Understanding the Determination of Meat Quality Using Biochemical Characteristics of the Muscle: Stress at Slaughter and Other Missing Keys
Abstract
1. Introduction
2. Stress and Meat Quality
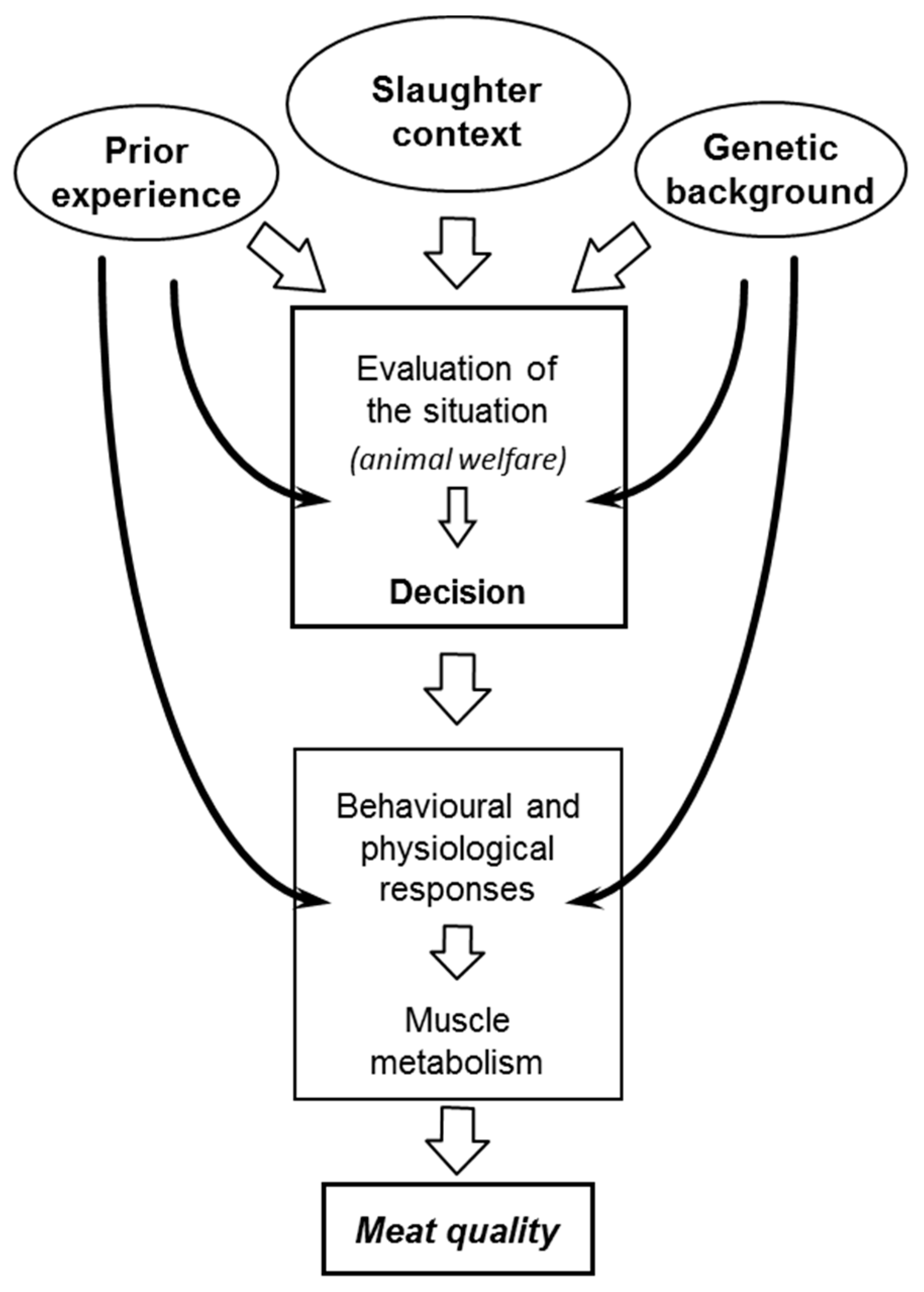
2.1. Physiological Mechanisms Underlying the Effects of Stress on Meat Quality
2.2. Tell Me Who’s Least Stressed, I’ll Tell You Whose Meat Is Best
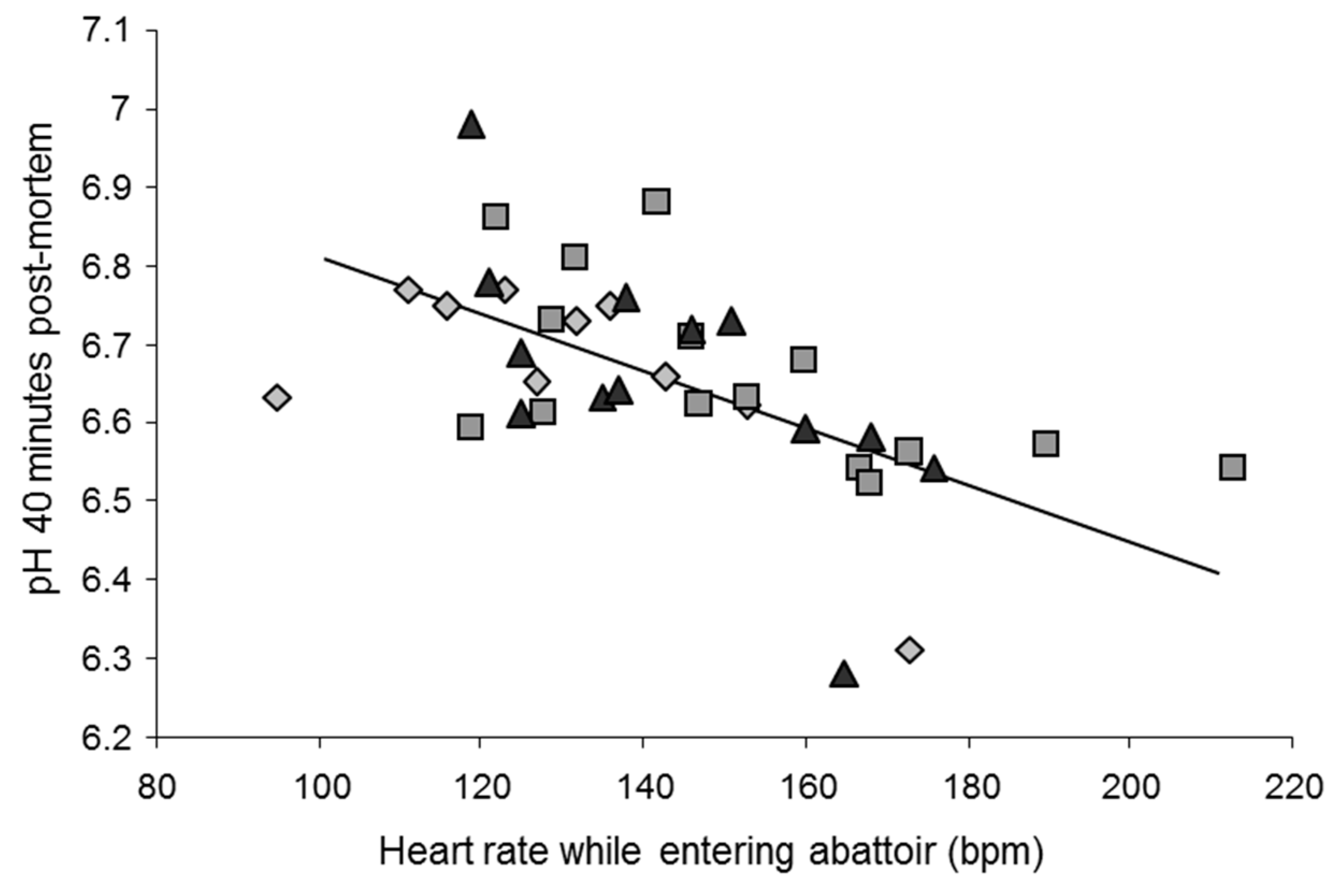
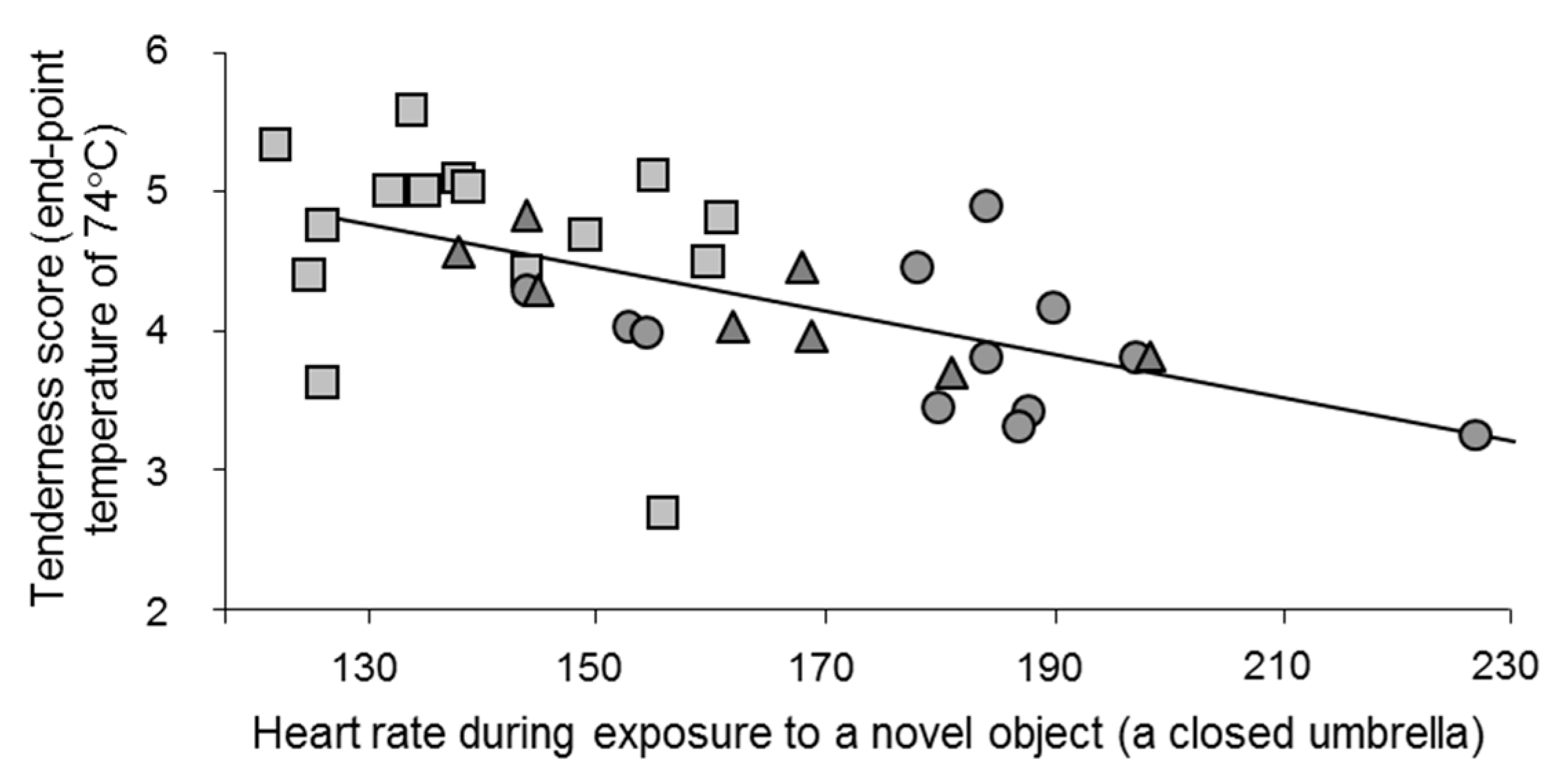
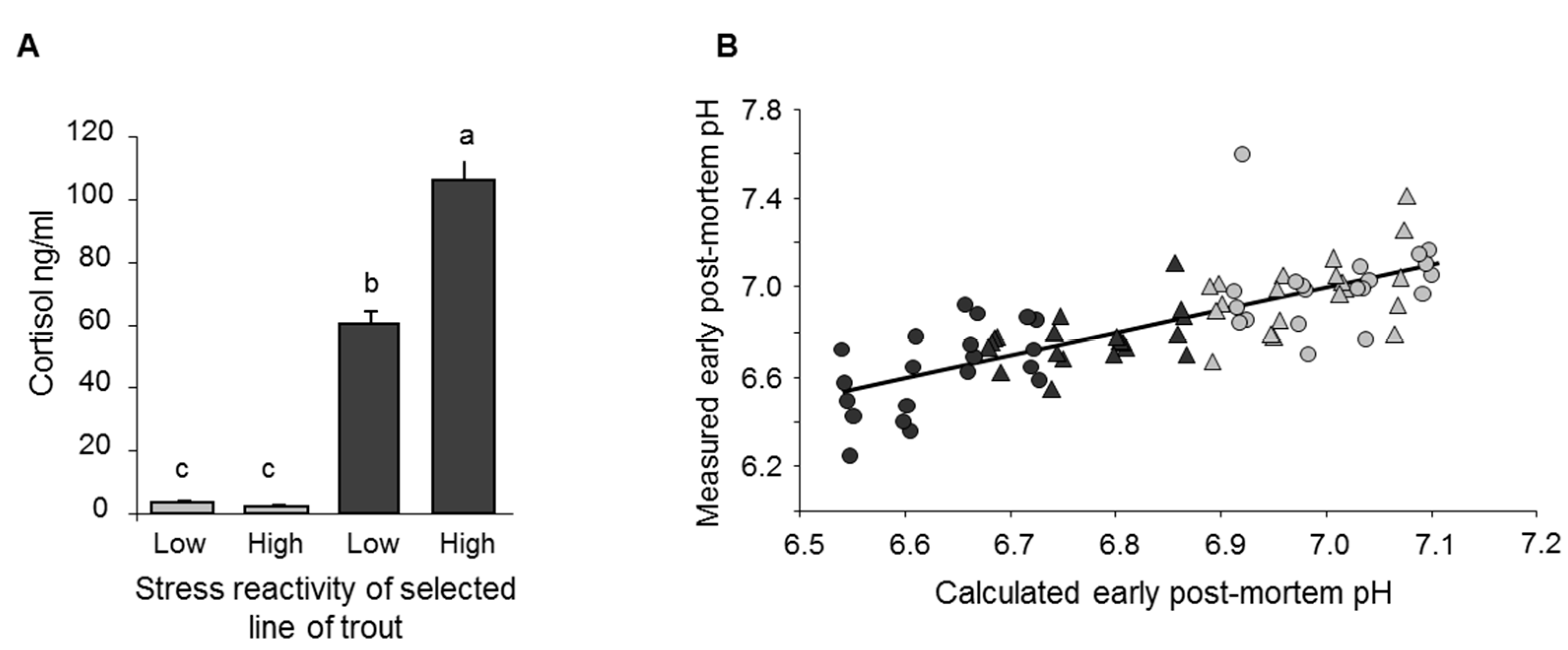
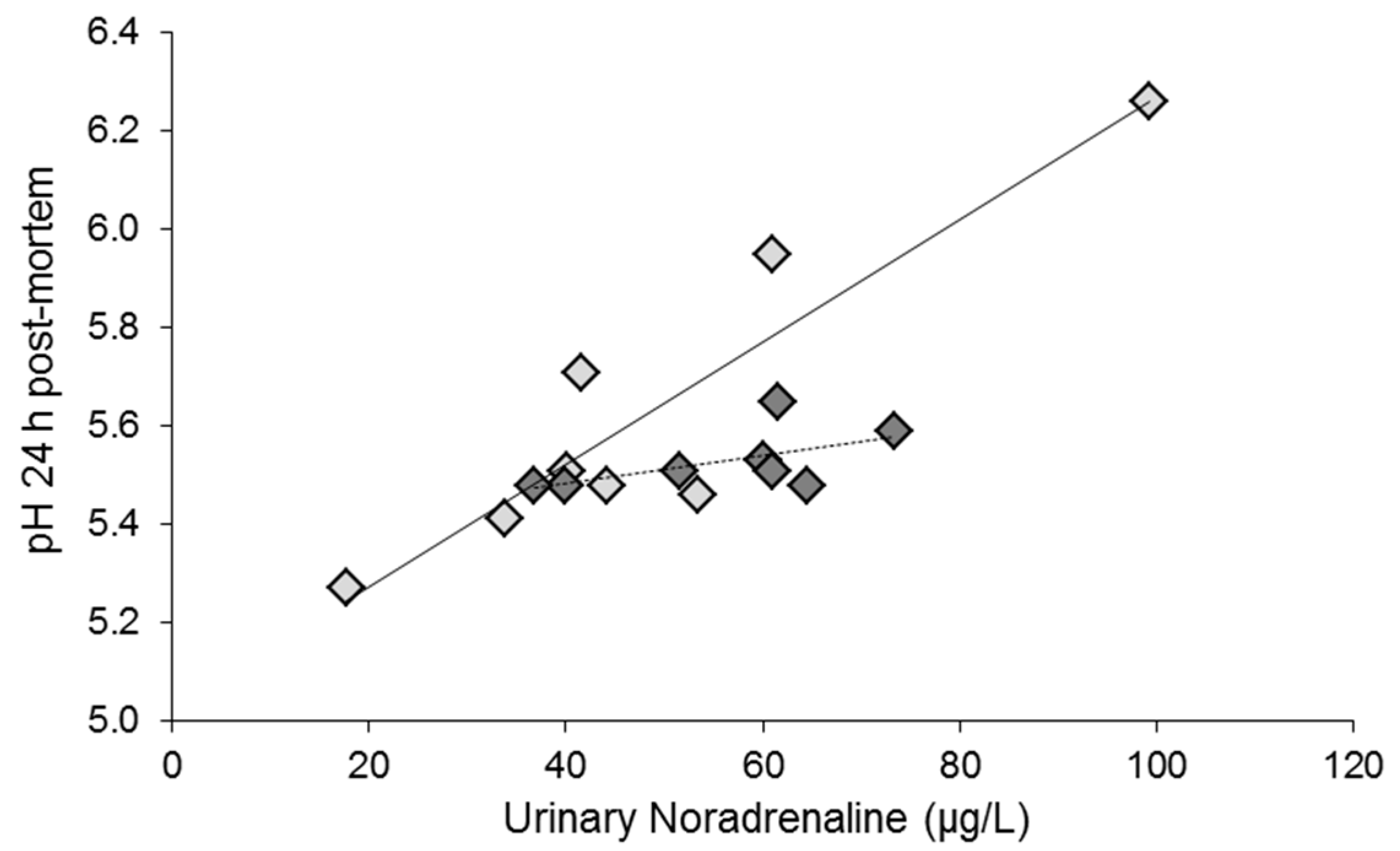
2.3. Predicting Stress Reactions Is Predicting Meat Quality
2.4. Stress at Slaughter: Lessons to Be Learned
3. Multiple Factors Begging Our Attention
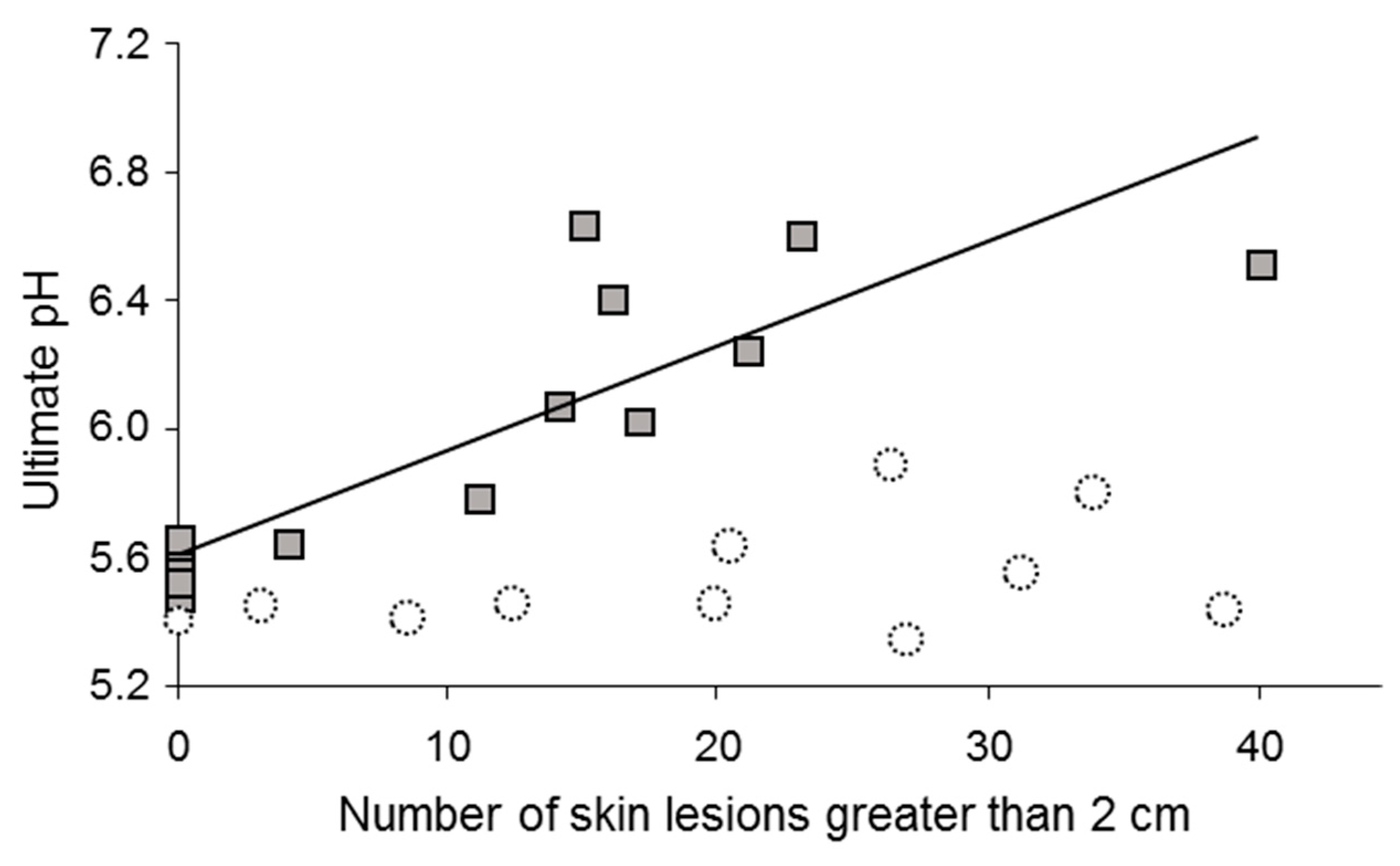
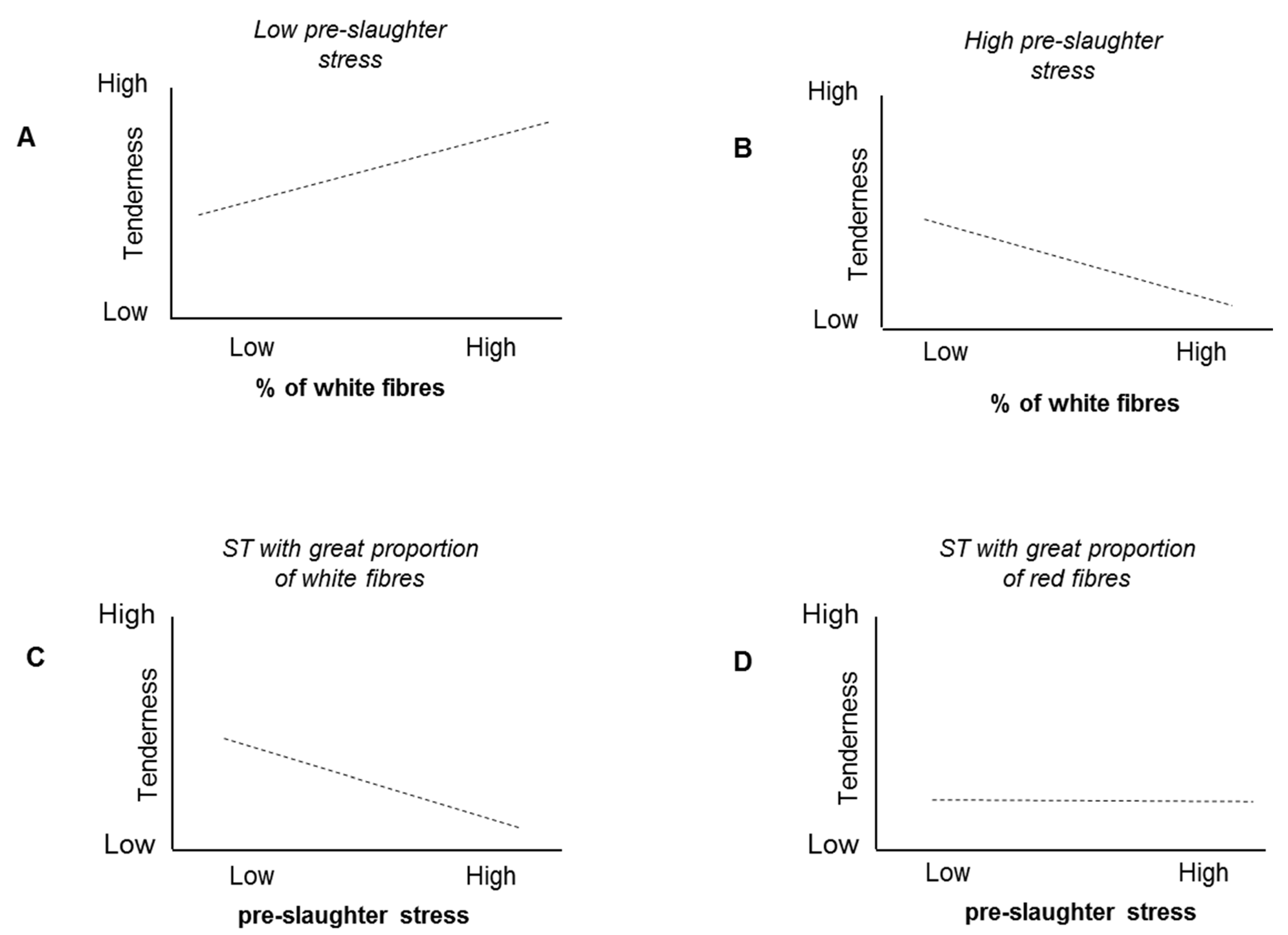
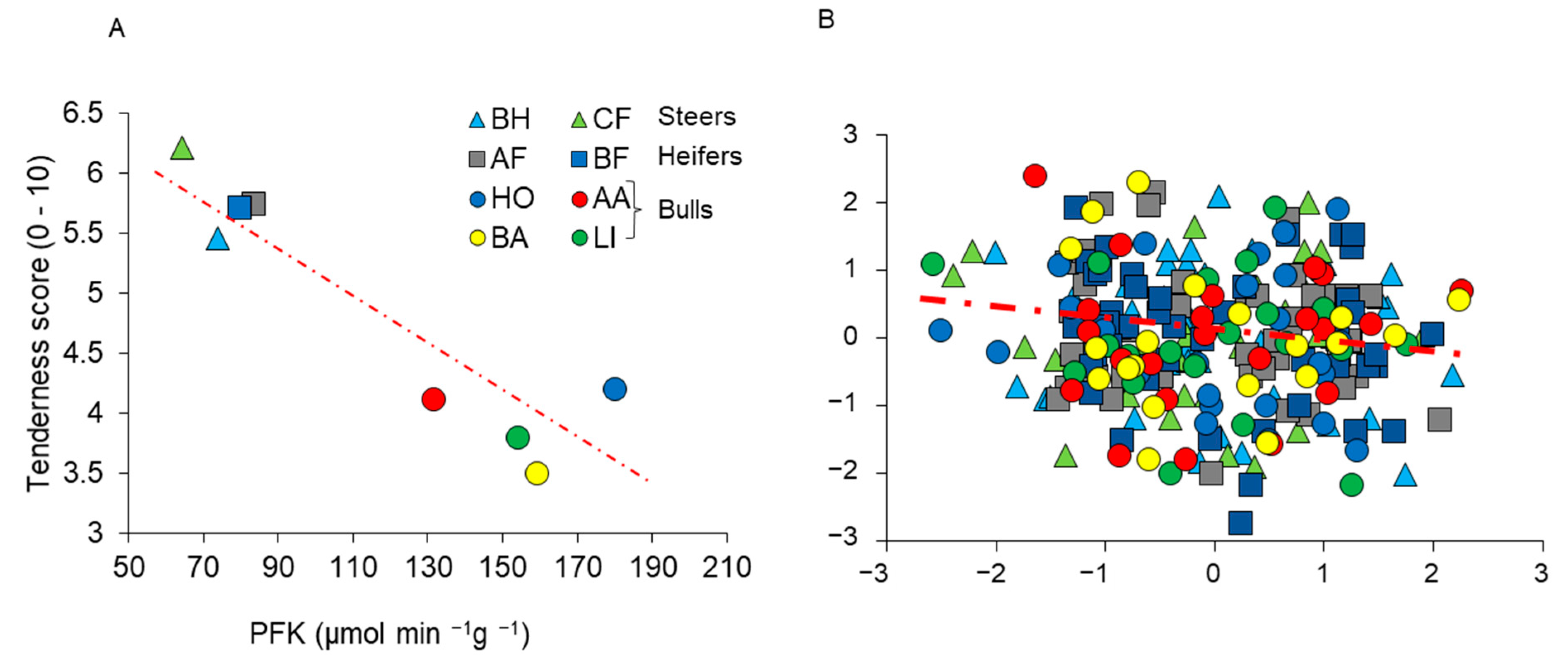
3.1. Grasping the Erratic Behavior of Correlations: Too Many Uncontrolled Factors!
3.2. How Many Pathways Lead to a Phenotype? How Many Ways to Make a Tasty Soup?
3.3. Multifactorial Phenotypes: Lessons to Be Learned
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ouali, A.; Gagaoua, M.; Boudida, Y.; Becila, S.; Boudjellal, A.; Herrera-Mendez, C.H.; Sentandreu, M.A. Biomarkers of meat tenderness: Present knowledge and perspectives in regards to our current understanding of the mechanisms involved. Meat Sci. 2013, 95, 854–870. [Google Scholar] [CrossRef]
- Gagaoua, M.; Terlouw, E.M.C.; Boudjellal, A.; Picard, B. Coherent correlation networks among protein biomarkers of beef tenderness: What they reveal. J. Proteom. 2015, 128, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Gagaoua, M.; Terlouw, E.M.C.; Picard, B. The study of protein biomarkers to understand the biochemical processes underlying beef color development in young bulls. Meat Sci. 2017, 134, 18–27. [Google Scholar] [CrossRef]
- Gagaoua, M.; Terlouw, E.M.C.; Mullen, A.M.; Franco, D.; Warner, R.D.; Lorenzo, J.M.; Purslow, P.P.; Gerrard, D.; Hopkins, D.L.; Troy, D.; et al. Molecular signatures of beef tenderness: Underlying mechanisms based on integromics of protein biomarkers from multi-platform proteomics studies. Meat Sci. 2021, 172, 108311. [Google Scholar] [CrossRef]
- Lovatt, S.J.; Devine, C.E. MODELING IN MEAT SCIENCE|Meat Quality. In Encyclopedia of Meat Sciences, 2nd ed.; Dikeman, M., Devine, C., Eds.; Academic Press: Oxford, UK, 2014; pp. 425–429. [Google Scholar] [CrossRef]
- Berri, C.; Picard, B.; Lebret, B.; Andueza, D.; Lefèvre, F.; Le Bihan-Duval, E.; Beauclercq, S.; Chartrin, P.; Vautier, A.; Legrand, I.; et al. Predicting the quality of meat: Myth or reality? Foods 2019, 8, 436. [Google Scholar] [CrossRef]
- Hocquette, J.-F.; Oury, M.; Legrand, I.; Pethick, D.; Gardner, G.; Wierzbicki, J.; Polkinghorne, R. Research in beef tenderness and palatability in the era of big data. Meat Muscle Biol. 2020, 4, 1–13. [Google Scholar] [CrossRef]
- Gagaoua, M.; Picard, B.; Soulat, J.; Monteils, V. Clustering of sensory eating qualities of beef: Consistencies and differences within carcass, muscle, animal characteristics and rearing factors. Livest. Sci. 2018, 214, 245–258. [Google Scholar] [CrossRef]
- Gagaoua, M.; Picard, B.; Monteils, V. Assessment of cattle inter-individual cluster variability: The potential of continuum data from the farm-to-fork for ultimate beef tenderness management. J. Sci. Food Agric. 2019, 99, 4129–4141. [Google Scholar] [CrossRef]
- Offer, G. Modelling of the formation of pale, soft and exudative meat: Effects of chilling regime and rate and extent of glycolysis. Meat Sci. 1991, 30, 157–184. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Hopkins, D.L.; Bruce, H.; Li, D.; Baldi, G.; El-din Bekhit, A. Causes and contributing factors to dark cutting meat: Current trends and future directions: A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 400–430. [Google Scholar] [CrossRef]
- Aalhus, J.L.; Best, D.R.; Murray, A.C.; Jones, S.D.M. A comparison of the quality characteristics of pale, soft and exudative beef and pork. J. Muscle Foods 1998, 9, 267–280. [Google Scholar] [CrossRef]
- Lawrie, R. Metabolic stresses which affect muscle. Physiol. Biochem. Muscle Food 1966, 137–164. [Google Scholar]
- Bendall, J.R. Post-Mortem Changes in Muscle; Structure and Function of Muscle; Academic Press: New York, NY, USA, 1973; pp. 243–309. [Google Scholar]
- Robergs, R.A.; Ghiasvand, F.; Parker, D. Biochemistry of exercise-induced metabolic acidosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Hambrecht, E.; Eissen, J.J.; Newman, D.J.; Smits, C.H.M.; Verstegen, M.W.A.; den Hartog, L.A. Preslaughter handling effects on pork quality and glycolytic potential in two muscles differing in fiber type composition. J. Anim. Sci. 2005, 83, 900–907. [Google Scholar] [CrossRef]
- Fraser, D.; Ritchie, J.S.D.; Faser, A.F. The term stress in a veterinary context. Br. Vet. J. 1975, 131, 653–662. [Google Scholar] [CrossRef]
- Duncan, I.J.H. Animal welfare defined in terms of feelings. Acta Agric. Scand. Sect. A Anim. Sci. Suppl. (Denmark) 1996, 29–35. [Google Scholar]
- Bekoff, M. Animal emotions-Exploring passionate natures. Bioscience 2000, 50, 861–870. [Google Scholar] [CrossRef]
- Dantzer, R. Can farm animal welfare be understood without taking into account the issues of emotion and cognition? J. Anim Sci. 2002, 80, 1–9. [Google Scholar]
- Desire, L.; Boissy, A.; Veissier, I. Emotions in farm animals: A new approach to animal welfare in applied ethology. Behav. Process. 2002, 60, 165–180. [Google Scholar]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef]
- Terlouw, C.; Bourguet, C.; Deiss, V. Consciousness, unconsciousness and death in the context of slaughter. Part I. Neurobiological mechanisms underlying stunning and killing. Meat Sci. 2016, 118, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Terlouw, E.M.C.; Arnould, C.; Auperin, B.; Berri, C.; Le Bihan-Duval, E.; Deiss, V.; Lefevre, F.; Lensink, B.J.; Mounier, L. Pre-slaughter conditions, animal stress and welfare: Current status and possible future research. Animal 2008, 2, 1501–1517. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000, 23, 155–184. [Google Scholar] [CrossRef] [PubMed]
- Morgane, P.J.; Galler, J.R.; Mokler, D.J. A review of systems and networks of the limbic forebrain/limbic midbrain. Prog. Neurobiol. 2005, 75, 143–160. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J.E.; Brown, R. A higher-order theory of emotional consciousness. Proc. Natl. Acad. Sci. USA 2017, 114, 2016–2025. [Google Scholar] [CrossRef] [PubMed]
- Lowndes, M.; Davies, D.C. The effect of archistriatal lesions on open field and fear/avoidance behaviour in the domestic chick. Behav. Brain Res. 1996, 72, 25–32. [Google Scholar] [CrossRef]
- Jarvis, E.D.; Gunturkun, O.; Bruce, L.; Csillag, A.; Karten, H.; Kuenzel, W.; Medina, L.; Paxinos, G.; Perkel, D.J.; Shimizu, T.; et al. Avian brains and a new understanding of vertebrate brain evolution. Nat. Rev. Neurosci. 2005, 6, 151–159. [Google Scholar] [CrossRef]
- Zimmerman, P.H.; Buijs, S.A.F.; Bolhuis, J.E.; Keeling, L.J. Behaviour of domestic fowl in anticipation of positive and negative stimuli. Anim. Behav. 2011, 81, 569–577. [Google Scholar] [CrossRef]
- Rose, J.D.; Arlinghaus, R.; Cooke, S.J.; Diggles, B.K.; Sawynok, W.; Stevens, E.D.; Wynne, C.D.L. Can fish really feel pain? Fish Fish. 2014, 15, 97–133. [Google Scholar] [CrossRef]
- Mok, E.Y.M.; Munro, A.D. Effects of dopaminergic drugs on locomotor activity in teleost fish of the genus Oreochromis (Cichlidae): Involvement of the telencephalon. Physiol. Behav. 1998, 64, 227–234. [Google Scholar] [CrossRef]
- Chandroo, K.P.; Duncan, I.J.H.; Moccia, R.D. Can fish suffer? Perspectives on sentience, pain, fear and stress: International society for applied ethology special issue: A selection of papers from the 36th ISAE International Congress. Appl. Anim. Behav. Sci. 2004, 86, 225–250. [Google Scholar] [CrossRef]
- Portavella, M.; Vargas, J.P.; Torres, B.; Salas, C. The effects of telencephalic pallial lesions on spatial, temporal, and emotional learning in goldfish. Brain Res. Bull. 2002, 57, 397–399. [Google Scholar] [CrossRef]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Ramsay, J.M.; Feist, G.W.; Varga, Z.M.; Westerfield, M.; Kent, M.L.; Schreck, C.B. Whole-body cortisol is an indicator of crowding stress in adult zebrafish, Danio rerio. Aquaculture 2006, 258, 565–574. [Google Scholar] [CrossRef]
- Terlouw, E.M.C.; Rybarczyk, P. Explaining and predicting differences in meat quality through stress reactions at slaughter: The case of large white and duroc pigs. Meat Sci. 2008, 79, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Bourguet, C.; Deiss, V.; Boissy, A.; Terlouw, E.M.C. Young Blond d’Aquitaine, Angus and Limousin bulls differ in emotional reactivity: Relationships with animal traits, stress reactions at slaughter and post-mortem muscle metabolism. Appl. Anim. Behav. Sci. 2015, 164, 41–55. [Google Scholar] [CrossRef]
- Christensen, N.J.; Galbo, H. Sympathetic nervous activity during exercise. Annu. Rev. Physiol. 1983, 45, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Febbraio, M.A.; Lambert, D.L.; Starkie, R.L.; Proietto, J.; Hargreaves, M. Effect of epinephrine in trained men. J. Appl. Physiol. 1998, 84, 465–470. [Google Scholar] [CrossRef]
- Mcveigh, J.M.; Tarrant, P.V. Glycogen-content and repletion rates in beef muscle, effect of feeding and fasting. J. Nutr. 1982, 112, 1306–1314. [Google Scholar] [CrossRef]
- Immonen, K.; Kauffman, R.G.; Schaefer, D.M.; Puolanne, E. Glycogen concentrations in bovine longissimus dorsi muscle. Meat Sci. 2000, 54, 163–167. [Google Scholar] [CrossRef]
- Immonen, K.; Puolanne, E. Variation of residual glycogen-glucose concentration at ultimate pH values below 5.75. Meat Sci. 2000, 55, 279–283. [Google Scholar] [CrossRef]
- Warriss, P.D.; Kestin, S.C.; Brown, S.N.; Bevis, E.A. Depletion of glycogen reserves in fasting broiler-chickens. Br. Poult. Sci. 1988, 29, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Warriss, P.D.; Kestin, S.C.; Brown, S.N.; Knowles, T.G.; Wilkins, L.J.; Edwards, J.E.; Austin, S.D.; Nicol, C.J. The depletion of glycogen stores and indices of dehydration in transported broilers. Br. Vet. J. 1993, 149, 391–398. [Google Scholar] [CrossRef]
- Jia, X.; Ekman, M.; Grove, H.; Faergestad, E.M.; Aass, L.; Hildrum, K.I.; Hollung, K. Proteome changes in bovine longissimus thoracis muscle during the early postmortem storage period. J. Proteome. Res. 2007, 6, 2720–2731. [Google Scholar] [CrossRef]
- Hwang, I.H.; Thompson, J.M. The interaction between pH and temperature decline early postmortem on the calpain system and objective tenderness in electrically stimulated beef longissimus dorsi muscle. Meat Sci. 2001, 58, 167–174. [Google Scholar] [CrossRef]
- Jeleníková, J.; Pipek, P.; Staruch, L. The influence of ante-mortem treatment on relationship between pH and tenderness of beef. Meat Sci. 2008, 80, 870–874. [Google Scholar] [CrossRef]
- Le Bihan-Duval, E.; Debut, M.; Berri, C.M.; Sellier, N.; Sante-Lhoutellier, V.; Jego, Y.; Beaumont, C. Chicken meat quality: Genetic variability and relationship with growth and muscle characteristics. BMC Genet. 2008, 9, 53. [Google Scholar] [CrossRef]
- Lefevre, F.; Bugeon, J.; Auperin, B.; Aubin, J. Rearing oxygen level and slaughter stress effects on rainbow trout flesh quality. Aquaculture 2008, 284, 81–89. [Google Scholar] [CrossRef]
- Bjørnevik, M.; Solbakken, V. Preslaughter stress and subsequent effect on flesh quality in farmed cod. Aquac. Res. 2010, 41, 467–474. [Google Scholar] [CrossRef]
- Gagaoua, M.; Terlouw, E.M.C.; Micol, D.; Boudjellal, A.; Hocquette, J.-F.; Picard, B. Understanding early post-mortem biochemical processes underlying meat color and pH decline in the longissimus thoracis muscle of young blond d’Aquitaine bulls using protein biomarkers. J. Agric. Food Chem. 2015, 63, 6799–6809. [Google Scholar] [CrossRef]
- Terlouw, E.M.C.; Porcher, J.; Fernandez, X. Repeated handling of pigs during rearing. II. Effect of reactivity to humans on aggression during mixing and on meat quality. J. Anim. Sci. 2005, 83, 1664–1672. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Foury, A.; Lebret, B.; Chevillon, P.; Vautier, A.; Terlouw, C.; Mormede, P. Alternative rearing systems in pigs: Consequences on stress indicators at slaughter and meat quality. Animal 2011, 5, 1620–1625. [Google Scholar] [CrossRef] [PubMed]
- Lebret, B.B.; Delgado-Andrade, C.; Claude, S.; Prunier, A.A. Quality of meat from entire, castrated or immunocastrated male pigs as affected by preslaughter handling. Journées de la Recherche Porcine en France 2012, 44, 49–50. [Google Scholar]
- Terlouw, C.; Berne, A.; Astruc, T. Effect of rearing and slaughter conditions on behaviour, physiology and meat quality of large white and duroc-sired pigs. Livest. Sci. 2009, 122, 199–213. [Google Scholar] [CrossRef]
- Bourguet, C.; Deiss, V.; Gobert, M.; Durand, D.; Boissy, A.; Terlouw, E.M.C. Characterising the emotional reactivity of cows to understand and predict their stress reactions to the slaughter procedure. Appl. Anim. Behav. Sci. 2010, 125, 9–21. [Google Scholar] [CrossRef]
- Warner, R.D.; Ferguson, D.M.; Cottrell, J.J.; Knee, B.W. Acute stress induced by the preslaughter use of electric prodders causes tougher beef meat. Aust. J. Exp. Agric. 2007, 47, 782–788. [Google Scholar] [CrossRef]
- Gruber, S.L.; Tatum, J.D.; Engle, T.E.; Chapman, P.L.; Belk, K.E.; Smith, G.C. Relationships of behavioral and physiological symptoms of preslaughter stress to beef longissimus muscle tenderness. J. Anim. Sci. 2010, 88, 1148–1159. [Google Scholar] [CrossRef]
- Reiche, A.M.; Oberson, J.L.; Silacci, P.; Messadene-Chelali, J.; Hess, H.D.; Dohme-Meier, F.; Dufey, P.A.; Terlouw, E.M.C. Pre-slaughter stress and horn status influence physiology and meat quality of young bulls. Meat Sci. 2019, 158, 107892. [Google Scholar] [CrossRef]
- Terlouw, E.M.C.; Cassar-Malek, I.; Picard, B.; Bourguet, C.; Deiss, V.; Arnould, C.; Berri, C.; Le Bihan-Duval, E.; LefÈVre, F.; Lebret, B. Stress en élevage et à l’abattage: Impacts sur les qualités des viandes. Inrae Prod. Anim. 2015, 28, 169–182. [Google Scholar] [CrossRef]
- Ruiz-de-la-Torre, J.L.; Velarde, A.; Diestre, A.; Gispert, M.; Hall, S.J.G.; Broom, D.M.; Manteca, X. Effects of vehicle movements during transport on the stress responses and meat quality of sheep. Vet. Rec. 2001, 148, 227–229. [Google Scholar] [CrossRef]
- Miranda-de la Lama, G.C.; Monge, P.; Villarroel, M.; Olleta, J.L.; Garcia-Belenguer, S.; Maria, G.A. Effects of road type during transport on lamb welfare and meat quality in dry hot climates. Trop. Anim. Health Prod. 2011, 43, 915–922. [Google Scholar] [CrossRef]
- Zhong, R.Z.; Liu, H.W.; Zhou, D.W.; Sun, H.X.; Zhao, C.S. The effects of road transportation on physiological responses and meat quality in sheep differing in age. J. Anim. Sci. 2011, 89, 3742–3751. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, A.; Di Nardo, A.; Realini, C.E.; Rodríguez, P.; Llonch, P.; Temple, D.; Velarde, A.; Giansante, D.; Messori, S.; Dalla Villa, P. Effect of the duration of road transport on the physiology and meat quality of lambs. Anim. Prod. Sci. 2014, 54, 179–186. [Google Scholar] [CrossRef]
- Warner, R.D.; Ferguson, D.M.; McDonagh, M.B.; Channon, H.A.; Cottrell, J.J.; Dunshea, F.R. Acute exercise stress and electrical stimulation influence the consumer perception of sheep meat eating quality and objective quality traits. Aust. J. Exp. Agric. 2005, 45, 553–560. [Google Scholar] [CrossRef]
- Castellini, C.; Mattioli, S.; Piottoli, L.; Mancinelli, A.C.; Ranucci, D.; Branciari, R.; Amato, M.G.; Dal Bosco, A. Effect of transport length on in vivo oxidative status and breast meat characteristics in outdoor-reared chicken genotypes. Ital. J. Anim. Sci. 2016, 15, 191–199. [Google Scholar] [CrossRef]
- Bonou, G.; Ahounou, S.; Folakè, C.; Salifou, A.; Paraïso, H.; Bachabi, K.; Konsaka, B.; Dahouda, M.; Dougnon, J.; Farougou, S.; et al. Influence of pre-slaughter transportation duration stress on carcass and meat quality of indigenous chicken reared under traditional system in Benin. Livest. Res. Rural Dev. 2018, 30, 1–12. [Google Scholar]
- Mitchell, M.; Kettlewell, P. Welfare of poultry during transport—A review. In Proceedings of the 8th Poultry Welfare Symposium, Cervia, Italy, 18–22 May 2009. [Google Scholar]
- Debut, M.; Berri, C.; Baeza, E.; Sellier, N.; Arnould, C.; Guemene, D.; Jehl, N.; Boutten, B.; Jego, Y.; Beaumont, C.; et al. Variation of chicken technological meat quality in relation to genotype and preslaughter stress conditions. Poult. Sci. 2003, 82, 1829–1838. [Google Scholar] [CrossRef]
- Berri, C.; Debut, M.; Sante-Lhoutellier, V.; Arnould, C.; Boutten, B.; Sellier, N.; Baeza, E.; Jehl, N.; Jego, Y.; Duclos, M.J.; et al. Variations in chicken breast meat quality: Implications of struggle and muscle glycogen content at death. Br. Poult. Sci. 2005, 46, 572–579. [Google Scholar] [CrossRef]
- Schneider, B.L.; Renema, R.A.; Betti, M.; Carney, V.L.; Zuidhof, M.J. Effect of holding temperature, shackling, sex, and age on broiler breast meat quality. Poult. Sci. 2012, 91, 468–477. [Google Scholar] [CrossRef]
- Dadgar, S.; Crowe, T.G.; Classen, H.L.; Watts, J.M.; Shand, P.J. Broiler chicken thigh and breast muscle responses to cold stress during simulated transport before slaughter. Poult. Sci. 2012, 91, 1454–1464. [Google Scholar] [CrossRef]
- Gregory, N.G.; Wilkins, L.J. Broken bones in domestic-fowl–handling and processing damage in end-of-lay battery hens. Br. Poult. Sci. 1989, 30, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Kannan, G.; Heath, J.L.; Wabeck, C.J.; Mench, J.A. Shackling of broilers: Effects on stress responses and breast meat quality. Br. Poult. Sci. 1997, 38, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Gentle, M.J.; Tilston, V.L. Nociceptors in the legs of poultry: Implications for potential pain in pre-slaughter shackling. Anim. Welf. 2000, 9, 227–236. [Google Scholar]
- Bedanova, I.; Voslarova, E.; Chloupek, P.; Pistekova, V.; Suchy, P.; Blahova, J.; Dobsikova, R.; Vecerek, V. Stress in broilers resulting from shackling. Poult. Sci. 2007, 86, 1065–1069. [Google Scholar] [CrossRef]
- Ngoka, D.A.; Froning, G.W. Effect of free struggle and pre-slaughter excitement on color of turkey breast muscles. Poult. Sci. 1982, 61, 2291–2293. [Google Scholar] [CrossRef]
- Marx, H.; Brunner, B.; Weinzierl, W.; Hoffmann, R.; Stolle, A. Methods of stunning freshwater fish: Impact on meat quality and aspects of animal welfare. Z. Für Lebensm. Und Forsch. A 1997, 204, 282–286. [Google Scholar] [CrossRef]
- Morzel, M.; Sohier, D.; De Vis, H. Evaluation of slaughtering methods for turbot with respect to animal welfare and flesh quality. J. Sci. Food Agric. 2003, 83, 19–28. [Google Scholar] [CrossRef]
- Lefèvre, F.; Cos, I.; Pottinger, T.G.; Bugeon, J. Selection for stress responsiveness and slaughter stress affect flesh quality in pan-size rainbow trout, Oncorhynchus mykiss. Aquaculture 2016, 464, 654–664. [Google Scholar] [CrossRef]
- Thomas, P.M.; Pankhurst, N.W.; Bremner, H.A. The effect of stress and exercise on post-mortem biochemistry of Atlantic salmon and rainbow trout. J. Fish Biol. 1999, 54, 1177–1196. [Google Scholar] [CrossRef]
- Erikson, U.; Misimi, E. Atlantic salmon skin and fillet color changes effected by perimortem handling stress, rigor mortis, and ice storage. J. Food Sci. 2008, 73, 50–59. [Google Scholar] [CrossRef]
- Gatica, M.C.; Monti, G.; Gallo, C.; Knowles, T.G.; Warriss, P.D. Effects of well-boat transportation on the muscle pH and onset of rigor mortis in Atlantic salmon. Vet. Rec. 2008, 163, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Skjervold, P.O.; Fjaera, S.O.; Ostby, P.B.; Einen, O. Live-chilling and crowding stress before slaughter of Atlantic salmon (Salmo salar). Aquaculture 2001, 192, 265–280. [Google Scholar] [CrossRef]
- Kiessling, A.; Espe, M.; Ruohonen, K.; Morkore, T. Texture, gaping and colour of fresh and frozen Atlantic salmon flesh as affected by pre-slaughter iso-eugenol or CO2 anaesthesia. Aquaculture 2004, 236, 645–657. [Google Scholar] [CrossRef]
- Terlouw, C. Stress reactions at slaughter and meat quality in pigs: Genetic background and prior experience: A brief review of recent findings. Livest. Prod. Sci. 2005, 94, 125–135. [Google Scholar] [CrossRef]
- Gagaoua, M.; Terlouw, E.M.C.; Micol, D.; Hocquette, J.F.; Moloney, A.P.; Nuernberg, K.; Bauchart, D.; Boudjellal, A.; Scollan, N.D.; Richardson, R.I.; et al. Sensory quality of meat from eight different types of cattle in relation with their biochemical characteristics. J. Integr. Agric. 2016, 15, 1550–1563. [Google Scholar] [CrossRef]
- Gagaoua, M.; Micol, D.; Picard, B.; Terlouw, C.E.M.; Moloney, A.P.; Juin, H.; Meteau, K.; Scollan, N.; Richardson, I.; Hocquette, J.-F. Inter-laboratory assessment by trained panelists from France and the United Kingdom of beef cooked at two different end-point temperatures. Meat Sci. 2016, 122, 90–96. [Google Scholar] [CrossRef]
- Lawrence, A.B.; Terlouw, E.M.C.; ILlius, A.W. Individual differences in behavioural responses of pigs exposed to non-social and social challenges. Appl. Anim. Behav. Sci. 1991, 30, 73–86. [Google Scholar] [CrossRef]
- Olsson, I.A.S.; De Jonge, F.; Schuurman, T.; Helmond, F. Poor rearing conditions and social stress in pigs: Repeated social challenge and the effect on behavioural and physiological responses to stressors. Behav. Process. 1999, 46, 201–215. [Google Scholar] [CrossRef]
- O’Connell, N.E.; Beattie, V.E.; Moss, B.W. Influence of social status on the welfare of growing pigs housed in barren and enriched environments. Anim. Welf. 2004, 13, 425–431. [Google Scholar]
- Boissy, A.; Bouix, J.; Orgeur, P.; Poindron, P.; Bibe, B.; Le Neindre, P. Genetic analysis of emotional reactivity in sheep: Effects of the genotypes of the lambs and of their dams. Genet. Sel. Evol. 2005, 37, 381–401. [Google Scholar] [CrossRef]
- Deiss, V.; Temple, D.; Ligout, S.; Racine, C.; Bouix, J.; Terlouw, C.; Boissy, A. Can emotional reactivity predict stress responses at slaughter in sheep? Appl. Anim. Behav. Sci. 2009, 119, 193–202. [Google Scholar] [CrossRef]
- Forkman, B.; Boissy, A.; Meunier-Salaun, M.C.; Canali, E.; Jones, R.B. A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol. Behav. 2007, 92, 340–374. [Google Scholar] [CrossRef] [PubMed]
- Gade, B.P. Effect of rearing system and mixing at loading on transport and lairage behaviour and meat quality: Comparison of outdoor and conventionally raised pigs. Animal 2008, 2, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Rault, J.-L.; Waiblinger, S.; Boivin, X.; Hemsworth, P. The power of a positive human–animal relationship for animal welfare. Front. Vet. Sci. 2020, 7. [Google Scholar] [CrossRef]
- D’Souza, D.N.; Warner, R.D.; Dunshea, F.R.; Leury, B.J. Effect of on-farm and pre-slaughter handing of pigs on meat quality. Aust. J. Agric. Res. 1998, 49, 1022–1025. [Google Scholar]
- Lensink, B.J.; Fernandez, X.; Cozzi, G.; Florand, L.; Veissier, I. The influence of farmers’ behavior on calves’ reactions to transport and quality of veal meat. J. Anim Sci. 2001, 79, 642–652. [Google Scholar] [CrossRef]
- Hemsworth, P.H.; Barnett, J.L.; Hofmeyr, C.; Coleman, G.J.; Dowling, S.; Boyce, J. The effects of fear of humans and pre-slaughter handling on the meat quality of pigs. Aust. J. Agric. Res. 2002, 53, 493–501. [Google Scholar] [CrossRef]
- Mounier, L.; Colson, S.; Roux, M.; Dubroeucq, H.; Boissy, A.; Veissier, I. Positive attitudes of farmers and pen-group conservation reduce adverse reactions of bulls during transfer for slaughter. Animal 2008, 2, 894–901. [Google Scholar] [CrossRef]
- Grandin, T. Recommended Animal Handling Guidelines and Audit Guide: A Systematic Approach to Animal Welfare; American Meat Institute: Washington, DC, USA, 2019. [Google Scholar]
- Murphey, R.M.; Moura Duarte, F.A.; Torres Penedo, M.C. Responses of cattle to humans in open spaces: Breed comparisons and approach-avoidance relationships. Behav. Genet. 1981, 11, 37–48. [Google Scholar] [CrossRef]
- Fordyce, G.; Whythes, J.R.; Shorthose, W.R.; Underwood, D.W.; Shepherd, R.K. Cattle temperaments in extensive beef herds in Northern Queensland 2. Effect of temperament on carcass and meat quality. Aust. J. Exp. Agric. 1988, 28, 689–693. [Google Scholar] [CrossRef]
- Voisinet, B.D.; Grandin, T.; Tatum, J.D.; O’Connor, S.F.; Struthers, J.J. Feedlot cattle with calm temperaments have higher avergare daily gains than cattle with excitable temperaments. J. Anim. Sci. 1997, 75, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Gauly, M.; Mathiak, H.; Hoffmann, K.; Kraus, M.; Erhardt, G. Estimating genetic variability in temperamental traits in German Angus and Simmental cattle. Appl. Anim. Behav. Sci. 2001, 74, 109–119. [Google Scholar] [CrossRef]
- Kjaer, J.B.; Guemene, D. Adrenal reactivity in lines of domestic fowl selected on feather pecking behavior. Physiol. Behav. 2009, 96, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Bourguet, C.; Deiss, V.; Tannugi, C.C.; Terlouw, E.M.C. Behavioural and physiological reactions of cattle in a commercial abattoir: Relationships with organisational aspects of the abattoir and animal characteristics. Meat Sci. 2011, 88, 158–168. [Google Scholar] [CrossRef]
- Ndlovu, T.; Chimonyo, M.; Okoh, A.I.; Muchenje, V. A comparison of stress hormone concentrations at slaughter in Nguni, Bonsmara and Angus steers. Afr. J. Agric. Res. 2008, 3, 96–100. [Google Scholar]
- Muchenje, V.; Dzama, K.; Chimonyo, M.; Strydom, P.E.; Raats, J.G. Relationship between pre-slaughter stress responsiveness and beef quality in three cattle breeds. Meat Sci. 2009, 81, 653–657. [Google Scholar] [CrossRef]
- O’Neill, H.A.; Webb, E.C.; Frylinck, L.; Strydom, P. Urinary catecholamine concentrations in three beef breeds at slaughter. S. Afr. J. Anim. Sci. 2012, 42, 545–549. [Google Scholar] [CrossRef]
- Andersen, H.J.; Oksbjerg, N.; Young, J.F.; Therkildsen, M. Feeding and meat quality–A future approach. Meat Sci. 2005, 70, 543–554. [Google Scholar] [CrossRef]
- Picard, B.; Gagaoua, M.; Micol, D.; Cassar-Malek, I.; Hocquette, J.F.; Terlouw, C.E. Inverse relationships between biomarkers and beef tenderness according to contractile and metabolic properties of the muscle. J. Agric. Food Chem. 2014, 62, 9808–9818. [Google Scholar] [CrossRef]
- Hocquette, J.F.; Botreau, R.; Picard, B.; Jacquet, A.; Pethick, D.W.; Scollan, N.D. Opportunities for predicting and manipulating beef quality. Meat Sci. 2012, 92, 197–209. [Google Scholar] [CrossRef]
- Gagaoua, M.; Monteils, V.; Couvreur, S.; Picard, B. Beef tenderness prediction by a combination of statistical methods: Chemometrics and supervised learning to manage integrative farm-to-meat continuum data. Foods 2019, 8, 274. [Google Scholar] [CrossRef] [PubMed]
- Lebret, B.; Ecolan, P.; Bonhomme, N.; Meteau, K.; Prunier, A. Influence of production system in local and conventional pig breeds on stress indicators at slaughter, muscle and meat traits and pork eating quality. Animal 2015, 9, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Bonny, S.P.F.; Gardner, G.E.; Pethick, D.W.; Legrand, I.; Polkinghorne, R.J.; Hocquette, J.F. Biochemical measurements of beef are a good predictor of untrained consumer sensory scores across muscles. Animal 2015, 9, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Enfalt, A.C.; Lundstrom, K.; Karlsson, A.; Hansson, I. Estimated frequency of the RN- allele in Swedish Hampshire pigs and comparison of glycolytic potential, carcass composition, and technological meat quality among Swedish Hampshire, Landrace, and Yorkshire pigs. J. Anim. Sci. 1997, 75, 2924–2935. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.T.; Hamill, R.M.; O’Halloran, A.M.; Davey, G.C.; McBryan, J.; Mullen, A.M.; McGee, C.; Gispert, M.; Southwood, O.I.; Sweeney, T. SNP variation in the promoter of the PRKAG3gene and association with meat quality traits in pig. BMC Genet. 2012, 13, 66. [Google Scholar] [CrossRef]
- Kwasiborski, A.; Sayd, T.; Chambon, C.; Sant-Lhoutellier, V.; Rocha, D.; Terlouw, C. Pig Longissimus lumborum proteome: Part I. Effects of genetic background, rearing environment and gender. Meat Sci. 2008, 80, 968–981. [Google Scholar] [CrossRef]
- Kwasiborski, A.; Rocha, D.; Terlouw, C. Gene expression in large white or duroc-sired female and castrated male pigs and relationships with pork quality. Anim. Genet. 2009, 40, 852–862. [Google Scholar] [CrossRef]
- Fibiger, W.; Singer, G.; Miller, A.J. Relationships between catecholamines in urine and physical and mental effort. Int. J. Psychophysiol. 1984, 1, 325–333. [Google Scholar] [CrossRef]
- Joo, S.T.; Kim, G.D.; Hwang, Y.H.; Ryu, Y.C. Control of fresh meat quality through manipulation of muscle fiber characteristics. Meat Sci. 2013, 95, 828–836. [Google Scholar] [CrossRef]
- Picard, B.; Gagaoua, M. Muscle fiber properties in cattle and their relationships with meat qualities: An overview. J. Agric. Food Chem. 2020, 68, 6021–6039. [Google Scholar] [CrossRef]
- Picard, B.; Gagaoua, M. Meta-proteomics for the discovery of protein biomarkers of beef tenderness: An overview of integrated studies. Food Res. Int. 2020, 127, 108739. [Google Scholar] [CrossRef] [PubMed]
- Gagaoua, M.; Hughes, J.; Terlouw, E.M.C.; Warner, R.D.; Purslow, P.P.; Lorenzo, J.M.; Picard, B. Proteomic biomarkers of beef colour. Trends Food Sci. Technol. 2020, 101, 234–252. [Google Scholar] [CrossRef]
- Picard, B.; Gagaoua, M.; Al-Jammas, M.; De Koning, L.; Valais, A.; Bonnet, M. Beef tenderness and intramuscular fat proteomic biomarkers: Muscle type effect. PeerJ 2018, 6, e4891. [Google Scholar] [CrossRef]
- Picard, B.; Gagaoua, M.; Al Jammas, M.; Bonnet, M. Beef tenderness and intramuscular fat proteomic biomarkers: Effect of gender and rearing practices. J. Proteom. 2019, 200, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Paula, F.M.M.; Ferreira, S.M.; Boschero, A.C.; Souza, K.L.A. Modulation of the peroxiredoxin system by cytokines in insulin-producing RINm5F cells: Down-regulation of PRDX6 increases susceptibility of beta cells to oxidative stress. Mol. Cell. Endocrinol. 2013, 374, 56–64. [Google Scholar] [CrossRef]
- Cori, C.F. The glucose–lactic acid cycle and gluconeogenesis. In Current Topics in Cellular Regulation; Estabrook, R.W., Srere, P., Eds.; Academic Press: New York, NY, USA, 1981; Volume 18, pp. 377–387. [Google Scholar]
- Jang, C.; Hui, S.; Zeng, X.F.; Cowan, A.J.; Wang, L.; Chen, L.; Morscher, R.J.; Reyes, J.; Frezza, C.; Hwang, H.Y.; et al. Metabolite exchange between mammalian organs quantified in pigs. Cell Metab. 2019, 30, 594–606. [Google Scholar] [CrossRef]
- Watt, M.J.; Howlett, K.F.; Febbraio, M.A.; Spriet, L.L.; Hargreaves, M. Adrenaline increases skeletal muscle glycogenolysis, pyruvate dehydrogenase activation and carbohydrate oxidation during moderate exercise in humans. J. Physiol. 2001, 534, 269–278. [Google Scholar] [CrossRef]
- Qvisth, V.; Hagström-Toft, E.; Enoksson, S.; Moberg, E.; Arner, P.; Bolinder, J. Human skeletal muscle lipolysis is more responsive to epinephrine than to norepinephrine stimulation in vivo. J. Clin. Endocrinol. Metab. 2006, 91, 665–670. [Google Scholar] [CrossRef]
- Nattie, E.; Li, A. Central chemoreceptors: Locations and functions. Compr. Physiol. 2012, 2, 221–254. [Google Scholar] [CrossRef]
- Skelton, L.A.; Boron, W.F.; Zhou, Y.H. Acid-base transport by the renal proximal tubule. J. Nephrol. 2010, 23, S4–S18. [Google Scholar]
- Roberts, D.J.; Miyamoto, S. Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell Death Differ. 2015, 22, 364. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Lu, S.Y.; Hu, P.; Fu, B.Q.; Li, Y.S.; Zhai, F.F.; Ju, D.D.; Zhang, S.J.; Su, B.; Zhou, Y.; et al. Construction and activity analyses of single functional mouse peroxiredoxin 6 (Prdx6). J. Vet. Res. 2019, 63, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Monin, G.; Sellier, P. Pork of low technological quality with a normal rate of muscle pH fall in the immediate post-mortem period: The case of the Hampshire breed. Meat Sci. 1985, 13, 49–63. [Google Scholar] [CrossRef]
- Terlouw, C.; Astruc, T.; Deiss, V.; Ferreira, C. Anesthésie gazeuse des porcs. Expertise d’un abattoir équipé du système BACK LOADER d’anesthésie en groupe au CO2. Viandes Et Prod. Carnés 2006, 25, 216–223. [Google Scholar]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Bonny, S.P.F.; O’Reilly, R.A.; Pethick, D.W.; Gardner, G.E.; Hocquette, J.F.; Pannier, L. Update of meat standards Australia and the cuts based grading scheme for beef and sheepmeat. J. Integr. Agric. 2018, 17, 1641–1654. [Google Scholar] [CrossRef]
- Gagaoua, M.; Monteils, V.; Picard, B. Decision tree, a learning tool for the prediction of beef tenderness using rearing factors and carcass characteristics. J. Sci. Food Agric. 2019, 99, 1275–1283. [Google Scholar] [CrossRef]
- England, E.M.; Scheffler, T.L.; Kasten, S.C.; Matarneh, S.K.; Gerrard, D.E. Exploring the unknowns involved in the transformation of muscle to meat. Meat Sci. 2013, 95, 837–843. [Google Scholar] [CrossRef]
- Ji, Z.; Yan, K.; Li, W.; Hu, H.; Zhu, X. Mathematical and computational modeling in complex biological systems. Biomed. Res. Int. 2017, 2017. [Google Scholar] [CrossRef]
- Shin, S.Y.; Rath, O.; Choo, S.M.; Fee, F.; McFerran, B.; Kolch, W.; Cho, K.H. Positive- and negative-feedback regulations coordinate the dynamic behavior of the Ras-Raf-MEK-ERK signal transduction pathway. J. Cell Sci. 2009, 122, 425–435. [Google Scholar] [CrossRef]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Lo Muzio, L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (Review). Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef]
- Qiu, P.; Wang, Z.J.; Liu, K.J.R.; Hu, Z.Z.; Wu, C.H. Dependence network modeling for biomarker identification. Bioinformatics 2007, 23, 198–206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guo, N.L.; Wan, Y.W. Network-based identification of biomarkers coexpressed with multiple pathways. Cancer Inf. 2014, 13, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-Y.; Cripps, A.W.; West, N.P.; Cox, A.J.; Zhang, P. A correlation-based network for biomarker discovery in obesity with metabolic syndrome. BMC Bioinform. 2019, 20, 477. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terlouw, E.M.C.; Picard, B.; Deiss, V.; Berri, C.; Hocquette, J.-F.; Lebret, B.; Lefèvre, F.; Hamill, R.; Gagaoua, M. Understanding the Determination of Meat Quality Using Biochemical Characteristics of the Muscle: Stress at Slaughter and Other Missing Keys. Foods 2021, 10, 84. https://doi.org/10.3390/foods10010084
Terlouw EMC, Picard B, Deiss V, Berri C, Hocquette J-F, Lebret B, Lefèvre F, Hamill R, Gagaoua M. Understanding the Determination of Meat Quality Using Biochemical Characteristics of the Muscle: Stress at Slaughter and Other Missing Keys. Foods. 2021; 10(1):84. https://doi.org/10.3390/foods10010084
Chicago/Turabian StyleTerlouw, E. M. Claudia, Brigitte Picard, Véronique Deiss, Cécile Berri, Jean-François Hocquette, Bénédicte Lebret, Florence Lefèvre, Ruth Hamill, and Mohammed Gagaoua. 2021. "Understanding the Determination of Meat Quality Using Biochemical Characteristics of the Muscle: Stress at Slaughter and Other Missing Keys" Foods 10, no. 1: 84. https://doi.org/10.3390/foods10010084
APA StyleTerlouw, E. M. C., Picard, B., Deiss, V., Berri, C., Hocquette, J.-F., Lebret, B., Lefèvre, F., Hamill, R., & Gagaoua, M. (2021). Understanding the Determination of Meat Quality Using Biochemical Characteristics of the Muscle: Stress at Slaughter and Other Missing Keys. Foods, 10(1), 84. https://doi.org/10.3390/foods10010084







