Effect of Homogenization Method and Carvacrol Content on Microstructural and Physical Properties of Chitosan-Based Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
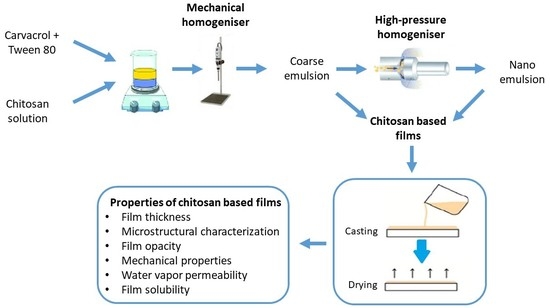
2.2. Preparation of Chitosan Solutions and Film-Forming Emulsions
2.3. Preparation of Emulsified Films
2.4. Film Thickness
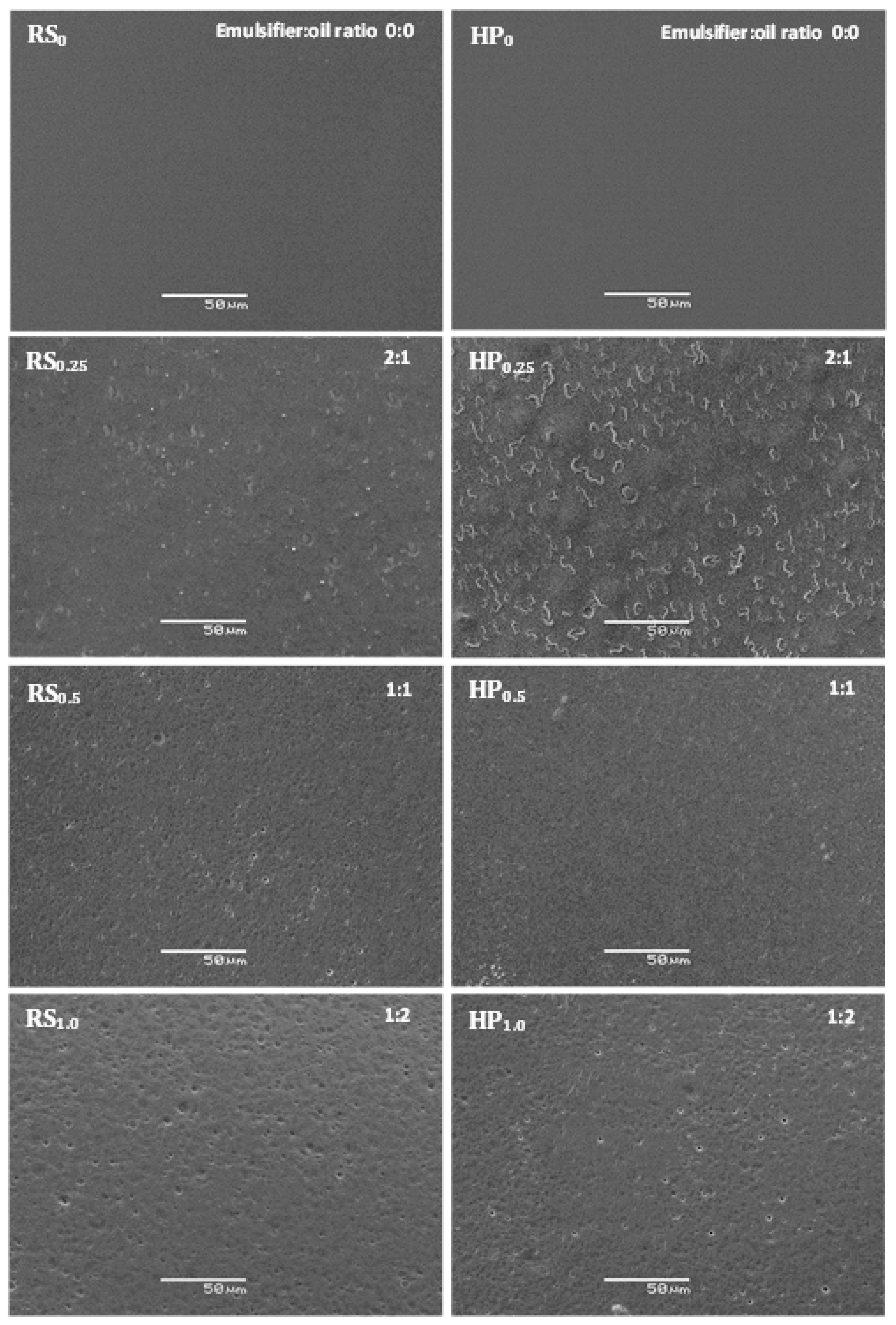
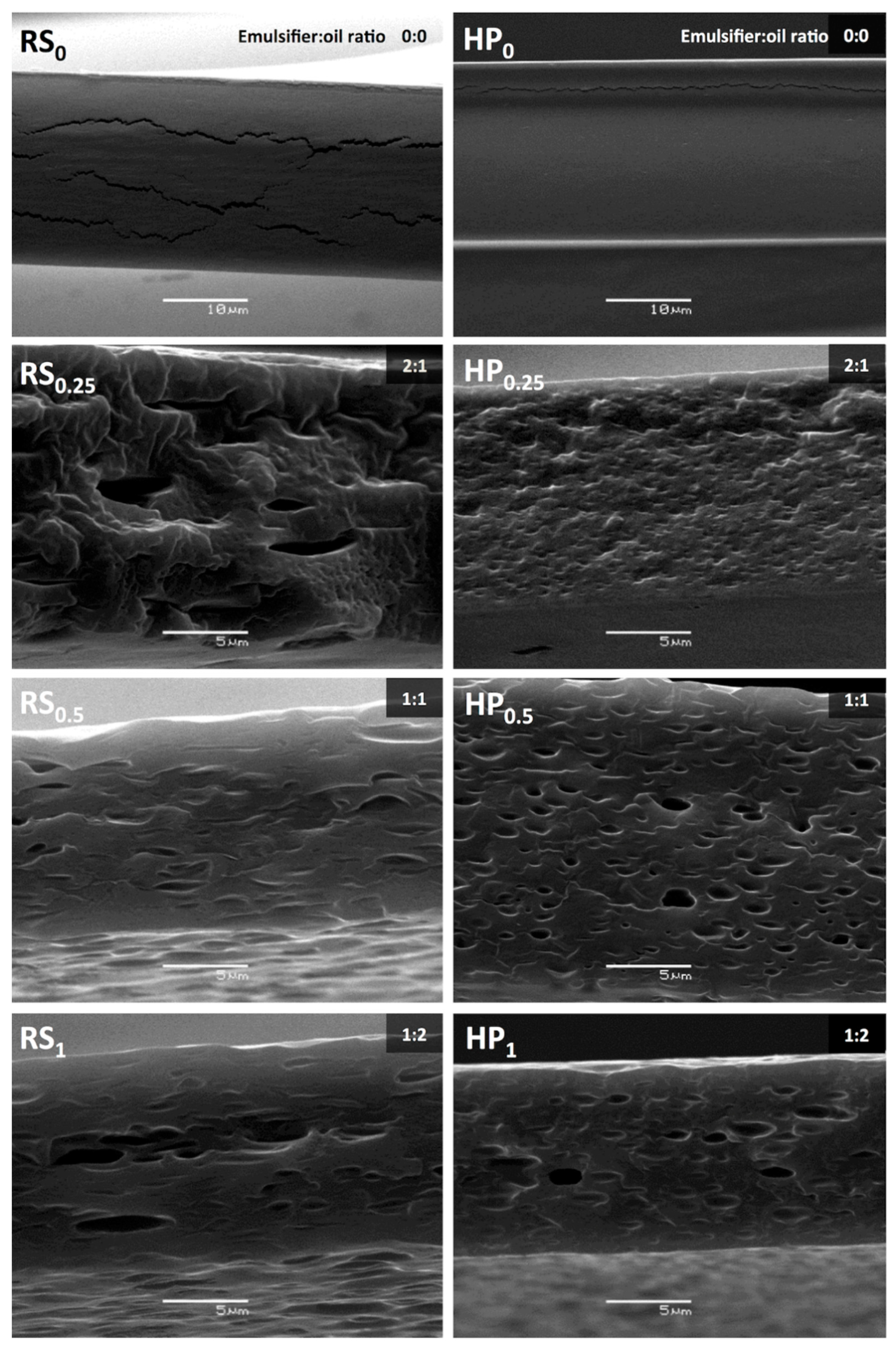
2.5. Microstructural Characterization
2.6. Film Opacity
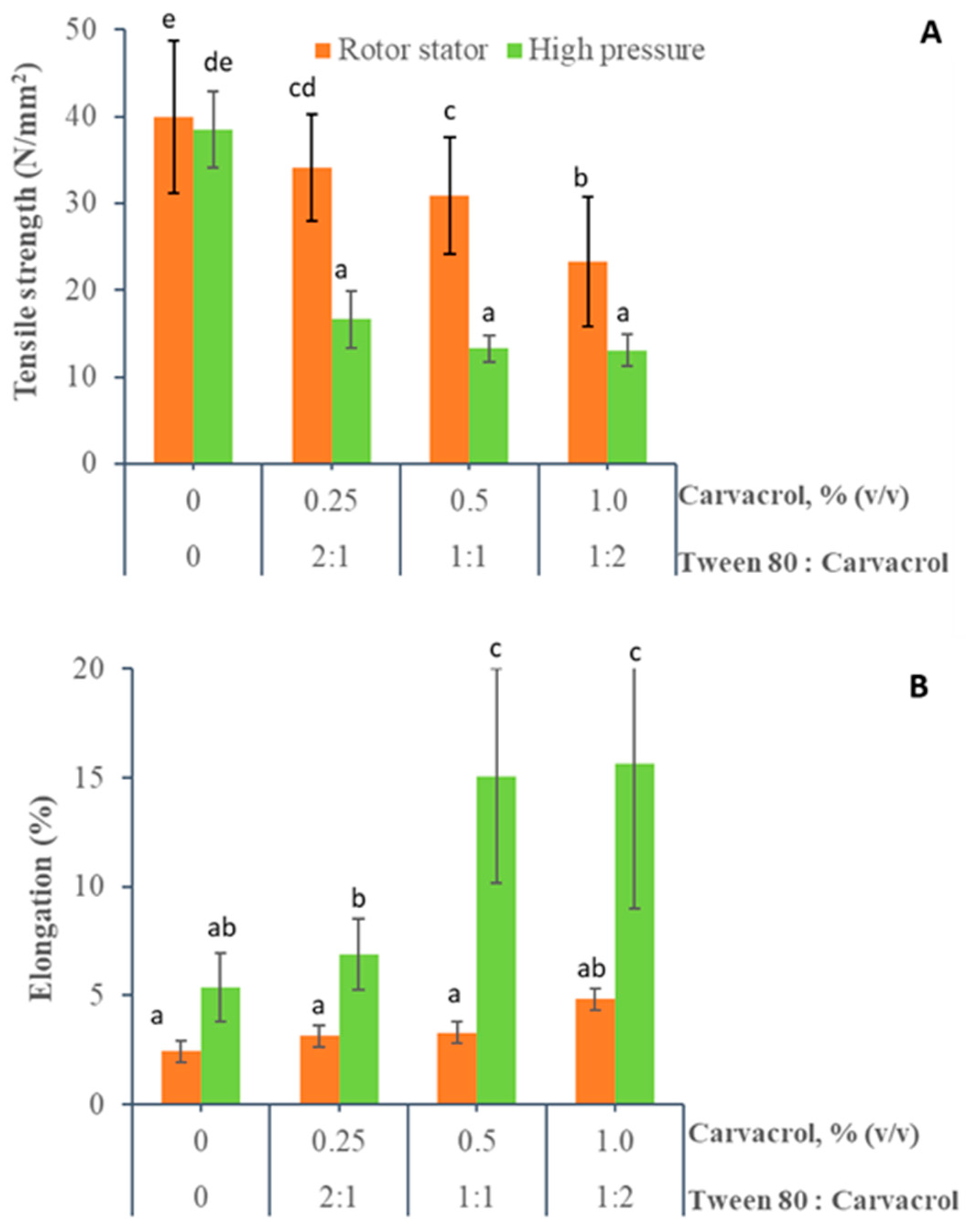
2.7. Mechanical Properties
2.8. Water Vapor Permeability
2.9. Film Solubility
2.10. Statistical Analysis
3. Results and Discussion
3.1. Physical and Optical Properties
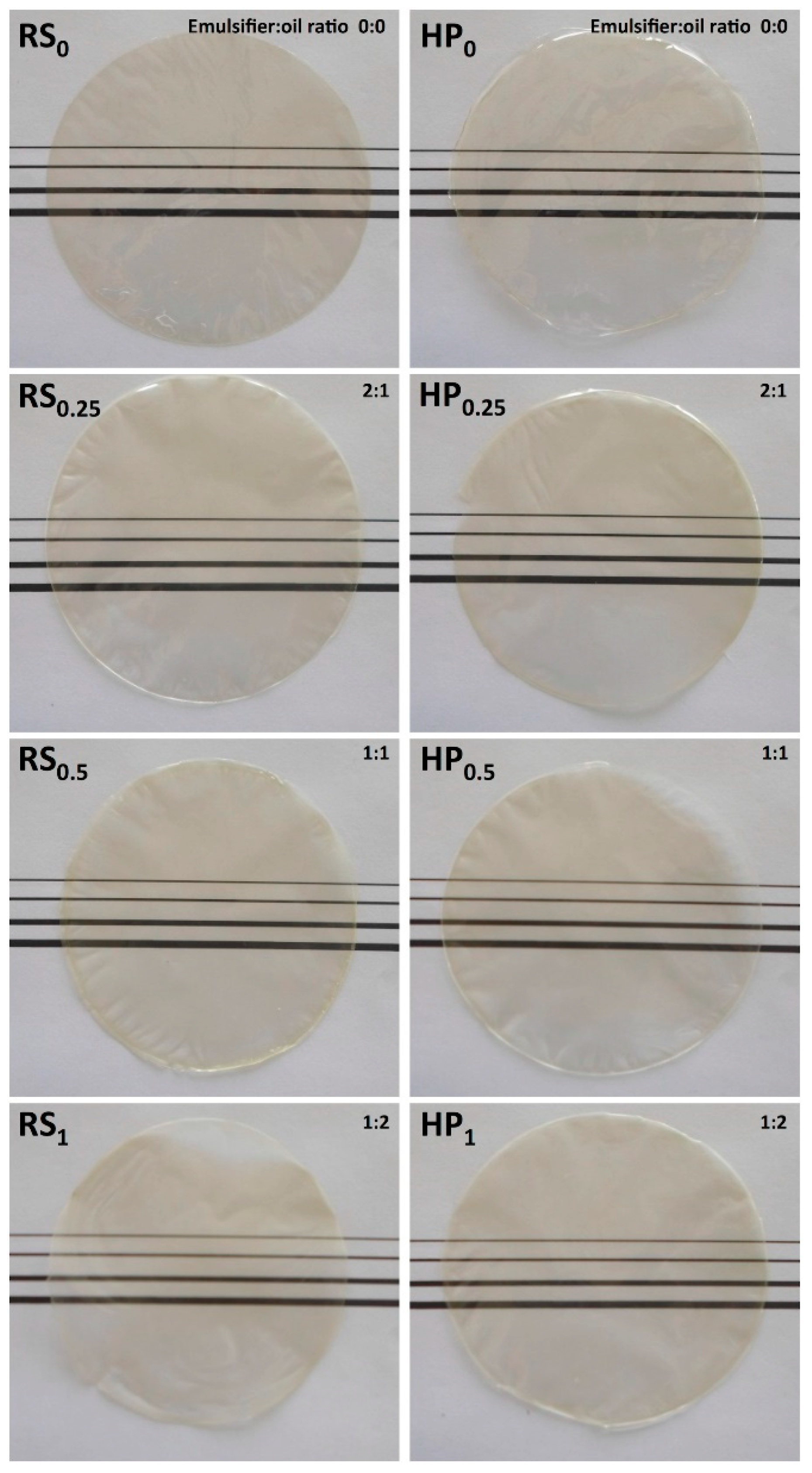
3.1.1. Thickness
3.1.2. Microstructural Characteristics
3.1.3. Mechanical Properties
3.1.4. Opacity
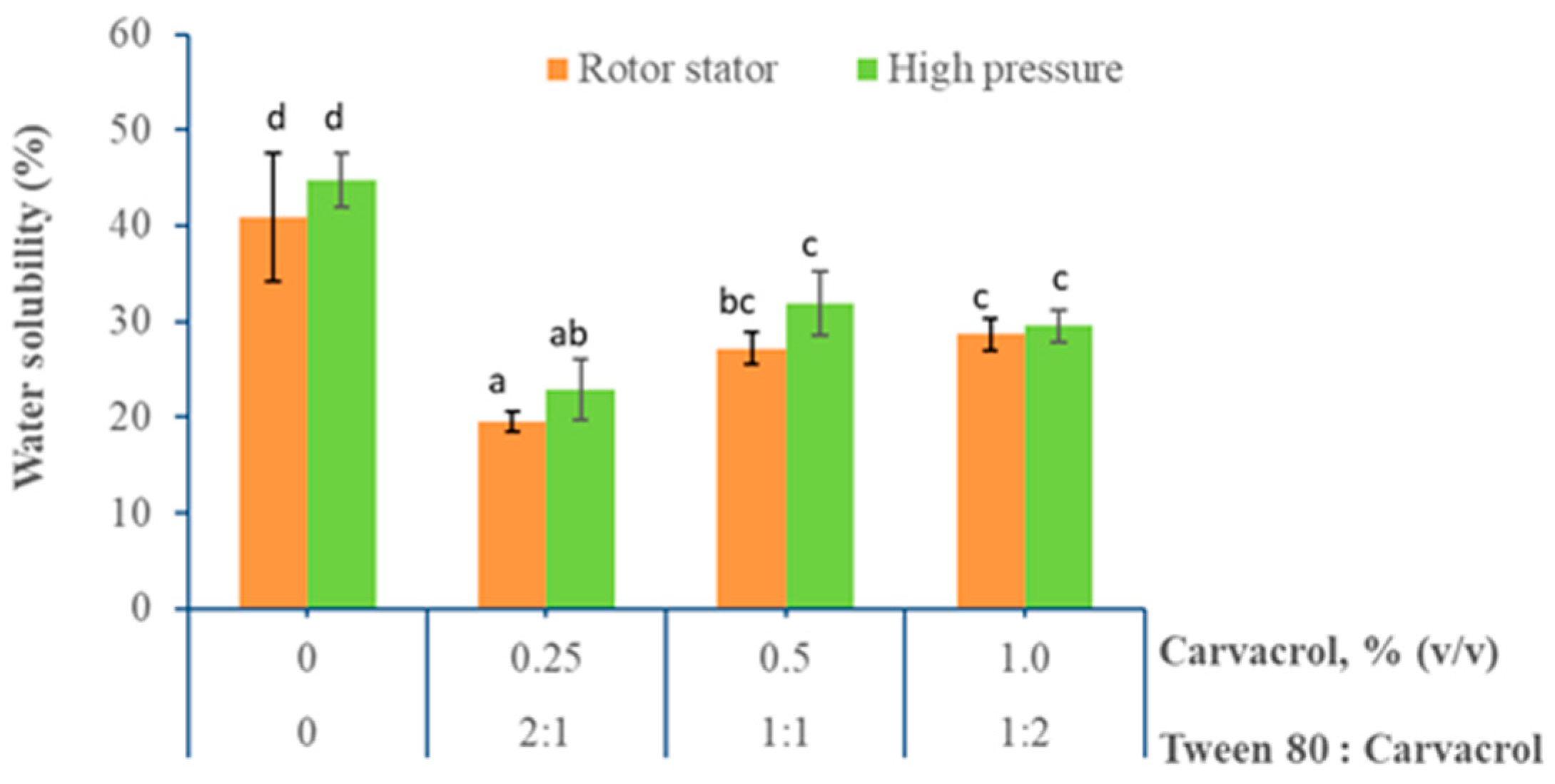
3.1.5. Water Solubility
3.1.6. Water Vapor Permeability (WVP)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Thakhiew, W.; Champahom, M.; Devahastin, S.; Soponronnarit, S. Improvement of mechanical properties of chitosan-based films via physical treatment of film-forming solution. J. Food Eng. 2015, 158, 66–72. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Nisperos-Carriedo, M.O.; Baker, R.A. Edible Coatings for Lightly Processed Fruits and Vegetables. HortScience 2019, 30, 35–38. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of essential oils and homogenization conditions on properties of chitosan-based films. Food Hydrocoll. 2012, 26, 9–16. [Google Scholar] [CrossRef]
- Sablani, S.S.; Dasse, F.; Bastarrachea, L.; Dhawan, S.; Hendrix, K.M.; Min, S.C. Apple peel-based edible film development using a high-pressure homogenization. J. Food Sci. 2009, 74. [Google Scholar] [CrossRef] [PubMed]
- Durango, A.M.; Soares, N.F.F.; Andrade, N.J. Microbiological evaluation of an edible antimicrobial coating on minimally processed carrots. Food Control 2006, 17, 336–341. [Google Scholar] [CrossRef]
- Li, Q.; Dunn, E.T.; Grandmaison, E.W.; Goosen, M.F.A. Applications and Properties of Chitosan. J. Bioact. Compat. Polym. 1992, 7, 370–397. [Google Scholar] [CrossRef]
- Park, S.I.; Zhao, Y. Incorporation of a High Concentration of Mineral or Vitamin into Chitosan-Based Films. J. Agric. Food Chem. 2004, 52, 1933–1939. [Google Scholar] [CrossRef]
- Ribeiro, C.; Vicente, A.A.; Teixeira, J.A.; Miranda, C. Optimization of edible coating composition to retard strawberry fruit senescence. Postharvest Biol. Technol. 2007, 44, 63–70. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Development and evaluation of a novel biodegradable film made from chitosan and cinnamon essential oil with low affinity toward water. Food Chem. 2010, 122, 161–166. [Google Scholar] [CrossRef]
- Bourtoom, T. Edible films and coatings: Characteristics and properties. Int. Food Res. J. 2008, 15, 237–248. [Google Scholar]
- Baldwin, E.A.; Nisperos-Carriedo, M.O.; Baker, R.A. Use of Edible Coatings to Preserve Quality of Lightly (and Slightly) Processed Products. Crit. Rev. Food Sci. Nutr. 1995, 35, 509–524. [Google Scholar] [CrossRef]
- Yuan, G.; Chen, X.; Li, D. Chitosan films and coatings containing essential oils: The antioxidant and antimicrobial activity, and application in food systems. Food Res. Int. 2016, 89, 117–128. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Use of Essential Oils in Bioactive Edible Coatings: A Review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C.A. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J. Appl. Microbiol. 2006, 101, 1232–1240. [Google Scholar] [CrossRef]
- Alvarez, I.; Niemira, B.A.; Fan, X.; Sommers, C.H. Inactivation of Salmonella Enteritidis and Salmonella Senftenberg in Liquid Whole Egg Using Generally Recognized as Safe Additives, Ionizing Radiation, and Heat. J. Food Prot. 2007, 70, 1402–1409. [Google Scholar] [CrossRef]
- Wu, Y.; Weller, C.L.; Hamouz, F.; Cuppett, S.L.; Schnepf, M. Development and application of multicomponent edible coatings and films: A review. Adv. Food Nutr. Res. 2002, 44, 347–394. [Google Scholar] [CrossRef]
- Villalobos, R.; Chanona, J.; Hernández, P.; Gutiérrez, G.; Chiralt, A. Gloss and transparency of hydroxypropyl methylcellulose films containing surfactants as affected by their microstructure. Food Hydrocoll. 2005, 19, 53–61. [Google Scholar] [CrossRef]
- Fabra, M.J.; Talens, P.; Chiralt, A. Microstructure and optical properties of sodium caseinate films containing oleic acid-beeswax mixtures. Food Hydrocoll. 2009, 23, 676–683. [Google Scholar] [CrossRef]
- Mcclements, D.J. Critical Review of Techniques and Methodologies for Characterization of Emulsion Stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga, R.N.; Skurtys, O.; Osorio, F.; Aguilera, J.M.; Pedreschi, F. Physical properties of emulsion-based hydroxypropyl methylcellulose films: Effect of their microstructure. Carbohydr. Polym. 2012, 90, 1147–1158. [Google Scholar] [CrossRef]
- Perdones, Á.; Chiralt, A.; Vargas, M. Properties of film-forming dispersions and films based on chitosan containing basil or thyme essential oil. Food Hydrocoll. 2016, 57, 271–279. [Google Scholar] [CrossRef]
- Debeaufort, F.; Martin-Polo, M.; Voilley, A. Polarity Homogeneity and Structure Affect Water Vapor Permeability of Model Edible Films. J. Food Sci. 1993, 58, 426–429. [Google Scholar] [CrossRef]
- Kralova, I.; Sjöblom, J. Surfactants used in food industry: A review. J. Dispers. Sci. Technol. 2009, 30, 1363–1383. [Google Scholar] [CrossRef]
- McClements, D.J. (Ed.) Food Emulsions, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015; ISBN 978-1-4987-2668-9. [Google Scholar]
- Qian, C.; McClements, D.J. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: Factors affecting particle size. Food Hydrocoll. 2011, 25, 1000–1008. [Google Scholar] [CrossRef]
- Rao, J.; McClements, D.J. Formation of flavor oil microemulsions, nanoemulsions and emulsions: Influence of composition and preparation method. J. Agric. Food Chem. 2011, 59, 5026–5035. [Google Scholar] [CrossRef]
- Rubilar, J.F.; Cruz, R.M.S.; Silva, H.D.; Vicente, A.A.; Khmelinskii, I.; Vieira, M.C. Physico-mechanical properties of chitosan films with carvacrol and grape seed extract. J. Food Eng. 2013, 115, 466–474. [Google Scholar] [CrossRef]
- López-Mata, M.A.; Ruiz-Cruz, S.; Silva-Beltrán, N.P.; Ornelas-Paz, J.D.J.; Zamudio-Flores, P.B.; Burruel-Ibarra, S.E. Physicochemical, antimicrobial and antioxidant properties of chitosan films incorporated with carvacrol. Molecules 2013, 18, 13735–13753. [Google Scholar] [CrossRef] [PubMed]
- Kurek, M.; Guinault, A.; Voilley, A.; Galić, K.; Debeaufort, F. Effect of relative humidity on carvacrol release and permeation properties of chitosan based films and coatings. Food Chem. 2014, 144, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Flores, Z.; San Martín, D.; Villalobos-Carvajal, R.; Tabilo-Munizaga, G.; Osorio, F.; Leiva-Vega, J. Physicochemical characterization of chitosan-based coating-forming emulsions: Effect of homogenization method and carvacrol content. Food Hydrocoll. 2016, 61, 851–857. [Google Scholar] [CrossRef]
- Kurek, M.; Moundanga, S.; Favier, C.; Gali, K.; Debeaufort, F. Antimicrobial efficiency of carvacrol vapour related to mass partition coefficient when incorporated in chitosan based films aimed for active packaging. Food Control 2013, 32, 168–175. [Google Scholar] [CrossRef]
- Han, J.H.; Floros, J.D. Casting Antimicrobial Packaging Films and Measuring Their Physical Properties and Antimicrobial Activity. J. Plast. Film Sheeting 1997, 13, 287–298. [Google Scholar] [CrossRef]
- Gennadios, A.; Weller, C.L.; Gooding, C.H. Measurement Errors in Water Vapor Permeability of Highly Permeable, Hydrophilic Edible Films. J. Food Eng. 1994, 21, 395–409. [Google Scholar] [CrossRef]
- Benavides, S.; Villalobos-Carvajal, R.; Reyes, J.E. Physical, mechanical and antibacterial properties of alginate film: Effect of the crosslinking degree and oregano essential oil concentration. J. Food Eng. 2012, 110, 232–239. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Effect of Oxidized Potato Starch on the Physicochemical Properties of Soy Protein Isolate-Based Edible Films. Food Technol. Biotechnol. 2013, 51, 403–409. [Google Scholar]
- Pérez-Gago, M.B. Protein-based films and coating. In Edible Coatings and Films to Improve Food Quality; Baldwin, E.A., Hagenmaier, R., Bai, J., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 13–78. ISBN 9781420059663. [Google Scholar]
- Han, J.H.; Krochta, J.M. Wetting properties and water vapor permeability of whey-protein-coated paper. Trans. ASAE 1999, 42, 1375–1382. [Google Scholar] [CrossRef]
- Fernández-Pan, I.; Maté, J.I.; Gardrat, C.; Coma, V. Effect of chitosan molecular weight on the antimicrobial activity and release rate of carvacrol-enriched films. Food Hydrocoll. 2015, 51, 60–68. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Y.; Zhong, Q. Physical and antimicrobial properties of chitosan fi lms incorporated with lauric arginate, cinnamon oil, and ethylenediaminetetraacetate. LWT Food Sci. Technol. 2016, 65, 173–179. [Google Scholar] [CrossRef]
- Guaregua, J.; Ruiz, B.L.F.; Castellanos, A. Actividad Superficial de Mezclas de Soluciones de Surfactantes No-Iónicos Tween 20 y Tween 80 en Interfaz Líquido-Aire. Available online: https://docplayer.es/50646152-Actividad-superficial-de-mezclas-de-soluciones-de-surfactantes-no-ionicos-tween-20-y-tween-80-en-interfaz-liquido-aire.html (accessed on 11 November 2020).
- Kasaai, M.; Charlet, G.; Paquin, P.; Arul, J. Fragmentation of chitosan by microfluidization process. Innov. Food Sci. Emerg. Technol. 2003, 4, 403–413. [Google Scholar] [CrossRef]
- Vargas, M.; Perdones, Á.; Chiralt, A.; Cháfer, M.; González-martínez, C. Effect of homogenization conditions on physicochemical properties of chitosan-based film-forming dispersions and films. Food Hydrocoll. 2011, 25, 1158–1164. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Physicochemical characterization and antimicrobial activity of food-grade emulsions and nanoemulsions incorporating essential oils. Food Hydrocoll. 2015, 43, 547–556. [Google Scholar] [CrossRef]
- Villay, A.; Lakkis de Filippis, F.; Picton, L.; Le Cerf, D.; Vial, C.; Michaud, P. Comparison of polysaccharide degradations by dynamic high-pressure homogenization. Food Hydrocoll. 2012, 27, 278–286. [Google Scholar] [CrossRef]
- Lagoueyte, N.; Paquin, P. Effects of microfluidization on the functional properties of xanthan gum. Food Hydrocoll. 1998, 12, 365–371. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Edible films from essential-oil-loaded nanoemulsions: Physicochemical characterization and antimicrobial properties. Food Hydrocoll. 2015, 47, 168–177. [Google Scholar] [CrossRef]
- Norajit, K.; Myong, K.; Hyung, G. Comparative studies on the characterization and antioxidant properties of biodegradable alginate films containing ginseng extract. J. Food Eng. 2010, 98, 377–384. [Google Scholar] [CrossRef]
- Shojaee-aliabadi, S.; Hosseini, H.; Amin, M.; Mohammadi, A.; Ghasemlou, M.; Marzieh, S.; Khaksar, R. Characterization of k-carrageenan films incorporated plant essential oils with improved antimicrobial activity. Carbohydr. Polym. 2014, 101, 582–591. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Chiralt, A.; González-Martínez, C.; Cháfer, M. Effect of essential oils on properties of film forming emulsions and films based on hydroxypropylmethylcellulose and chitosan. J. Food Eng. 2011, 105, 246–253. [Google Scholar] [CrossRef]
- Atarés, L.; Pérez-Masiá, R.; Chiralt, A. The role of some antioxidants in the HPMC film properties and lipid protection in coated toasted almonds. J. Food Eng. 2011, 104, 649–656. [Google Scholar] [CrossRef]
- Kurek, M.; Brachais, C.H.; Scetar, M.; Voilley, A.; Galic, K.; Couvercelle, J.P.; Debeaufort, F. Carvacrol affects interfacial, structural and transfer properties of chitosan coatings applied onto polyethylene. Carbohydr. Polym. 2013, 97, 217–225. [Google Scholar] [CrossRef]
- Shit, S.C.; Shah, P.M. Edible Polymers: Challenges and Opportunities. J. Polym. 2014, 2014, 427259. [Google Scholar] [CrossRef]
- Hosseini, M.H.; Razavi, S.H.; Mousavi, M.A. Antimicrobial, physical and mechanical properties of chitosan-based films incorporated with thyme, clove and cinnamon essential oil. J. Food Process. Preserv. 2009, 33, 727–743. [Google Scholar] [CrossRef]
- Shi, Y.; Luo, H.Q.; Li, N.B. Determination of the critical premicelle concentration, first critical micelle concentration and second critical micelle concentration of surfactants by resonance Rayleigh scattering method without any probe. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Shaw, N.B.; Monahan, F.J.; Riordan, E.D.O.; Sullivan, M.O. Effect of soya oil and glycerol on physical properties of composite WPI. J. Food Eng. 2002, 51, 299–304. [Google Scholar] [CrossRef]
- Guerrero, P.; Hanani, Z.A.N.; Kerry, J.P.; Caba, K. De Characterization of soy protein-based films prepared with acids and oils by compression. J. Food Eng. 2011, 107, 41–49. [Google Scholar] [CrossRef]
- Pereda, M.; Amica, G.; Marcovich, N.E. Development and characterization of edible chitosan/olive oil emulsion films. Carbohydr. Polym. 2012, 87, 1318–1325. [Google Scholar] [CrossRef]
- Abdollahi, M.; Rezaei, M.; Farzi, G. A novel active bionanocomposite film incorporating rosemary essential oil and nanoclay into chitosan. J. Food Eng. 2012, 111, 343–350. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Y.; Critzer, F.; Davidson, P.M.; Zivanovic, S.; Zhong, Q. Physical, mechanical, and antimicrobial properties of chitosan fi lms with microemulsions of cinnamon bark oil and soybean oil. Food Hydrocoll. 2016, 52, 533–542. [Google Scholar] [CrossRef]
- Du, W.; Olsen, C.W.; Friedman, M.; Mchugh, T.H. Physical and Antibacterial Properties of Edible Films Formulated with Apple Skin Polyphenols. J. Food Sci. 2011, 76, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, Y. Combined effects of two kinds of essential oils on physical, mechanical and structural properties of chitosan fi lms. Food Hydrocoll. 2014, 36, 287–293. [Google Scholar] [CrossRef]
- Morillon, V.; Debeaufort, F.; Blond, G.; Capelle, M.; Voilley, A. Factors Affecting the Moisture Permeability of Lipid-Based Edible Films: A Review. Crit. Rev. Food Sci. Nutr. 2002, 42, 67–89. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gago, M.B.; Krochta, J.M. Lipid Particle Size Effect on Water Vapor Permeability and Mechanical Properties of Whey Protein/Beeswax Emulsion Films. J. Agric. Food Chem. 2001, 49, 996–1002. [Google Scholar] [CrossRef] [PubMed]


| Chitosan (g) | Glycerol (mL) | Tween 80 (mL) | Carvacrol (mL) | Emulsifier: Oil Ratio |
|---|---|---|---|---|
| 1.5 | 0.225 | 0.0 | 0.0 | 0:0 |
| 1.5 | 0.225 | 0.5 | 0.25 | 2:1 |
| 1.5 | 0.225 | 0.5 | 0.5 | 1:1 |
| 1.5 | 0.225 | 0.5 | 1.0 | 1:2 |
| Carvacrol (% v/v) | Thickness (mm) | |
|---|---|---|
| Rotor-Stator | High-Pressure | |
| 0.0 | 0.013 ± 0.001 a,x | 0.012 ± 0.002 a,x |
| 0.25 | 0.012 ± 0.001 a,x | 0.014 ± 0.002 a,x |
| 0.5 | 0.011 ± 0.002 a,x | 0.013 ± 0.004 a,x |
| 1.0 | 0.012 ± 0.004 a,x | 0.012 ± 0.002 a,x |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores, Z.; San-Martin, D.; Beldarraín-Iznaga, T.; Leiva-Vega, J.; Villalobos-Carvajal, R. Effect of Homogenization Method and Carvacrol Content on Microstructural and Physical Properties of Chitosan-Based Films. Foods 2021, 10, 141. https://doi.org/10.3390/foods10010141
Flores Z, San-Martin D, Beldarraín-Iznaga T, Leiva-Vega J, Villalobos-Carvajal R. Effect of Homogenization Method and Carvacrol Content on Microstructural and Physical Properties of Chitosan-Based Films. Foods. 2021; 10(1):141. https://doi.org/10.3390/foods10010141
Chicago/Turabian StyleFlores, Zoila, Diego San-Martin, Tatiana Beldarraín-Iznaga, Javier Leiva-Vega, and Ricardo Villalobos-Carvajal. 2021. "Effect of Homogenization Method and Carvacrol Content on Microstructural and Physical Properties of Chitosan-Based Films" Foods 10, no. 1: 141. https://doi.org/10.3390/foods10010141
APA StyleFlores, Z., San-Martin, D., Beldarraín-Iznaga, T., Leiva-Vega, J., & Villalobos-Carvajal, R. (2021). Effect of Homogenization Method and Carvacrol Content on Microstructural and Physical Properties of Chitosan-Based Films. Foods, 10(1), 141. https://doi.org/10.3390/foods10010141