Abstract
The main food-origin antigens that the infant’s body is in contact with are cow’s milk proteins (CMP). Still, CMP are one of the main sources of beneficial biologically active peptides that play a role in treatment of non-communicable diseases. Safe methods to quickly predict the sensitizing potential of food proteins among their range of health-promoting properties are essential. The aim of study was to adapt an integrated approach combining several in silico (IS) studies and in vitro (IV) assays to screen the multifunctionality of CMP-derived peptides. Major histocompatability complex type II MHC II-binders, interleukin-4 and -10 inducers, interferon γ -inducers and immunobioactivity tools were used to predict the peptide-power of inducing allergies or tolerance. A comparison of the peptide profiless revealed the presence of one identical and one overlapping sequence in IS and IV hydrolysate. By IS analysis, four of 24 peptides were found to have high affinity and stimulate IL-4 expression, and by IV, one of seven peptides had this potential (Bos d9 peptide DIPNPIGSENSEK (195–208)). Three IV peptides may induce IL-10 expression. The IV/IS assessment seems promising agents for peptides’ potential determination dedicated only to preliminary screening of peptides. The IV verification is still crucial in further steps of studies.
1. Introduction
In the 21st century, the world is facing two major problems. On the one hand, the growing population of the world needs a new source of nutritious food [1]. On the other hand, the problem of diet-related diseases and people requiring special medical-purpose food makes it difficult to adapt and use new food sources [2]. The potentially health-promoting activity of food derived peptides seems also to be a top notch aim of studies in that context [3]. However, it is already known that the human immune system is immensely complex but is not always able to develop tolerance to food proteins subjected to various processing technologies [4]. Not entirely known is why developing an immune system that may recognize and respond to infections creates the potential for hypersensitivity reactions. These manifest as allergic responses to environmental agents or autoimmune responses to self-antigens. Therefore, there is a need for an efficient and at the same time safe system to verify the immunoreactive potential of proteins and their resulting peptides [5]. The main food-origin antigens that the infant’s body is in contact with are cow’s milk proteins (CMP). CMP allergy ranges from 0.5% to 3% in developed countries [6]. It has been observed lately that there is an earlier and more severe failure of oral tolerance induction protocols during the first year of life, as well as a likelihood of decrease in the outgrowing of allergy, which forces changes in the approach to conducting therapy [7]. Therefore, highly efficient and at the same time precise and safe methods of testing both new sources of functional proteins/peptides and their usefulness in possible specific immunotherapy are needed.
Along with this, a lot of effort is put into implementing the principles of the 3Rs (replacement, reduction and refinement strategy) in in vivo studies. The effectiveness and repeatability of animal models used to test the allergenic potential of new foods is questionable [8]. Therefore, in silico (IS) research aimed at faithfully replacing research in this field with the use of animal and cell models can be helpful in the screening of peptides obtained after protein digestion. Each of the available methods has its advantages and disadvantages, and to a varying degree copes with challenges such as cross-reactivity and de novo sensitization [9]. Still, the complexity of the immune reactions limits definitive data interpretation so a single analysis may be insufficient. The matter is even more complicated due to several possible mechanisms of hypersensitivity by which the allergy may proceed depending on the dose of the allergen, its digestive resistance, or the route of administration to the body [5,10].
Nevertheless, an allergy is still the adverse response of the body’s immune system to otherwise harmless substances, mostly proteins. The allergenic proteins follow the same path as other foreign antigens that enter the human body. They are broken down into small fragments by human proteases. Some of these fragments bind to human MHC (Major histocompatability complex) class II proteins. A very useful technique to monitor this phenomenon is proteochemometrics [11]. The complexes between the peptides of allergenic origin and MHC class II proteins are expressed on the human cell surface. It is also important to know the alleles of MHC class II involved in the reaction with a specific type of allergen, because the response to individual allergens can be associated with different alleles [12]. The peptide-MHC class II complex can be recognized by antigen-specific armed helper T cells, stimulating them to make proteins that, in turn, cause the B cell to proliferate and its progeny to differentiate into antibody-secreting cells. It is important to understand that even bound epitopes do not always induce the same type of cytokine response. In the context of humoral reaction it is important to verify the potential to induce interleukin-4 (IL4) inducing MHC II binders [13]. The ability and strength of induction of interferon γ (IFNγ) secretion is important in the context of accompanying pro-inflammatory reaction but also, depending on the dose of allergen, its significant role in suppression of the immune system and induction of tolerance to allergenic proteins [14]. Also, interleukin-10 (IL10) induction plays a significant role in tolerance development through the suppressing of inflammatory process, changing the profile of activated effector cells, increasing the expression of proteins forming tight junctions in mucosa and an increased amount of Goblet cells. Reducing the secretion of pro-inflammatory cytokines, e.g., IL-4 and IFNγ with a simultaneous increasing the level of regulatory cytokines (including IL-10) in the context of discrimination of potential of peptides gives hope to more targeted and efficient diagnosis and immunotherapy.
In this article, the authors therefore attempted to develop the algorithm to assess the possibility of an allergic reaction /tolerance to well-known CMP allergens using several IS tools effective in vaccine development. Several models in a sequence as a potential pathway to which peptides are subjected in a living organism were begun from simulated digestion. The immunoreactive potential of obtained peptides were determined based on potential to bind MHC class II and IL-4/-10 and IFNγ secretion induction. At the same time beneficial activities of the peptides on the immune system were determined. An analogous procedure was also applied to the cocktail of peptides obtained during the spectral analysis of a cow’s milk sample submitted to simulated digestion by the in vitro (IV) method.
The authors agree that no existing IS solution can replace the IV assessment, and cannot be even compared with in vivo approach, but they made an attempt to prepare the theoretical decisive procedure that maximally mimicked conditions prevailing in a living organism. It was decided not to replace IV testing but to limit the amount of optional peptides for that procedure. This protocol was especially developed to reduce the use of allergic human sera that are routinely used to test products immunoreactivity. Human sera are invaluable material, and their purchasing is more and more difficult due to the increasing ethical rigors. Also, the sera lifetime and reproducibility are limited so it is necessary to limit to minimum the number of tested formulas.
The main aim was to assess the usefulness of sequential action on IS tools in order to find key data that allow determination of whether a specific peptide has a higher sensitizing or modulating potential on the immune system. Such a personalized approach in terms of MHC II alleles can be helpful in predicting the effectiveness and susceptibility to specific immunotherapy and a peptide-based formulas intended for therapy.
2. Materials and Methods
2.1. Selection of Proteins for In Silico Analysis
Major milk allergens were selected based on AllergenOnline version 20 [15] and from Allergome database [16] and the further steps of analysis are shown in Figure 1. Sequences of those allergens were downloaded from Uniprot database [17]. The chosen CMP with allergenic potential and known primary structure (α -lactalbumin (Bos d 4, uniprot id: P00711), β-lactoglobulin (Bos d 5, uniprot id: P02754), serum albumin (Bos d 6, uniprot id: P02769), α -S1-casein (Bos d 9 uniprot id: P02662), α-S2-casein (Bos d 10, uniprot id:P02663), β-casein (Bos d 11, uniprot id: P02666), κ-casein (Bos d 12, uniprot id: P02668) were submitted to further analysis.
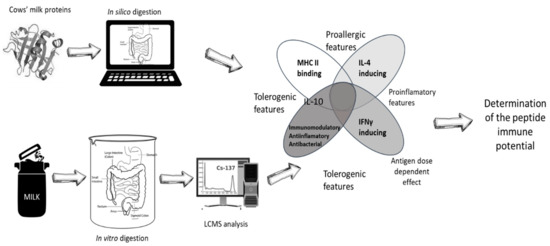
Figure 1.
Scheme of in silico (IS) and integrated in vitro/in silico (IV/IS) analyses.
2.2. In Silico Digestion
IS digestion was carried out using PeptideCutter (http://web.expasy.org/peptide_cutter/) as it was described before [12]. Briefly, the protocol mimicking gastrointestinal enzyme digestion with pepsin at pH -2, trypsin and chymotrypsin was carried out to determine a larger pool of possible peptides. The FASTA format of CMP were uploaded on the PeptideCutter server. PeptideCutter predicts potential cleavage sites cleaved by proteases or chemicals in a given protein sequence and returns the query sequence with the possible cleavage sites. After this protocol, each protein is presented as a set of peptides with different lengths. As the peptide-binding groove of MHC class II proteins is able to accept peptides of 9 amino acid residues or longer, in the next step only those peptides were selected and used which resulted from previously established protocols [18].
2.3. In Vitro Milk Digestion
Physicochemical properties of commercially purchased 2% fat milk were tested using MilkoScan™ FT2 infrared milk analyzer (Foss, Hilleroed, Denmark). In vitro (IV) digestion was carried out using Minekus et al., 2014 static IV digestion protocol. A simulated gastric and intestinal fluid was added to aliquots of bovine normalized (2% fat, 3.5% proteins, 3.2% lactose) milk [19]. Briefly simulated gastric fluid was added to achieve a concentration of 2000 U/mL pepsin (P700, Sigma-Aldrich, Warsaw, Poland) in the final mixture. After 2h incubation at 37 °C with constant stirring, a pancreatin (P7545, Sigma-Aldrich, Warsaw, Poland) containing simulated intestinal fluid to a final 100 U/mL trypsin activity and bile (B8631, Sigma-Aldrich, Warsaw, Poland) 10 mM concentration was added. After another 2 h incubation under the conditions as specified above the samples were immediately frozen to stop the reaction and freeze dried. The obtained digested samples were used for the subsequent analysis. Undigested milk samples were also lyophilized and subjected to further spectral analysis in an analogous manner. In IS models there is unfortunately no option to model digestion of proteins in matrix so in their emulsified form. We need to know then the protein composition that we can separately test in the mathematical IS model. That is why we parallel performed an analysis of raw milk (raw material) that was not submitted to IV digestion prior MS screening (that allows also to identify trace and labile proteins). Using such a protocol, we get the maximally precise screening of proteins profile in raw material and for further IS steps we can choose only those that perform the functions desired for us (like a strong sensitizing potential, or expected tolerogenic potential).
2.4. Spectral Analyses of Milk Peptides
Freeze dried samples of proteins and peptides after IV digestion were dissolved in 100 μL of 100 mM ammonium bicarbonate buffer, reduced in 100 mM S-Methyl methanethiosulfonate for 30 min at 57 °C, alkylated in 100 mM Tris(2-carboxyethyl)phosphine hydrochloride for 40 min, and digested overnight with 10 ng/mL trypsin (Promega, Madison, WI, USA) at 37 °C. Finally, trifluroaceti acid was added at a final concentration of 0.1%. Mass spectrometry (MS) analysis was performed by online liquid chromatography-mass spectrometry LC-MS in the Laboratory of Mass Spectrometry (IBB PAS, Warsaw, Poland) using a nanoAcquity ultra-performance LC (UPLC) system (Waters, Milford Sound, MA, USA) coupled to an LTQ-Orbitrap Velos mass spectrometer (Thermo Scientific, Waltham, MA, USA). Peptides were separated by a 180-min linear gradient of 95% solution A (0.1% (v/v) formic acid in water) to 35% solution B (acetonitrile with 0.1% formic acid). The measurement of each sample was preceded by three washing runs to avoid cross-contamination; the final LC-MS washing run was searched for the presence of cross-contamination between samples. Raw data were searched by Mascot (Matrix Science) against SwissProt database, tax.: Mammals (556 006 sequences). Search parameters: unspecific digest (“enzyme none”) due to simulated digestion pretreatment in hydrolyzed samples and peptides mass tolerance 30 ppm, fragment ion tolerance 0.1 Da, Higher-energy collisional dissociation (HCD) with a normalized collision energy value of 35%, variable modification: methionine oxidation, methylation. The score threshold cut-off- calculated for this analysis was 32. The signals obtained from analysis of hydrolyzed products were compared with signal of proteins and peptides of non-hydrolyzed milk. Obtained peptides were screened in in terms of length to select those structures that also had a minimum of 9 amino acid residues.
2.5. In Silico Analysis of Immunoreactive and Bioactive Peptides
2.5.1. The Evaluation of Binding Affinities to MHC II and T- and B-Cells Inducers
Peptides that survived after IS and IV digestion were tested for binding to human MHC class II proteins. Each peptide was uploaded on EpiTOP3 server [20] (http://www.ddg-pharmfac.net/EpiTOP3/). EpiTOP is a server for human leukocyte antigen (HLA) class II binding prediction using proteochemometric models. It is a web-based application written in Python and HTML and integrating the MySQL database environment. It uses quantitative structure–activity relationship (QSAR) analysis that is a powerful method due to its high and fast throughput and good hit rate. This tool analyzes the primary structure of proteins and predicts potential binders to the most common alleles among human population (12 HLA-DRB1, 5 HLA -DQ and 7 HLA- DP proteins). Based on previous experience, obtained peptides were tested in the context of their binding affinity to DRB1 *01: 01, DQ7 (DQA1*05: 01/DQB1*03: 01) and DQ8 (DQA1*03: 01/DQB1*03: 02) in the context of allergic reactions and DRB1*03:01, DRB1*14:19 and *14:21 which seem protective against cow’s milk allergy [21]. For this confirmation, another IS tool (http://tools.iedb.org/mhcii/result/) was used that employs several methods to predict MHC Class II epitopes, including a consensus approach which combines NN-align, SMM-align and combinatorial library methods [22]. Also, B-cell and T-cell epitopes prediction was made using DiscoTope tool and Algpred, respectively. DiscoTope is a method for predicting discontinuous epitopes from 3D structures of proteins in PDB format (http://tools.iedb.org/discotope/). It incorporates solvent-accessible surface area calculations, as well as contact distances into its prediction of B cell epitope potential along the length of a protein sequence. Moreover, thirteen proteins in the training data set were used and prediction methods achieved highly significant predictive performances [23]. Algpred tool (http://crdd.osdd.net/raghava/algpred/) allows prediction of allergens based on similarity of known epitope with any region of protein. It uses a Support Vector Machine (SVM) based method. Five-fold cross validation technique with four sets of data were used for training and testing of this tool by its authors. A hybrid option of analysis was chosen as recommended in [24].
2.5.2. The Determination of IL4 Inducing MHC II Binders
The IL4pred tool (http://crdd.osdd.net/raghava/il4pred/) was used in the next step as a method that can distinguish if the peptide has potential interleukin-4 (IL4) secretion induction. For the analysis, the set of peptides with proven high strength of MHC II binding was used. IL4pred uses also a SVM based method. Five-fold cross validation technique with four sets of data were used for training and testing of this tool by its authors [13]. This approach is important due to the fact that the secretion of interleukin-4 (IL4) is a characteristic of T-helper 2 response, but MHC II epitope binding does not always induce IL-4.
2.5.3. The Determination of IFNγ Epitopes
An online module for the IFNγ inducing peptide/epitope was used (https://webs.iiitd.edu.in/raghava/ifnepitope/). IFNepitope software is an online prediction server that aims to predict and design the peptides from protein sequences having the capacity to induce IFNγ release from CD4+ T cells. This tool also uses a SVM based method [14]. The processes of training and validation of this model are analogous to those described in the IL4pred tool. In this analysis, all peptides obtained during the simulated digestion were tested.
2.5.4. The Determination of IL10 Inducing Binders
The IL10pred tool (http://crdd.osdd.net/raghava/IL-10pred/) was used in the next step as a method that can distinguish if the peptide has potential interleukin-10 (IL10) secretion induction [25]. For the analysis, the set of peptides with proven high strength of MHC II binding was used. IL10pred uses also SVM based method. The processes of training and validation of this model are analogous to those described in the IL4pred tool.
2.5.5. The Determination of Immune-Bioactive Peptides
For this analysis the FeptideDB tool (http://www4g.biotec.or.th/FeptideDB) was used for determination of bioactive peptides with proven benefit for immune system activities. The digestion module in this tool provides its own algorithm for IS enzymatic digestion. Steps downloaded from Uniprot database sequences were used. Peptides cleaved from query protein sequences via Pepsin 2pH/trypsin option were analyzed. Here, the same peptides, previously characterized and meeting the length criterion of peptides not smaller than 9 amino acids, were also used for the analysis.
2.6. Statistical Analysis and Data Calculation
The IV study along with the spectrometric analysis was performed in triplicate. All the data were gathered from separate aliquots of raw material obtained from different containers but purchased at this same time from this same part of dairy beverage (batch no.000237/2018/_G). The IV digestion both with spectrometric analysis were conducted parallel. The homogeneity of the model due to the used qualitative analysis of MS allows to estimate the homogeneity based on the presence of peptides in all replicates. The analogical peptides (characteristic for particular protein) found in all the repetitions were tested. The comparison was based on those proteins that were identified basing on peptides considered by Mascot as significant using score value. Basing on indirect quantitative parameters for those peptides the homogeneity of digesta was tested with the Manhattan method of distance analysis where the first dimension was the score value for peptide and the second value was the exponentially modified protein abundance index (emPAI). Based on those variables, the minimal distance between obtained parameters was tested. The accuracy and precision of IS models were tested by their creators as described in Section 2.7. The result of integrated analysis was presented in the form of a radial chart where the values for individual variables were normalized to one and the potential was assessed on the basis of the area under the chart. The area was framed by the curve connecting the individual points on the graph. The area saw higher values when several features describing specific properties of the peptides got higher (more significant SVM scores) than the area for a single feature that is presented in the form of a single radius. Factors #4 to #9 were rated as stronger pro-allergic and #10 to #16 as tolerogenic. Factors #1 to #3 as discriminants of weak binging to different alleles of HLA might be significant in both types of reactions. IFN epitopes (#11) no matter if it was stated positive or negative induction were normalized also to one but interpreted in the context of pro-inflammatory activity both with IL-4 and as tolerogenic both with IL-10- however in tested group of peptides the second regularity was not noted. An important role in the nature of the IFN mediated response plays the dose of allergenic peptide that still must be tested empirically.
2.7. In Silico Models Training and Validation
The accuracy and precision of the IS models were tested by their authors. The developed classification model of IL4pred, IL10pred and IFNγ epitope used the hybrid method of amino acid pairs and motif information. The main dataset used in IL4pred training comprised 904 experimentally validated IL4 inducing and 742 noninducing MHC class II binders. IFNγ contained 3705 IFNγ inducing and 6728 non-IFN-γ inducing MHC class II binders. Another dataset used in training was IFNgOnly, containing 4483 IFN-γ inducing epitopes and 2160 epitopes that induce cytokines other than IFN-γ.
The main dataset used in IL10pred training comprised 394 IL-10 inducing and 848 non-inducing peptides. That approach gave the maximum accuracy of method 75.76% with Matthew’s correlation coefficience (MCC) of 0.51 and 82.10% with MCC of 0.62 for the IL4pred and IFNγ epitopes, respectively. Default settings were used in those systems with SVM threshold: 0.2 and overlapping peptides/epitopes window length: 15 or 9. For Algpred tool, the training and testing process dataset consisted of 578 allergens and 700 non-allergens. The recommended hybrid method enables sensitivity of 93.94% with 33.34% specificity characteristic for motif-based method and 17.47% sensitivity at specificity of 98.14% based on known IgE epitopes [24]. This hybrid method achieves a golden balance between the sensitivity and specificity of individual methods. For the Immune Epitope Database (IEDB) MHC II tool, the recommended 2.22 Consensus approach, combining NN-align, SMM-align, CombLib, and Sturniolo was used [26]. EpiTOP3 returns the binding affinity in pIC50 (negative logarithm of IC50) units. Peptides with pIC50 > 6.3 (IC50 < 500 nM) were considered as strong binders. Peptides with 6.3 >pIC50 > 5.3 (IC50 < 500 nM) were considered as weak binders. In IEDB MHC II tool the predicted output is given in units of IC50 (nM). Therefore, a lower number indicates higher affinity. Peptides with IC50values <50 nM are considered high affinity, <500 nM intermediate affinity and <5000 nM low affinity. The default value of threshold in DiscoTope tool for version 1.1 was −7.7 corresponds to a specificity of 75% and sensitivity of 47% [23].
3. Results
3.1. In Silico Digestion
Seven bovine milk proteins, allergens with known primary structure underwent IS digestion by pepsin, trypsin and chymotrypsin. Enzyme degradation of allergens significantly reduced the number of peptides with antigenic potential. Digestion of the proteins resulted in 24 peptides longer than nine amino acids (Table 1).

Table 1.
In silico antigenicity assessment of in silico digested peptides with major MHC II alleles according the EpiTOP3 tool.
α-lactalbumin (Bos d 4) consists of 142 amino acids. After IS digestion, two peptides longer than nine amino acids have survived: DTQAIVQNNDSTE (56–68) and SSNICNISCDK (88–98).
β-lactoglobulin (Bos d 5) consists of 178 amino acids. After IS digestion, one peptide longer than nine amino acids has survived: ENSAEPEQS (124–132).
Serum albumin (Bos d 6) consists of 607 amino acids. After IS digestion, five peptides longer than nine amino acids have survived: QECCQAEDK (189–197), ICDNQDTISSK (287–297), DAIPENLPPL (319–328), VPQVSTPTL (438–446), CCTKPESER (460–468).
α-S1-casein (Bos d 9) consists of 214 amino acids. After IS digestion, four peptides longer than nine amino acids have survived: DIGSESTEDQAM (58–69), EAESISSSEEIVPNSVEQK (76–94), EIVPNSAEER (125–134), SDIPNPIGSENSEK (195–208).
α-S2-casein (Bos d 10) consists of 222 amino acids. After IS digestion, three peptides longer than nine amino acids have survived: VSSSEESIISQET (22–34), SIGSSSEESAEVATEEVK (68–85), and NAVPITPTL (130–138).
β-casein (Bos d 11) consists of 224 amino acids. After IS digestion, five peptides longer than nine amino acids have survived: NVPGEIVES (22–30), SSSEESITR (32–40), QSEEQQQTEDE (49–59), PFPGPIPNSL (76–85), TQTPVVVPPF (93–102).
κ-casein (Bos d 12) consists of 190 amino acids. After IS digestion, four peptides longer than nine amino acids have survived: GAQEQNQEQPIR (20–31), SCQAQPTTM (108–116), TEIPTINTIASGEPTSTPTTEAVESTVAT (138–166), EDSPEVIESPPEINTVQVTSTAV (168–190).
3.2. In Silico Antigenicity Assessment of In Silico Digested Proteins
The 24 peptides originating from IS digestion of milk proteins were tested for binding to the most common HLA-DR, HLA DQ and HLA DP alleles (Table 1).
Bos d 4: The peptide DTQAIVQNNDSTE (56–68) binds to 20 alleles: 7 HLA DP, 5 HLA DQ and 8 HLA DR. It matches a T cell epitope QAIVQNNDSTEYGLFQIN (58–75) and is a part of the known IgE epitope GYGGVSLPEWVCTTFHTSGYDTQAIVQNNDSTEYGLFQINNK (36–77). The peptide SSNICNISCDK (88–98) binds only to HLA DQ alleles. It partially matches a T cell epitope INNKIWCKDDQNPHSSNI (74–91) and is a part of the known IgE epitope GYGGVSLPEWVCTTFHTSGYDTQAIVQNNDSTEYGLFQINNK (36–77).
Bos d 5: ENSAEPEQS (124–132) is a weak binder to 5 DQ and 3 DP allele. Although its sequence couldn’t be matched to any of the known IgE epitopes, it matches a T cell epitope: TDYKKYLLFCMENSAEPEQSL (113–133).
Bos d 6: QECCQAEDK (189–197) shows weak binding affinity only to three of the DQ alleles. ICDNQDTISSK (287–297) binds to 11 of DR, six of DP, and five DR alleles. DAIPENLPPL (319–328) does not bind to any of the studied DR alleles, but shows strong binding affinity to all of the DQ and DP alleles. VPQVSTPTL (438–446) binds to nine DR and all of the DQ and DP alleles. CCTKPESER (460–468) is a strong binder to one DQ allele and a weak binder to one DP and the rest of DQ alleles. None of the peptides that survived after the IS digestion of Bos d 6 are found to match any IgE epitopes. DAIPENLPPL (319–328) is the only binder that matched a T cell epitope: (317–336).
Bos d 9: DIGSESTEDQAM (58–69) binds to five DQ alleles, eight DR and six DP alleles. It is part of the known T cell epitope DIGSESTEDQAMEDIKQMEAESIS (58–81). Parts of it can also be found in two known IgE epitopes, ELSKDIGSES (54–63) and TEDQAMEDIKQMEAE (64–78). EAESISSSEEIVPNSVEQK (76–94) binds to 8 DR, and all DQ and DP alleles. A ten amino acid sequence from it matches the known IgE epitope, EEIVPNSVEQ (84–93), while another part of it matches the T cell epitope PNSVEQKHIQKEDVPSERYLGYLE (88–111). EIVPNSAEER (125–134) binds to five DR, five DQ and seven DP alleles. It matches the known IgE epitope LEIVPNSAEERL (124–135) and the T cell epitope YLGYLEQLLRLKKYKSESTEDQAM (106–129). SDIPNPIGSENSEK (195–208) binds to eight DR, five DQ and seven DP alleles. It matches known IgE epitopes YTDAPSFSDIPNPIGSENSE (188–207), DAPSFSDIPNPIGSENSEKT (190–209) and FSDIPNPIGSENSEKTTMPL (194–213).
Bos d 10: VSSSEESIISQET (22–34) is recognized by all of the DQ alleles, seven DP and three DR alleles. It matches the IgE epitope: KNTMEHVSSSEESIISQETY (16–34). SIGSSSEESAEVATEEVK (68–85) binds to eight DR, five DQ and 7 DP of the studied alleles. Its sequence partially matches two known IgE epitopes: EVVRNANEEEYSIGS (57–71) and EEVKITVDDKHYQKALNEIN (82–101) and the T cell epitope SEESAEVATEEV (73–84). NAVPITPTL (130–138) binds to all DQ and one DP alleles and partially matches a known IgE epitope VPITPTLNREQL (132–143).
Bos d 11: NVPGEIVES (22–30) binds to five DQ alleles and one DP allele. Its sequence fully matches a known IgE epitope, RELEELNVPGEIVESL (16–31). SSSEESITR (32–40) is a weak binder to one DQ and one DP allele. Its sequence matched with a known IgE epitope, LSSSEESITRINKKIEKFQS (31–50). QSEEQQQTEDE (49–59) is a weak binder to all of the studied DQ alleles and one DP allele. Its sequence matched a known IgE epitope, QSEEQQQTEDELQDKIH (49–65). PFPGPIPNSL (76–85) binds to nine DR alleles and all of the DQ and DP alleles. Its sequence matches the known IgE epitope TQSLVYPFPGPIPNSL (70–85). TQTPVVVPPF (93–102) does not bind to DR. It is a strong binder to all of the DQ alleles and a weak binder to all of the DP alleles. Its sequence partially matches a known IgE epitope VVPPFLQPEV (98–107).
Bos d 12 GAQEQNQEQPIR (20–31) and SCQAQPTTM (108–116) do not bind to any of the studied DR alleles, but are recognized by all of the studied DQ and DP alleles. Their sequence does not match any of the known IgE epitopes. TEIPTINTIASGEPTSTPTTEAVESTVAT (138–166) binds to nine of the DR alleles and all of the DQ and DP alleles with high affinity. It partially matches two known IgE epitopes: KKNQDKTEIPTINTIA (132–147) and EAVESTVATLED (158–169).
EDSPEVIESPPEINTVQVTSTAV (168–190) binds to 11 of the DR alleles and all of the DQ and DP alleles. Its sequence almost fully covers the sequence of the known IgE epitope SPEVIESPPEINTVQVTSTA (170–189).
3.3. In Vitro Digestion and Peptides Determination
Six allergenic bovine milk proteins, three non-allergenic proteins of cow’s milk and a trace amount of collagen α-1 external contamination in milk were identified after the IV digestion by pepsin and pancreatin (Table 2). Pancreatin is a broad-spectrum enzymatic cocktail composed of amylase, trypsin, lipase, ribonuclease and protease obtaining a mixture of peptides even more accurately than with pepsin-trypsin digestion. Enzyme degradation of allergens significantly reduced the number of peptides with antigenic potential and the vast majority of the obtained peptides were shorter than seven amino acids. In in vivo conditions proteins might be less degraded but in static model the digestion of the proteins resulted in seven peptides longer than nine amino acids (Table 2).

Table 2.
Characteristics of the milk chydrolysate obtained after IV digestion and screening of peptides obtained from MASCOT in terms of inducers for T and B lymphocytes.
α-lactalbumin (Bos d 4)- after IV digestion produced one peptide longer than nine amino acids: NNKIWCKDDQNPSSNICNISCDK (76–98). It contained predicted IS digestion region SSNICNISCDK (88–98).
β-lactoglobulin (Bos d 5) after IV digestion produced one peptide longer than nine amino acids: LVRTPEVDDEALEK-FDKALKALPM (139–163).
Neither serum albumin nor allergen Bos d 6 fragments, longer than nine amino acids, remained after IV digestion. Shorter fragments in spectral analysis did not allow a significant identification of this protein in the sample. The score threshold cut-off-32 calculated for this analysis was still low and proper coverage was not obtained.
α-S1-casein (Bos d 9) after IV digestion produced 4 peptides longer than 9 amino acids. One of them confirmed the IS prognosed sequence SDIPNPIGSENSEK (195–208). The rest were much longer than the prognoses: QKHIQKEDVPSERYLGYLEQLLRL (94–116), GYLEQLLRLKKYKVPQLEIVPNSA (108–131), SMKEGIHAQQKEPMIGVNQELAYF (137–160).
A similar situation was observed for β-casein (Bos d 11). No allergenic fragments longer than 9 amino acids were found but shorter fragments in spectral analysis allowed identification of that protein with 33% coverage. The PAI value indicates that the content of this protein was also consistent with the values accepted for standardized milk.
Neither κ-casein nor allergen Bos d 12 fragments, longer than 9 amino acids, remained after IV digestion. Shorter fragments in spectral analysis did not allow identification of this protein in the sample.
However, the presence of four other CMP characteristic for the milk fat globule membrane (MFGM) a.o. was observed (butyrophilin, glycosylation-dependent cell adhesion molecule, osteopontin and allergenic α-1-glycoprotein (Bos d2)). No allergenic fragments longer than 9 amino acids were found but shorter fragments in spectral analysis allowed identifications of those proteins with 5–33% coverage.
During the analysis, the presence of fragments characteristic for collagen α-1was also found. What is more, this protein recognized as allergenic was digested in such a way that one peptide longer than nine amino acids was identified (GPVGNPGPAGPAGPRGEVGLPGLS (266–289)). The PAI value was 0.05 so it was a trace amount, but IS analysis of fragments capable of binding to T and B cells have many inducible regions.
3.4. In Silico Antigenicity Assessment of In Vitro Digested Proteins
The 7 peptides originating from IV digestion of milk proteins were tested for binding epitopes for T and B-cells (Table 2) as well to the major groups of HLA-DR, HLA DQ and HLA DP alleles (Table 3).

Table 3.
IS antigenicity assessment of IV digested peptides with major MHC II alleles according the EpiTOP3 tool.
Bos d 4 peptide NNKIWCKDDQNPHSSNICNISCDK (75–98) binds to 20 alleles: seven HLA DP, five HLA DQ, and eight HLA DR. Although SSNICNISCDK (88–98) peptide binds only to HLA DQ a longer structure binds to seven of the DR alleles, four of the DQ, and three of the DP alleles with high affinity. In Bos d 4 conformational structure, it has 18 regions of potential recognition to T-cells and six to B-cells and in this peptide, so it is six and two, respectively. The peptide SSNICNISCDK (88–98) as was the only one consistent with those predicted in IS digestion and binds only to HLA DQ alleles. In its structure it has only one region of potential recognition to T-cells, but two for B-cells.
Bos d 5 LVRTPEVDDEALEKFDKALKALPM (139–163) is a strong binder to 5 DQ and 7 DP allele but also to 9 DR allele. In Bos d 5 conformational structure, it has 21 regions of potential recognition to T-cells and seven to B-cells, but only four and tow in this peptide, respectively.
Bos d 9 peptide GYLEQLLRLKKYKVPQLEIVPNSA(108–131) is a strong binder to all major tested alleles. In Bos d 9 conformational structure it has 25 regions of potential recognition to T-cells and 12 to B-cells and in this peptide, it is five and two, respectively. SMKEGIHAQQKEPMIGVNQELAYF (137–160) is a strong binder to 5 DQ and 7 DP alleles but also to 8 DR alleles. In this peptide there are only threeregions of potential recognition to T-cells and none for B-cells. QKHIQKEDVPSERYLGYLEQLLRL (94–116) is a strong binder to five DQ and seven DP alleles but also to eight DR alleles. It has six regions of potential recognition to T-cells and three to B-cells.
Collagen α-1 peptide GPVGNPGPAGPAGPRGEVGLPGLS (266–289) is a strong binder mainly to DQ but rather weak for DR alleles. In collagen α-1 conformational structure it has 109 regions of potential recognition to T-cells but only three to B-cells and in this peptide, it is 8 and 1 respectively.
No strong binders for DRB1 * 03:01, and the two similar in structure HLA-DRB1*14:19 and *14:21 and also DRB1 * 04:04, DRB1 * 12:01 and DRB1 * 15:01 have been stated for IS digested peptides but binding of GYLEQLLRLKKYKVPQLEIVPNSA from IV digested peptides with DRB1 * 04:04, and DRB1 * 12:01 was strong (pIC50 −6.658 and 6.315, respectively).
3.5. Comparative In Silico Affinity Of Peptides to Chosen MHC II
Affinity of tested peptides to chosen DRB1 *01: 01, DRB1*03:01, DRB1*14:19, DRB1 *14:21 and DQ7, DQ8 is presented in Table 4.

Table 4.
IS tested affinity of obtained both (A) IS and (B) IV peptides to selected, defined MHC II according the IEDB Analysis Resource.
It was observed that only four peptides showed strong affinity to DQ8 belonging to α-S1-casein (Bos d 9) and α-S2-casein (Bos d 10) after IS digestion. Also, both peptides of α-S2-casein (Bos d 10) had strong affinity to DQ7, whereas most of the peptides after IV digestion, except Bos d 9 SDIPNPIGSENSEK peptide, had low affinity (500nM<IC50values <5000 nM). Importantly, calculated affinity for bolded SSNICNISCDK was 30-times stronger than for the longer peptide NNKIWCKDDQNPHSSNICNISCDK obtained after IV digestion.
3.6. Immunoreactive Activities Of Peptides
All of the described above peptides were submitted to further IS analyses to confirm their potential to induce IL-4 and/or IFNγ secretion and to check if there are, in their structures, fragments that can be involved in immunomodulation, antibacterial or anti-inflammatory activities (Table 5).

Table 5.
Immunoreactive activities of peptides.
DTQAIVQNNDSTE peptide belonging to Bos d 4, that has strong affinity to several HLA alleles and T cell epitopes, seems to be unable to induce IL-4 but able to induce IFNγ secretion. In its structure there are no other fragments involved in differential inhibition of Angiotensin-converting-enzyme (ACE) and Dipeptidyl Peptidase IV (DPP IV). On the other hand, SSNICNISCDK peptide is a strong binder only to HLA DQ, but it is a strong IL-4 secretion inducer.
ENSAEPEQS peptide belonging to Bos d 5 that is not able to bind with DRB1 and it is a weak binder to five DQ and three DP alleles, but it seems to have a strong IL-4 expression inductive potential. No other beneficial biological activities of this peptide have been reported so far.
ICDNQDTISSK peptide belonging to Bos d 6 with strong affinity to different types of HLA reveals also a strong potential to induce IL-4 expression but also is able to stimulate IFNγ expression. Only its inhibitory activity to Dipeptidyl Peptidase IV has been reported and deposed in database so far.
DIGSESTEDQAM peptide belonging to Bos d 9, strongly recognized by many HLA alleles, is able to induce IL-4 expression to a moderate degree. In its structure there are previously reported and deposed in database peptides with inhibitory potential to ACE and DPP IV but also stimulating vasoactive substance release. Another Bos d 9 peptide, EAESISSSEEIVPNSVEQK, shows a strong stimulation to IL-4 expression properties and at the same time stimulating IFNγ expression. Moreover, it has been reported to stimulate vasoactive substance release, glucose uptake, as well as thymosin-like peptide which is an immunomodulating activity. In the structure of Bos d 9, both in IV and IS obtained hydrolysate there was also SDIPNPIGSENSEK peptide. That peptide is a strong inducer of IL-4 expression but also it was reported to have antibacterial activity against Listeria innocua and stimulate vasoactive substance release.
VSSSEESIISQET belonging to Bos d 10 has a strong affinity for all tested HLA alleles and it has a moderate potential to induce IL-4 and IFNγ expression. It plays an important role in glucose uptake stimulation. The second tested peptide SIGSSSEESAEVATEEVK that has also strong affinity to tested HLA allele has, at the same time, strong potential to induce IL-4 and IFNγ expression and stimulates vasoactive substance release.
QSEEQQQTEDE peptide, belonging to Bos d 11, is a weak binder and is not reported to be able to induce IL-4 or IFNγ expression. It has no reported activities other than stimulation of vasoactive substance release and DPP IV inhibition.
GAQEQNQEQPIR peptide, belonging to Bos d 12, that in one predictive tool showed no affinity to HLA DR alleles in other models seems to have moderate affinity to some of the alleles. It is also able to stimulate IL-4 expression and its anti-inflammatory and anti-oxidative properties have been reported. Another two peptides from this protein, TEIPTINTIASGEPTSTPTTEAVESTVAT and EDSPEVIESPPEINTVQVTSTAV, show similar anti-oxidative and anti-inflammatory properties but at the same time its fragments EIPT and VQVTSTAV have antibacterial activity against mainly gram-positive bacteria with moderate ability to induce IL-4 and IFNγ expression.
The vast majority of tested peptides obtained during IV digestion, except described SDIPNPIGSENSEK, had moderate to weak potential to induce IL-4 and IFNγ expression, but 3 peptides show the ability to induce IL-10 expression.
NNKIWCKDDQNPHSSNICNISCDK peptide of Bos d 4 contains sequences with anti-inflammatory (PHS and TSTA) and antibacterial (CKDDQNPH) activity.
GYLEQLLRLKKYKVPQLEIVPNSA peptide of Bos d 9 contains anti-inflammatory and IL-10 expression boosting sequences (LLR, GYLEQ, RLKKY) and stimulating of glucose uptake dipeptides, or anxiolytic neuropeptides. SMKEGIHAQQKEPMIGVNQELAYF also contains dipeptides with anti-oxidative and anti-inflammatory potential among plenty of DPP IV and ACE inhibitors. One of the Bos d 9 QKHIQKEDVPSERYLGYLEQLLRL peptide contains in its structure the LGY fragment that has proven immunostimulating properties. In that peptide there are also three fragments with anti-inflammatory and one with IL-10 inducing properties.
GPVGNPGPAGPAGPRGEVGLPGLS belonging to collagen α-1, despite its proven allergenic potential, contains many fragments taking significant part in regulation of the stomach mucosal membrane activity, satiety regulation, antithrombotic activity and chemotaxis. With such strong activities, even a trace of such peptides in milk can be significant in inducing reaction.
3.7. Integrated Evaluation of Peptides Potential
The assessment was made by comparing the area under the graph prepared for 16 variables described by numerical values obtained thanks to the measurements using the algorithm SVM-score using threshold 0.2 and nine amino acids sequences analysis criterion (Figure 2). Based on this, proceeding peptides like NNKIWCKDDQNPHSSNIC-NISCDK (Bos d4), ENSAEPEQS (Bos d5), EAESISSSEEIVPNSVEQK (Bos d9), and GPVGNPGPAGPAGPRGEVGLPGLS (Collagen α1) can be verified in terms of their moderate tolerogenic potential whereas the rest of peptides needs empirical prove of their allergenicity. Basing on integrated model, peptide SSNICNISCDK (Bos d4), SIGSSSEESAEVATEEVK (Bos d10) and TEIPTINTIASGEPTSTPTTEAVESTVAT (Bos d12) may show particularly strong sensitizing properties. Some peptides like GYLEQLLRLKKYKVPQLEIVPNSA, and QKHIQKEDVPSERYLGYLEQLLRL of Bos d9, despite its potential to induce interleukin 10 expression, show a theoretical strong potential to induce allergies.
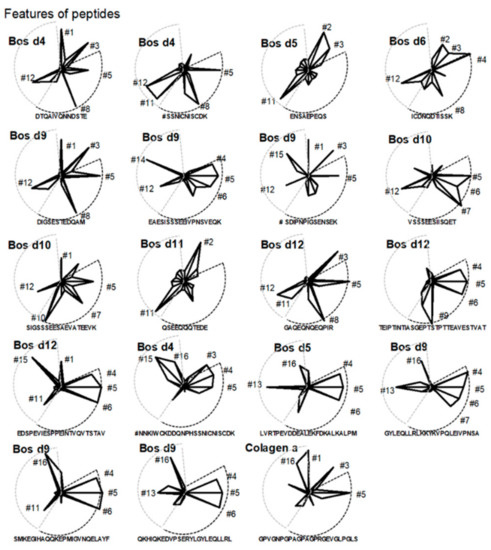
Figure 2.
Dominant peptides features integrated with the strength of individual feature in the form of a radial chart.
The area under the graph is a theoretically determined value based on the SVM score values of individual variables: 1# DRB1-Weak; 2# DQ-Weak; 3# DP-Weak; 4# DRB1-Strong; 5# DQ-Strong; 6# DP-Strong; 7# Strong Allergy involved Allels Inducer; 8# Weak Allergy involved Allels Inducer; 9# IL-4 inducers; 10# IFN gamma inducers; 11# Strong Tolerance involved Allels Inducer; 12# Weak Tolerance involved Allels Inducer; 13# IL-10 inducers; 14# proven Immunomodulative properties; 15# proven Antimicrobial properties; 16# proven Anti-inflammatory properties.
Areas in black dotted line denote strong allergenic features, and areas in gray dotted line, tolerogenic features.
4. Discussion
According to the WHO, allergy is currently the third most commonly diagnosed chronic disease [6]. The EAACI report that more than 150 million Europeans suffer from chronic allergic diseases as per the current scenario and half of the entire EU population is foreseen to be affected by 2025 [38]. The consequence of the progression of allergic diseases is a number of possible complications and the well characterized allergic march. However, the latest research mainly focuses on demonstrating possible links between allergic diseases and the development of subsequent eating disorders, psychosocial drawbacks [39] and inflammatory autoimmune diseases [40]. Substantial commonalities between allergy and autoimmune inflammatory diseases in terms of susceptibility loci, genetic pathways, and genomic regulatory sites may also explain the dramatic increase in the incidence of both disorders that are highly heritable. Some commonalities in the genetic architecture of transcriptional cofactors, cell-cycle regulators, regulatory T and helper Th17 cells differentiation factors explain a relationship between these diseases by pinpointing common disease mechanisms that are very complex in both types of diseases [7,39]. Notwithstanding, the only effective way to improve the condition of a patient with a detrimental reaction to food is to eliminate the source of antigen from the diet so far. Such a procedure, regardless of whether it is used during pregnancy, infancy or even adult life, does not always result in a reduction in the frequency of allergic reactions and the development of tolerance. Any deviation and elimination may result in a change in antibody and cytokines profile (including IL-4 and IFNγ) and response to other epitopes [41]. Various dietary changes can induce changes at the epigenetic level. The importance of maintaining allergens consumption, in terms of, e.g., CMP is emphasized for stimulating epigenetic changes that may cause tolerance [7]. As a result of the development of new processing technologies, the structure of some biological food components changes. It is also caused by the attempts to design and develop new multifunctional foods or nutraceuticals.
Animal food proteins are currently the main source of a range of biologically-active peptides which have gained special interest because they may also influence numerous physiological responses in the organism [42,43]. Therefore, it becomes necessary to develop minimally invasive and reliable systems for the initial screening of the properties of the tested peptides [3,5]. Most often, however, large discrepancies appear between IV and in vivo tests and even larger ones between tests using IS analysis. Numerous predictive models based on various calculation systems and neural networks has been developed so far [9,11,14,18,20,26]. Most often, a single system is used in the analysis. In the present study, an integrated approach combining several IS study and IV/IS assessment was used to screening the multifunctionality of milk protein-derived peptides. The main focus was on affinity of peptides to MHC II.
The MHC proteins in humans are encoded by the human leukocyte antigen (HLA) system. HLA genes are highly polymorphic which means that they have many different alleles. There are 3 major MHC class II proteins encoded by the HLA: HLA DR, HLA DQ and HLA DP. Not all binders to MHC class II proteins become epitopes but all the epitopes are binders. The structure of the MHC class II proteins’ binding site uses nine amino acids of the peptides’ sequences to bind. Therefore, in order to be recognized by the immune system the peptides should be equal or longer than nine amino acid residues [26,44]. The great majority of IS analyses are based solely on assessing the affinity of peptides for individual HLAs but as it was presented also in this study the calculated strength might be different depending on the used predictive method and tool what was observed in our research. Available methods are based on the linear structure of proteins/peptides, or involved more complex computational models, allowing also to take into account the spatial structures of proteins. The support vector machine (SVM) is a tool that allowed integration of properties and determination of regularity that is used in prediction. The difference in this matter to those based in neural network arises depending on the training and testing datasets and ways of developing libraries that reflects on specificity and sensitivity of the model [45]. In this study, almost all of the peptides that originate from IS digested allergens bind to all of the five DQ alleles. Taking into account that these alleles are the most common DQ alleles among the human population, man can expect high prevalence of allergic reaction to their parent proteins. The exception, Bos d 11 peptide SSSEESITR (32–40), binds only to one DQ, one DP and one DR allele. The difference in binding affinity to HLA alleles corresponds also to a difference in stability of the complex between allergen and MHC class II protein. Higher affinity supposes more stable complex, hence higher probability a binder becomes a T cell epitope and consequently its parent protein is recognized as an allergen. According to the previous study of the authors it has been stated that there is an association between the susceptibility to milk allergy and the HLA-DRB1/DQ polymorphism. It was stated based on the milk allergen’s strong affinity (average pIC 50 >7, IC 50 <100 nM) to DRB1 * 01:01, DQ7 and DQ8. These alleles could be considered as alleles of susceptibility to cow’s milk allergy. Whereas DRB1 * 03: 01, and two similar in structure HLA-DRB1*14:19 and *14:21 but also DRB1 * 04: 04, DRB1 * 12: 01 and DRB1 * 15:01 have been considered as alleles of protection against allergy due to weak binding to allergens [12,21]. GYLEQLLRLKKYKVPQLEIVPNSA turned out to be an interesting, in this context, peptide that originates from Bos d9. After the IV digestion it showed strong binding properties to DRB1 * 04:04 and DRB1 * 12:01. That observation contributed to the search for additional solutions to evaluate other immunological features of tested peptides.
Therefore, it was additionally proposed to verify the allergenic potential of peptides using the analysis of their potential to induce the expression of IL-4 and IFNγ. The secretion of IL-4 is a characteristic of T-helper 2 responses. It has a critical role in guiding antibody class switching, hematopoiesis and inflammation, and the development of appropriate effector T-cell responses. Th2 lymphocytes are formed as a result of the maturation of helper lymphocytes Th0 in the presence of IL-4 produced by previously activated Th2, mast cells, basophils and NKT cells (Natural Killer T cells). IL-4 plays a pivotal role in antibody isotype switching and stimulates the production of IgE [13]. We observed that even peptides defined as strong HLA binders seem not to always show a strong potential for IL-4 expression induction (DTQAIVQNNDSTE) but sometimes seem to induce IFNγ expression. On the other hand, some moderate or weak binders or peptides binding only one HLA allele type like DQ (SSNICNISCDK) show a strong potential to induce IL-4 expression. Thus, IS analysis of the ability to induce IL-4 expression appears to be a helpful tool in assessing the immunoreactive potential of peptides especially in the context of immunotherapy. Also, the role of peptides in IFNγ expression induction seems important due to the fact that IFN plays a significant role in tissue homeostasis, immune and inflammatory responses and tumor immunosurveillance. It is confirmed that it has important implications for autoimmunity, metabolic diseases, atherosclerosis, neurological diseases and immune checkpoint blockade cancer therapy [46]. In that context, the predictive tool both for IFNγ inductors as well as beneficial peptides properties was applied here. Alson IL10pred tool provided some information about tolerogenic potential of peptides. On the one hand, no particularly strong expression-inducing properties were found among the tested peptides but on the other hand plenty of anti-inflammatory dipeptides were present in tested structures.
In the conducted research, it was also important to use a comparative analysis of all features between the profile of peptides obtained during the IS and IV digestion. As has been previously reported by many groups, a small amount of peptides coincide with the obtained profiles. On the one hand, the digestibility of some proteins is very high, so they are quickly digested especially in static model of digestion, into peptides, most of them smaller than 9 amino acids [12,19]. On the other hand, some enzyme cleavage sites are inaccessible, e.g., due to the protective action of other milk components, such as emulsifying lipids [47].
In the mixture obtained after IV digestion one of the peptides belonging to the Bos d 4 allergen (SSNICNISCDK) remained in a longer structure (NNKIWCKDDQNPHSSNICNISCDK). In longer structure both allergic and tolerogenic features changed obtained higher numerical values in the used model for tolerogenic features and lower for allergic. In this context, the use of integrated, sequential IS analysis systems based on IV hydrolysates can be a helpful tool in predicting the potential of peptides and its dynamic changes. However, it should be remembered that it is necessary to test this potential with in vivo methods, but it allows for their preliminary screening. Additionally, the use of sensitive spectrometric methods helps to detect possible contaminants (collagen α-1) that probably got into the product as a result of the technological process of milk standardization. Such a combination of tools and protocols helps in a deeper understanding of various possibilities that cannot be achieved with the use of synthetic peptides. It still seems necessary to validate this approach and continue these studies in term of testing such protocols and their usefulness for other raw materials.
We should also keep in mind that all of these proteins will not be completely digested all of the time in vivo. There are factors such as food components interactions, kinetics involved in the reaction and the intestinal peristalsis constantly moving material through, regardless of whether it is broken down [48]. So, there will be many more peptides longer than 9 amino acid residues presented to MHC-II. The potential differences between the efficiency of digestive enzymes depending on age and health status (like allergy sufferers, people with gastric disorders) [49] will also be an important factor influencing efficiency of proteins degradation. In the context of such predictive difficulties, it would be helpful to include in this type of research a segregated, gastrointestinal flow models of digestion that allow not only to modulate the time of flow and retention of food, but also take into account the volumes of individual organs. Such approaches are improved and increasingly used in drug kinetics research [50]. Thus, they can also contribute to broadening the precision of this approach.
It should also be emphasized that verification of the immunoreactivity of individual model peptides should always be confirmed by IV and/or ex vivo methods, but this publication only aims to develop a method adapted to screening and selection of peptides for those confirming stage of research. Empirical tests of synthetic peptides with cell lines and animal models are expensive, so there is a need to limit choice and variety of tested peptides. Until now, human allergic sera are commonly used, also in our study [51] to verify the immunoreactivity of peptides. Sera remain a unique analytical material, so even the use of extremely sensitive detection methods, such as mass spectrometry, secondary antibodies with fluorescent dyes or quantum dots in immunohistofluorescence [52] does not eliminate the need of immunoreactivity verification with this problematic material which remains well characterized allergic human serum. The proper selection of peptides for further, more sensitive analysis, seems as important as non-targeted IV screening.
Some reports proving the IgE-immunoreactivity of certain peptides (reported in our study) confirmed with the use of human sera, are already available. Still, the etching model used in those publications were limited to single pepsin protocol or trypsin/chymotrypsin assessment. The isolated proteins like α-S1 casein and β- Lactoglobulin were tested, respectively [53,54]. Peptides obtained during that methods differed a bit from ours but mainly in their length and sequence of amino acids but not so much according their immunoreactivity. Some peptides were reported to be IgE-reactive (like: IGVNQELAYFYPELFRQFYQ) [53]. In our model after three-steps of IV digestion we observed the peptide (SMKEGIHAQQKEPMIGVNQELAYF) that contained the fragment IGVNQELAYF. In our study we concluded it might be a strong binder to moderate number of alleles of MHC II but also it was only a moderate inducer of IL-4 related allele. In referred studies of Elsayed et al., 2004 [53] that peptide was inducing only a reaction on moderate level class 1 and 2 of antibody content. That findings seems to be consistent. This same situation was observed with another peptide (DIPNPIGSENSEKTTMPLW) reported by that group as both IgG/IgE reactive and was interpreted as major inhibiting epitope. We also observed that SDIPNPIGSENSEK is a strong inducer of proallergenic and tollerogenic alleles, and a strong IL-4 allele inducer, but at this same time shows reported previously immunomodulative properties. This is a mentioned double face of peptide that can induce both types of reactions.
The results of another group also proved a deep hydrolysis of some allergenic proteins like β-Lactoglobulin and confirmed the lack of some tested, synthesized epitopes like β-Lactoglobulin 102–124. That IV test was consistent with our modelling and confirmed the applicability of IV digestion prior IS assessment utility in predicting and testing possible peptides. Still the low molecular mass of peptides <7kDa with moderate IgE-affinity was reported [54]. All that results show a high level of consistency with our protocol. The predictivity and applicability of our developed theoretical model might be a really promising. The limitation for this model might be all the possible modifications (glycation, phosphorylation and acetylation) that can both reduce or boast the immunoreactivity of peptides and that was already reported [55]. Our study provides a tool dedicated only to preliminary screening. Without a doubt, the further verification of peptides properties must be made, and we do not discuss that here.
5. Conclusions
The assessment of the affinity of peptides to MHC class II proteins allows identification of potentially antigenic peptides but still this approach needs to be multi-tool and validated with various methods. We found two common sequences for both IS and IV/IS integrated protocols. Both of those structures contain strong binders to MHC class II proteins but only one of them SDIPNPIGSENSEK was 100% identical to IS predicted. On the other hand, altering the enzyme cleavage frame may result in a change in the predicted properties of the peptides. The integration of the in vitro and IS methods creates a lot of possibilities to faithfully reproduce the reactions taking place in a living organism. To the best of the authors’ knowledge, such a sequential approach has been not applied yet.
Author Contributions
Conceptualization, B.W., A.M.O. and I.D.; methodology, A.M.O. and I.D.; software, A.M.O. and I.D.; validation, I.D.; formal analysis, A.M.O. and I.D.; investigation, A.M.O. and I.D.; resources, A.M.O. and I.D.; data curation, A.M.O. and I.D.; writing—A.M.O., I.D., B.W.; writing—Review and editing, A.M.O.; visualization, A.M.O.; supervision, B.W. and I.D.; project administration, B.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We would like to thank Benjamin Oyler for his methodical and editorial tips and correction of our work.
Conflicts of Interest
The authors declare no conflict of interest. There were no funders.
References
- WHO Report. As More Go Hungry and Malnutrition Persists, Achieving Zero Hunger by 2030 in Doubt, UN Report Warns. Available online: https://www.who.int/news/item/13-07-2020-as-more-go-hungry-and-malnutrition-persists-achieving-zero-hunger-by-2030-in-doubt-un-report-warns (accessed on 13 July 2020).
- Mazzucchelli, G.; Holzhauser, T.; Velickovic, T.C.; Diaz-perales, A.; Molina, E.; Roncada, P.; Rodrigues, P.; Verhoeckx, K.; Hoffmann-sommergruber, K. Current (Food) Allergenic Risk Assessment: Is It Fit for Novel Foods? Status Quo and Identification of Gaps. Mol. Nutr. Food Res. 2018, 1700278. [Google Scholar] [CrossRef] [PubMed]
- Amigo, L.; Martinez-Maqueda, D.; Hernandez-Ledessma, B. In silico and in vitro analysis of multifunctionality. Foods 2020, 9, 991. [Google Scholar] [CrossRef] [PubMed]
- Brossard, C.; Pineau, F.; Triballeau, S.; Pietri, M. Allergy to deamidated gluten in patients tolerant to wheat: Specific epitopes linked to deamidation. J. Allergy Clin. Immunol. 2012, 67, 1023–1032. [Google Scholar] [CrossRef]
- Verhoeckx, K.; Bøgh, K.L.; Constable, A.; Epstein, M.M.; Sommergruber, K.H.; Holzhauser, T.; Houben, G.; Kuehn, A.; Roggen, E.; Mahony, L.O.; et al. COST Action ‘ImpARAS’: What have we learnt to improve food allergy risk assessment. A summary of a 4 year networking consortium. Clin. Transl. Allergy 2020, 1–12. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J. Allergy Clin. Immunol. 2014, 133, 291–307. [Google Scholar] [CrossRef]
- Paparo, L.; Nocerino, R.; Bruno, C.; Di Scala, C.; Cosenza, L.; Bedogni, G.; Di Costanzo, M.; Mennini, M.; Argenio, V.D.; Salvatore, F.; et al. Randomized controlled trial on the influence of dietary intervention on epigenetic mechanisms in children with cow’s milk allergy: The EPICMA study. Nat. Sci. Rev. 2019, 1–10. [Google Scholar] [CrossRef]
- Bøgh, K.L.; Van Bilsen, J.; Głogowski, R.; Expósito, I.L.; Bouchaud, G.; Blanchard, C.; Bodinier, M.; Smit, J.; Pieters, R.; Net, S.B.; et al. Current challenges facing the assessment of the allergenic capacity of food allergens in animal models. Clin. Transl. Allergy 2016, 1–13. [Google Scholar] [CrossRef]
- Remington, B.; Broekman, H.C.H.; Blom, W.M.; Capt, A.; Crevel, R.W.R.; Dimitrov, I.; Faeste, C.K.; Fernandez-canton, R.; Giavi, S.; Houben, G.F.; et al. Approaches to assess IgE mediated allergy risks (sensitization and cross- reactivity) from new or modi fi ed dietary proteins. Food Chem. Toxicol. 2018, 112, 97–107. [Google Scholar] [CrossRef]
- Chatchatee, P.; Järvinen, K.M.; Bardina, L.; Beyer, K.; Sampson, H.A. Identification of IgE- and IgG-binding epitopes on αs1-casein: Differences patients with persistent and transient cow’s milk allergy. J. Allergy Clin. Immunol. 2001, 107, 379–383. [Google Scholar] [CrossRef]
- Yordanov, V.; Dimitrov, I.; Doytchinova, I. Proteochemometrics-Based Prediction of Peptide Binding to HLA-DP Proteins. J. Chem. Inf. Model. 2017. [Google Scholar] [CrossRef]
- Dimitrov, I.; Doytchinova, I. Associations between Milk and Egg Allergens and the HLA-DRB1/DQ Polymorphism: A Bioinformatics Approach. Int. Arch. Allergy Immunol. 2016, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Dhanda, S.K.; Gupta, S.; Vir, P.; Raghava, G.P.S. Prediction of IL4 Inducing Peptides. Clin. Dev. Immunol. 2013, 263952. [Google Scholar] [CrossRef] [PubMed]
- Dhanda, S.K.; Vir, P.; Raghava, G.P.S. Designing of interferon-gamma inducing MHC class-II binders. Biol. Direct 2013, 1–15. Available online: http://www.biology-direct.com/content/8/1/30 (accessed on 13 July 2020).
- Goodman, R.E. AllergenOnline Vesion 20; University of Nebraska: Lincoln, NV, USA, 2020; ISBN 1018736824. [Google Scholar]
- Mari, A.; Rasi, C.; Palazzo, P.; Scala, E. Allergen databases: Current status and perspectives. Curr. Allergy Asthma Rep. 2009, 9, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Uniprot Database. Available online: https://www.uniprot.org (accessed on 13 July 2020).
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server; Springer Protocols Handbooks; Humana Press: Geneva, Switzerland, 2005; pp. 571–607. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutro, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food–An international consensus. Food Funct. 2014, 1113–1124. [Google Scholar] [CrossRef]
- Dimitrov, I.; Garnev, P.; Flower, D.R.; Doytchinova, I. EpiTOP-a proteochemometric tool for MHC class II binding prediction. Bioinformatics 2010, 26, 2066–2068. [Google Scholar] [CrossRef]
- Kadiyska, T.; Mladenova, M.; Dimitrov, I.; Doytchinova, I. Milk allergy in Hla-Drb1* 14:19/14: 21 paediatric patients: A bioinformatics approach. Pharmacia 2018, 65, 23–27. [Google Scholar]
- Jensen, K.K.; Andreatta, M.; Marcatili, P.; Buus, S.; Greenbaum, J.A.; Yan, Z.; Sette, A.; Peters, B.N. Improved methods for predicting peptide binding affinity to MHC class II molecules. Immunology 2018, 154, 394–406. [Google Scholar] [CrossRef]
- Kringelum, J.V.; Lundegaard, C.; Lund, O.; Nielsen, M.; Reliable, B. Cell epitope predictions: Impacts of method development and improved benchmarking. PLoS Comput. Biol. 2012, 8. [Google Scholar] [CrossRef]
- Saha, S.; Raghava, G.P.S. AlgPred: Prediction of allergenic proteins and mapping of IgE epitopes. Nucleic Acids Res. 2006, 34, 202–209. [Google Scholar] [CrossRef]
- Nagpal, G.; Usmani, S.S.; Dhanda, S.K.; Kaur, H. Computer-aided designing of immunosuppressive peptides based on IL-10 inducing potential. Nat. Publ. Gr. 2017, 1–10. [Google Scholar] [CrossRef]
- Wang, P.; Sidney, J.; Kim, Y.; Sette, A.; Lund, O.; Nielsen, M.; Peters, B. Peptide binding predictions for HLA DR, DP and DQ molecules. BMC Bioinform. 2010, 11, 568. Available online: http://www.biomedcentral.com/1471-2105/11/568 (accessed on 13 July 2020). [CrossRef] [PubMed]
- Meulenbroek, L.A.P.M.; Den Hartog Jager, C.F.; Lebens, A.F.M.; Knulst, A.C.; Bruijnzeel-Koomen, C.A.F.M.; Garssen, J.; Knippels, L.M.J.; Van Hoffen, E. Characterization of T cell epitopes in bovine α-lactalbumin. Int. Arch. Allergy Immunol. 2014, 163, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Maynard, F.; Jost, R.; Wal, J.M. Human IgE binding capacity of tryptic peptides from bovine alpha-lactalbumin. Int. Arch Allergy Immunol. 1997, 113, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Hochwallner, H.; Schulmeister, U.; Swoboda, I.; Focke-Tejkl, M.; Civaj, V.; Balic, N.; Nystrand, M.; Härlin, A.; Thalhamer, J.; Scheiblhofer, S.; et al. Visualization of clustered IgE epitopes on α-lactalbumin. J. Allergy Clin. Immunol. 2010, 125. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, H.; Inoue, R.; Kaneko, H.; Watanabe, M.; Suzuki, K.; Kato, Z.; Matsushita, S.; Kondo, N. Interaction among human leucocyte antigen-peptide-T cell receptor complexes in cow’s milk allergy: The significance of human leucocyte antigen and T cell receptor-complementarity determining region 3 loops. Clin. Exp. Allergy 2002, 32, 762–770. [Google Scholar] [CrossRef]
- Inoue, R.; Matsushita, S.; Kaneko, H.; Shinoda, S.; Sakaguchi, H.; Nishimura, Y.; Kondo, N. Identification of β-lactoglobulin-derived peptides and class II HLA molecules recognized by T cells from patients with milk allergy. Clin. Exp. Allergy 2001, 31, 1126–1134. [Google Scholar] [CrossRef]
- Tanabe, S.; Kobayashi, Y.; Takahata, Y.; Morimatsu, F.; Shibata, R.; Nishimura, T. Some human B and T cell epitopes of bovine serum albumin, the major beef allergen. Biochem. Biophys. Res. Commun. 2002, 293, 1348–1353. [Google Scholar] [CrossRef]
- Ruiter, B.; Trégoat, V.; M’Rabet, L.; Garssen, J.; Bruijnzeel-Koomen, C.A.F.M.; Knol, E.F.; Van Hoffen, E. Characterization of T cell epitopes in αs1-casein in cow’s milk allergic, atopic and non-atopic children. Clin. Exp. Allergy 2006, 36, 303–310. [Google Scholar] [CrossRef]
- Lin, J.; Bardina, L.; Shreffler, W.G.; Andreae, D.A.; Ge, Y.; Wang, J.; Bruni, F.M.; Fu, Z.; Han, Y.; Sampson, H.A. Development of a novel peptide microarray for large-scale epitope mapping of food allergens. J. Allergy Clin. Immunol. 2009, 124, 315–322. [Google Scholar] [CrossRef]
- Cerecedo, I.; Zamora, J.; Shreffler, W.G.; Lin, J.; Bardina, L.; Dieguez, M.C.; Wang, J.; Muriel, A.; de la Hoz, B.; Sampson, H.A. Mapping of the IgE and IgG4 sequential epitopes of milk allergens with a peptide microarray-based immunoassay. J. Allergy Clin. Immunol. 2008, 122, 589–594. [Google Scholar] [CrossRef]
- Lisson, M.; Novak, N.; Erhardt, G. Immunoglobulin E epitope mapping by microarray immunoassay reveals differences in immune response to genetic variants of caseins from different ruminant species. J. Dairy Sci. 2014, 97, 1939–1954. [Google Scholar] [CrossRef] [PubMed]
- Wildner, G.; Diedrichs-Möhring, M. Autoimmune uveitis induced by molecular mimicry of peptides from rotavirus, bovine casein and retinal S-antigen. Eur. J. Immunol. 2003, 33, 2577–2587. [Google Scholar] [CrossRef] [PubMed]
- Food Allergy Diagnostics & Therapeutics Market Size, Share, & Trends Analysis Report By Product Type (Therapeutic), By Allergen Source (Peanut), By End Use (Hospitals & Clinics), By Region, And Segment Forecasts, 2019–2026. Available online: https://www.factmr.com/report/2456/stationary-fuel-cell-systems-market (accessed on 3 October 2019).
- Wróblewska, B.; Szyc, A.M.; Markiewicz, L.H.; Zakrzewska, M.; Romaszko, E. Increased prevalence of eating disorders as a biopsychosocial implication of food allergy. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Kreiner, E.; Waage, J.; Standl, M.; Brix, S.; Pers, T.H.; Couto Alves, A.; Warrington, N.M.; Tiesler, C.M.T.; Fuertes, E.; Franke, L.; et al. Shared genetic variants suggest common pathways in allergy and autoimmune diseases. J. Allergy Clin. Immunol. 2017, 140, 771–781. [Google Scholar] [CrossRef]
- Ogrodowczyk, A.M.; Zakrzewska, M.; Romaszko, E.; Wroblewska, B. Gestational Dysfunction-Driven Diets and Probiotic Supplementation Correlate with the Profile of Allergen-Specific Antibodies in the Serum of Allergy Sufferers. Nutrients 2020, 12, 2381. [Google Scholar] [CrossRef]
- Albenzio, M.; Santillo, A.; Caroprese, M.; Malva, A. Bioactive Peptides in Animal Food Products. Foods 2017, 6, 35. [Google Scholar] [CrossRef]
- Bottani, M.; Cattaneo, S.; Pica, V.; Stuknyt, M.; Gomarasca, M.; Banfi, G.; De Noni, I.; Ferraretto, A.; Lombardi, G. Gastrointestinal In Vitro Digests of Infant Biscuits Formulated with Bovine Milk Proteins Positively. Foods 2020, 9, 1510. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Sidney, J.; Assarsson, E.; Moore, C.; Ngo, S.; Pinilla, C.; Sette, A.; Peters, B. Quantitative peptide binding motifs for 19 human and mouse MHC class I molecules derived using positional scanning combinatorial peptide libraries. Immunome Res. 2008, 14, 1–14. [Google Scholar] [CrossRef]
- Ivashkiv, L.B.; Program, T.D.; Rosensweig, D.Z.; Program, M.P.; Medicine, W.C. HHS Public Access. Nat. Rev. Immunol. 2019, 18, 545–558. [Google Scholar] [CrossRef]
- Macierzanka, A.; Sancho, A.I.; Mills, E.N.C.; Rigby, N.M.; Mackie, A.R. Emulsification alters simulated gastrointestinal proteolysis of b -casein and b -lactoglobulin. Soft Matter 2009, 538–550. [Google Scholar] [CrossRef]
- Bøgh, K.L.; Madsen, C.B. Critical reviews in food science and nutrition food allergens: Is there a correlation between stability to digestion and allergenicity? Food Sci. Nutr. 2015, 37–41. [Google Scholar] [CrossRef]
- Verhoeckx, K.; Lindholm, K.; Dupont, D.; Egger, L.; Gadermaier, G.; Larré, C.; Mackie, A.; Menard, O.; Adel-patient, K.; Picariello, G.; et al. The relevance of a digestibility evaluation in the allergenicity risk assessment of novel proteins. Opinion of a joint initiative of COST action ImpARAS and COST action INFOGEST. Food Chem. Toxicol. 2019, 129, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.S.; Peng, H.B.; Noh, K. The Segregated Intestinal Flow Model (SFM) for drug absorption and drug metabolism: Implications on intestinal and liver metabolism and drug–drug interactions. Pharmaceutics 2020, 12, 312. [Google Scholar] [CrossRef] [PubMed]
- Fotschki, J.; Wróblewska, B.; Fotschki, B.; Kalicki, B.; Rigby, N.; Mackie, A. Microbial transglutaminase alters the immunogenic potential and cross-reactivity of horse and cow milk proteins. J. Dairy Sci. 2020, 103, 3. [Google Scholar] [CrossRef] [PubMed]
- Kalčáková, L.; Tremlová, B.; Pospiech, M.; Hostovský, M.; Dordević, D.; Javůrková, Z.; Běhalová, H.; Bartlová, M. Use of IHF-QD microscopic analysis for the detection of food allergenic components: Peanuts and wheat protein. Foods 2020, 9, 239. [Google Scholar] [CrossRef]
- Elsayed, S.; Hill, D.J.; Do, T.V. Evaluation of the allergenicity and antigenicity of bovine-milk αs1-casein using extensively purified synthetic peptides. Scand. J. Immunol. 2004, 60, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.M.; Kato, T.; Ohnishi, H.; Kawamoto, N.; Kato, Z.; Kaneko, H.; Kondo, N.; Nakano, T. T-cell epitope-containing hypoallergenic β-lactoglobulin for oral immunotherapy in milk allergy. Pediatr. Allergy Immunol. 2016, 27, 818–824. [Google Scholar] [CrossRef]
- Liu, J.; Chen, W.M.; Shao, Y.H.; Zhang, J.L.; Tu, Z.C. The mechanism of the reduction in allergenic reactivity of bovine α-lactalbumin induced by glycation, phosphorylation and acetylation. Food Chem. 2020, 310, 125853. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).