbiota in rats. Int. J. Biol. Macromol. 2019, 155, 1030–1039. [Google Scholar] [CrossRef]
- Pan, Y.-Y.; Zeng, F.; Guo, W.-L.; Li, T.-T.; Jia, R.-B.; Huang, Z.-R.; Lv, X.-C.; Zhang, J.; Liu, B. Effect of Grifola frondosa 95% ethanol extract on lipid metabolism and gut microbiota composition in high-fat diet-fed rats. Food Funct. 2018, 9, 6268–6278. [Google Scholar] [CrossRef] [PubMed]
- Kodama, N.; Murata, Y.; Nanba, H. Administration of a polysaccharide from Grifola frondosa stimulates immune function of normal mice. J. Med. Food 2004, 7, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.-I.; Miura, N.N.; Adachi, Y.; Ohno, N.; Yadomae, T. Relationship between solubility of Grifolan, a fungal 1,3-beta;-D-Glucan, and production of tumor necrosis factor by macrophages in vitro. Biosci. Biotechnol. Biochem. 2001, 65, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Su, C.H.; Lu, T.M.; Lai, M.N.; Ng, L.T. Inhibitory potential of Grifola frondosa bioactive fractions on α-amylase and α-glucosidase for management of hyperglycemia. Biotechnol. Appl. Biochem. 2013, 60, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Kabir, Y.; Yamaguchi, M.; Kimura, S. Effect of Shiitake (Lentinus edodes) and Maitake (Grjfola frondosa) mushrooms on blood pressure and plasma lipids of spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. 1987, 33, 341–346. [Google Scholar] [CrossRef]
- Kabir, Y.; Kimura, S. Dietary mushrooms reduce blood pressure in spontaneously hypertensive rats (SHR). J. Nutr. Sci. Vitaminol. 1989, 35, 91–94. [Google Scholar] [CrossRef]
- Jayachandran, M.; Xiao, J.; Xu, B. A critical review on health promoting benefits of edible mushrooms through gut microbiota. Int. J. Mol. Sci. 2017, 18, 1934. [Google Scholar] [CrossRef]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N. Dietary intervention impact on gut microbial gene richness. Nature 2013, 500, 585–588. [Google Scholar] [CrossRef]
- Friedman, M. Mushroom polysaccharides: Chemistry and antiobesity, antidiabetes, anticancer, and antibiotic properties in cells, rodents, and humans. Foods 2016, 5, 80. [Google Scholar] [CrossRef]
- Guo, W.-L.; Deng, J.-C.; Pan, Y.-Y.; Xu, J.-X.; Hong, J.-L.; Shi, F.-F.; Liu, G.-L.; Qian, M.; Bai, W.-D.; Zhang, W.; et al. Hypoglycemic and hypolipidemic activities of Grifola frondosa polysaccharides and their relationships with the modulation of intestinal microflora in diabetic mice induced by high-fat diet and streptozotocin. Int. J. Biol. Macromol. 2020, 153, 1231–1240. [Google Scholar] [CrossRef]
- Gangarapu, V.; Yildiz, K.; İnce, A.T.; Baysal, B. Role of gut microbiota: Obesity and NAFLD. Turk. J. Gastroenterol. 2014, 25, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, Y.; Kim, Y.; Lee, S.; Ryu, S.; Fukuda, S.; Hase, K.; Yang, C.; Lim, H.; Kim, M. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2017, 10, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.-L.; Chen, M.; Pan, W.-L.; Zhang, Q.; Xu, J.-X.; Lin, Y.-C.; Li, L.; Liu, B.; Bai, W.-D.; Zhang, Y.-Y.; et al. Hypoglycemic and hypolipidemic mechanism of organic chromium derived from chelation of Grifola frondosa polysaccharide-chromium (III) and its modulation of intestinal microflora in high fat-diet and STZ-induced diabetic mice. Int. J. Biol. Macromol. 2020, 145, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, W.; Yu, N.; Sun, J.; Yu, X.; Li, X.; Xing, Y.; Yan, D.; Ding, Q.; Xiu, Z. Anti-diabetic effect of baicalein is associated with the modulation of gut microbiota in streptozotocin and high-fat-diet induced diabetic rats. J. Funct. Foods 2018, 46, 256–267. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).