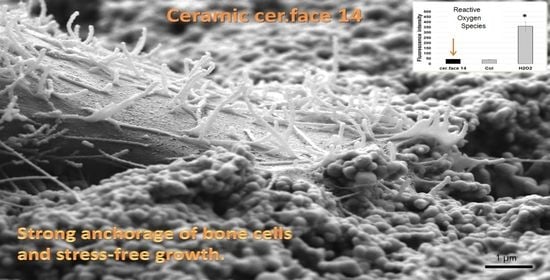
The Anchorage of Bone Cells onto an Yttria-Stabilized Zirconia Surface with Mild Nano-Micro Curved Profiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials: Yttria-Stabilized Zirconia
2.2. Cell Culture
2.3. Stress Level of Cells
2.4. Image Analysis
2.5. Statistics
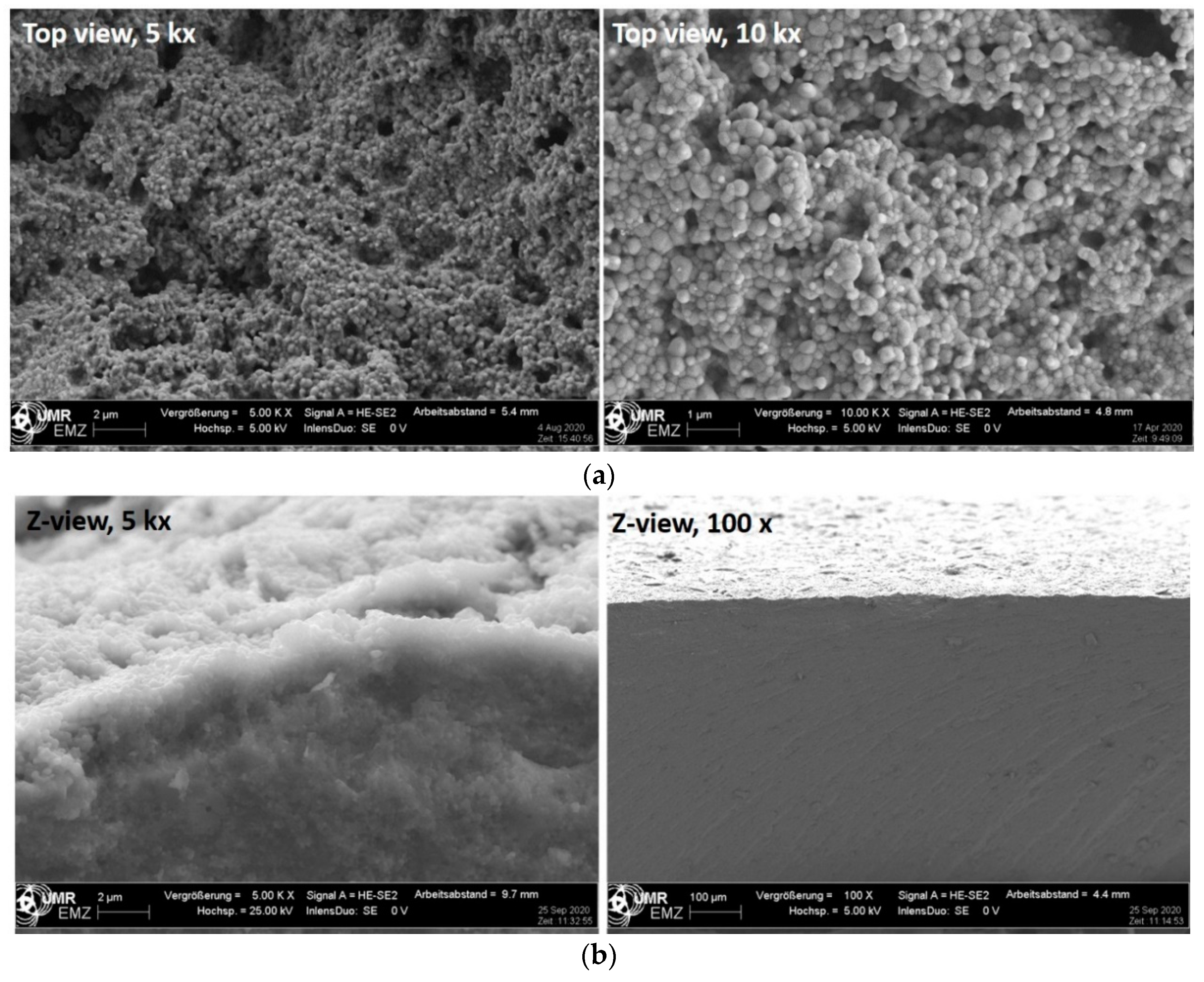
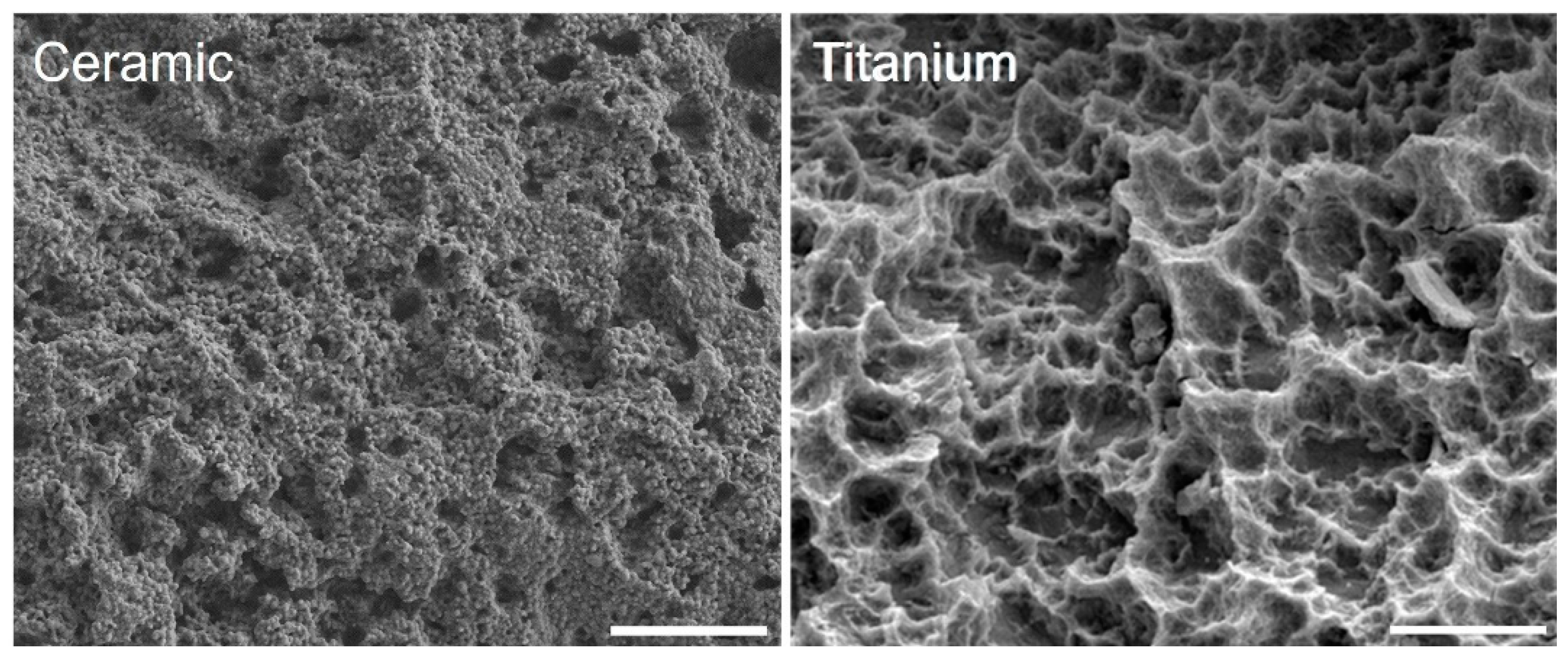
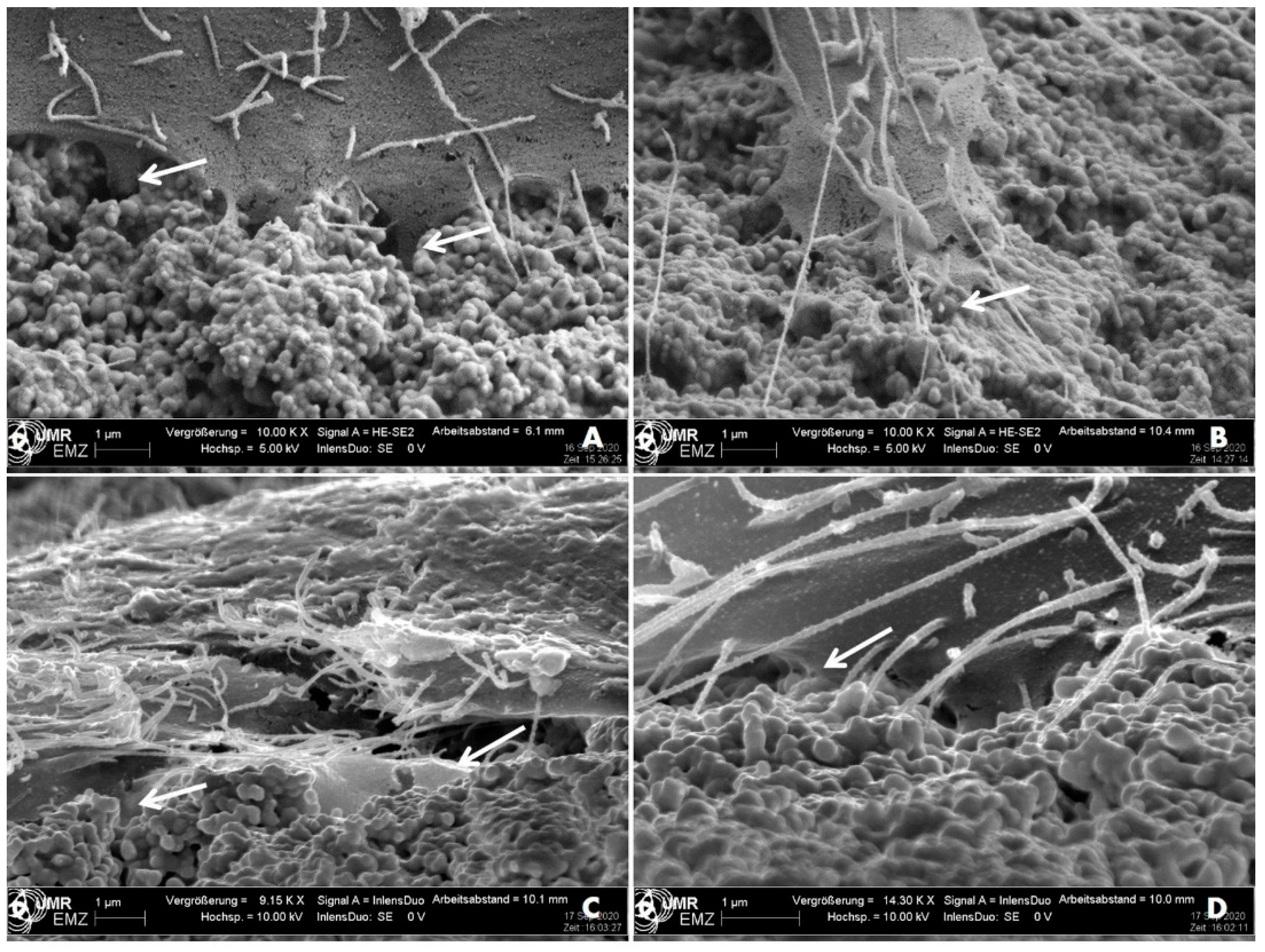
3. Results
3.1. Surface Characteristics
3.2. Cell Morphology
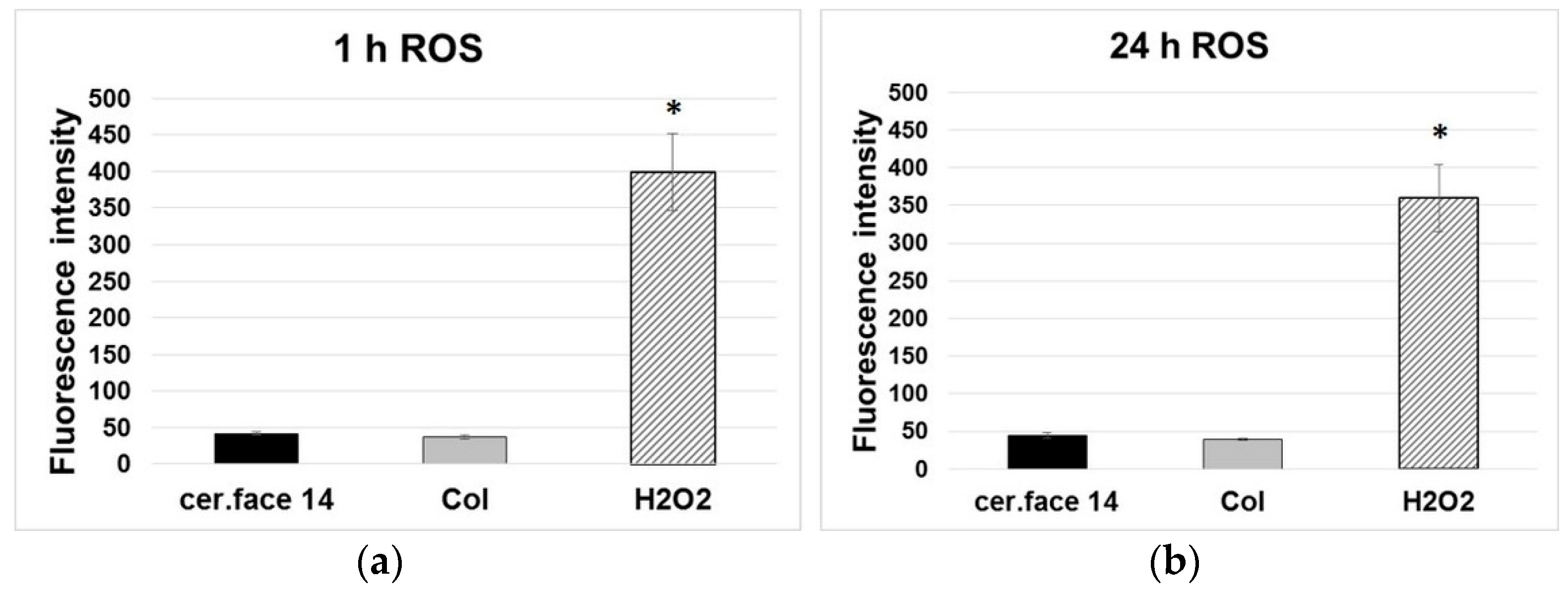
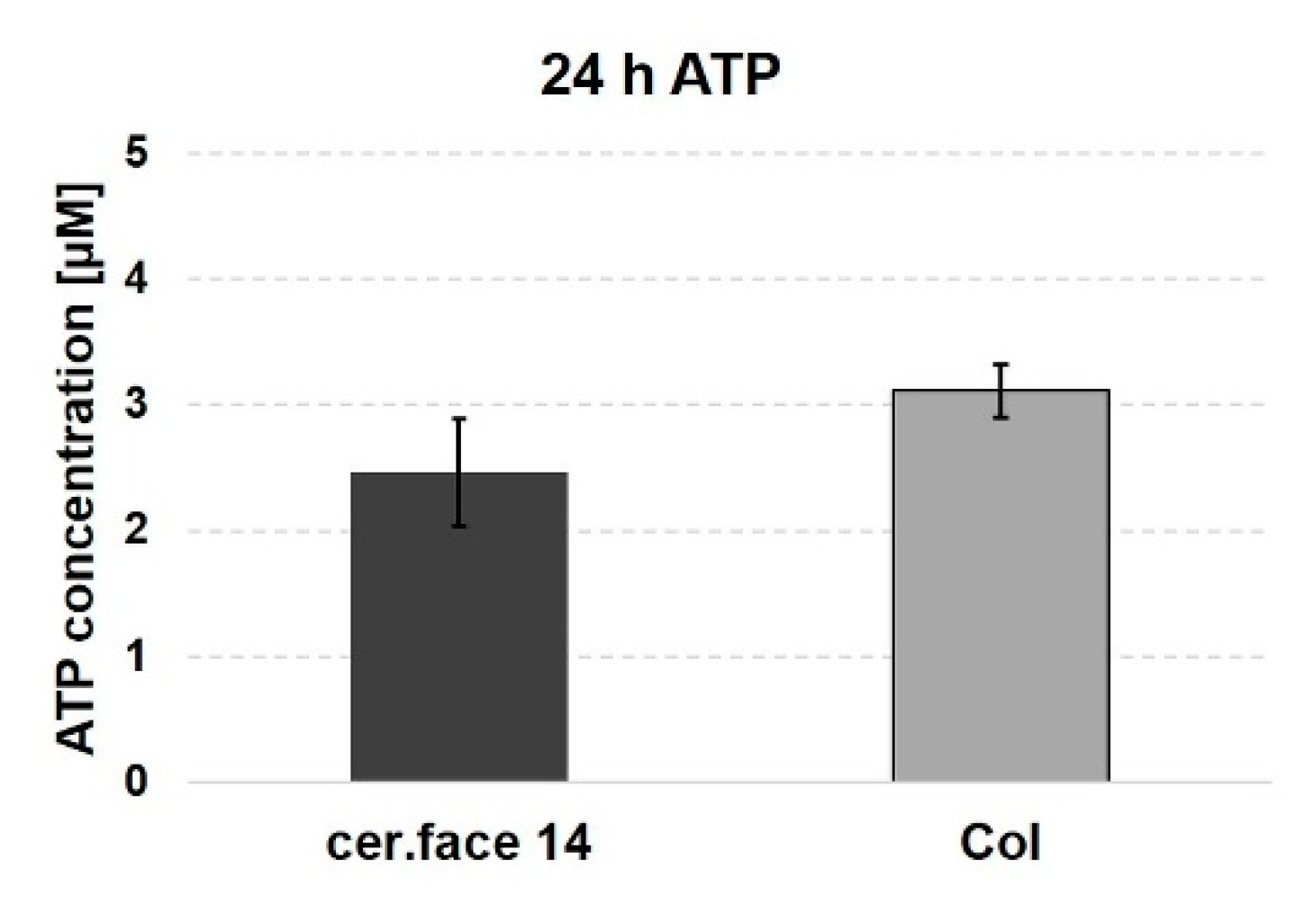
3.3. Stress Level of Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ritzert, B. Zahnimplantate Zunehmend Erste Wahl. Available online: https://idw-online.de/de/news707107 (accessed on 30 November 2018).
- Zhang, D.; Wong, C.S.; Wen, C.; Li, Y. Cellular responses of osteoblast-like cells to 17 elemental metals. J. Biomed. Mater. Res. Part A 2017, 105A, 148–158. [Google Scholar] [CrossRef]
- Fage, S.W.; Muris, J.; Jakobsen, S.S.; Thyssen, J.P. Titanium: A review on exposure, release, penetration, allergy, epidemiology, and clinical reactivity. Contact Dermat. 2016, 74, 323–345. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, F.S.-L.; Garaicoa-Pazmiño, C.; Fretwurst, T.; Castilho, R.M.; Squarize, C.H. Dental implants-associated release of titanium particles: A systematic review. Clin. Oral Implant. Res. 2018, 29, 1085–1100. [Google Scholar] [CrossRef] [PubMed]
- Nedjat, A. Rem Tene, Verba Sequentur. Available online: https://www.dimagazin-aktuell.de/marktplatz/kollegentipps/story/rem-tene-verba-sequentur_7845.html (accessed on 15 July 2019).
- Sadowsky, S.J. Has zirconia made a material difference in implant prosthodontics? A review. Dent. Mater. 2020, 36, 1–8. [Google Scholar] [CrossRef]
- Thoma, D.S.; Ioannidis, A.; Cathomen, E.; Hämmerle, C.H.F.; Husler, J.; Jung, R.E. Discoloration of the Peri-implant Mucosa Caused by Zirconia and Titanium Implants. Int. J. Periodontics Restor. Dent. 2016, 36, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Cosgarea, R.; Gasparik, C.; Dudea, D.; Culic, B.; Dannewitz, B.; Sculean, A. Peri-implant soft tissue colour around titanium and zirconia abutments: A prospective randomized controlled clinical study. Clin. Oral Implant. Res. 2014, 26, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Pieralli, S.; Kohal, R.; Jung, R.; Vach, K.; Spies, B. Clinical Outcomes of Zirconia Dental Implants: A Systematic Review. J. Dent. Res. 2016, 96, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Ritzert, B. Materialien Auf Dem Prüfstand: Keramikimplantate. Available online: https://www.dginet.de/web/dgi/presse-aktuelles (accessed on 1 December 2017).
- Rohr, N.; Bergemann, C.; Nebe, J.B.; Fischer, J. Crystal structure of zirconia affects osteoblast behavior. Dent. Mater. 2020, 36, 905–913. [Google Scholar] [CrossRef]
- Nebe, B. In Vitro Studies and Cell Adhesion to Biomaterials. In Materials for Medical Applications, 1st ed.; Heimann, R., Ed.; Walter de Gruyter GmbH: Berlin, Germany, 2020; Volume 1, pp. 385–396. ISBN 978-3-11-061919-5. [Google Scholar]
- Gruening, M.; Neuber, S.; Nestler, P.; Lehnfeld, J.; Dubs, M.; Fricke, K.; Schnabelrauch, M.; Helm, C.A.; Müller, R.; Staehlke, S.; et al. Enhancement of Intracellular Calcium Ion Mobilization by Moderately but Not Highly Positive Material Surface Charges. Front. Bioeng. Biotechnol. 2020, 8, 8. [Google Scholar] [CrossRef]
- Kirchhof, K.; Hristova, K.; Krasteva, N.; Altankov, G.; Groth, T. Multilayer coatings on biomaterials for control of MG-63 osteoblast adhesion and growth. J. Mater. Sci. Mater. Med. 2008, 20, 897–907. [Google Scholar] [CrossRef]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. Plasma Methods for the Generation of Chemically Reactive Surfaces for Biomolecule Immobilization and Cell Colonization—A Review. Plasma Process. Polym. 2006, 3, 392–418. [Google Scholar] [CrossRef]
- Rebl, H.; Finke, B.; Lange, R.; Weltmann, K.-D.; Nebe, J.B. Impact of plasma chemistry versus titanium surface topography on osteoblast orientation. Acta Biomater. 2012, 8, 3840–3851. [Google Scholar] [CrossRef] [PubMed]
- Zinger, O.; Zhao, G.; Schwartz, Z.; Simpson, J.; Wieland, M.; Landolt, D.; Boyan, B.D. Differential regulation of osteoblasts by substrate microstructural features. Biomaterials 2005, 26, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Pieuchot, L.; Marteau, J.; Guignandon, A.; Dos Santos, T.; Brigaud, I.; Chauvy, P.-F.; Cloatre, T.; Ponche, A.; Petithory, T.; Rougerie, P.; et al. Curvotaxis directs cell migration through cell-scale curvature landscapes. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mörke, C.; Rebl, H.; Finke, B.; Dubs, M.; Nestler, P.; Airoudj, A.; Roucoules, V.; Schnabelrauch, M.; Körtge, A.; Anselme, K.; et al. Abrogated Cell Contact Guidance on Amino-Functionalized Microgrooves. ACS Appl. Mater. Interfaces 2017, 9, 10461–10471. [Google Scholar] [CrossRef]
- Fujita, S.; Ohshima, M.; Iwata, H. Time-lapse observation of cell alignment on nanogrooved patterns. J. R. Soc. Interface 2009, 6, S269–S277. [Google Scholar] [CrossRef]
- Löffler, R.; Fleischer, M.; Kern, D.P.; Matschegewski, C.; Stählke, S.; Nebe, B.; Lange, R. Pyramid array substrates for biomedical studies. J. Vac. Sci. Technol. B 2012, 30, 06F901. [Google Scholar] [CrossRef]
- Matschegewski, C.; Staehlke, S.; Loeffler, R.; Lange, R.; Chai, F.; Kern, D.P.; Beck, U.; Nebe, J.B. Cell architecture–cell function dependencies on titanium arrays with regular geometry. Biomaterials 2010, 31, 5729–5740. [Google Scholar] [CrossRef]
- Staehlke, S.; Haack, F.; Waldner, A.-C.; Koczan, D.; Moerke, C.; Mueller, P.; Uhrmacher, A.M.; Nebe, J.B. ROS Dependent Wnt/β-Catenin Pathway and Its Regulation on Defined Micro-Pillars—A Combined In Vitro and In Silico Study. Cells 2020, 9, 1784. [Google Scholar] [CrossRef]
- Moerke, C.; Mueller, P.; Nebe, B. Attempted caveolae-mediated phagocytosis of surface-fixed micro-pillars by human osteoblasts. Biomaterials 2016, 76, 102–114. [Google Scholar] [CrossRef]
- Staehlke, S.; Koertge, A.; Nebe, B. Intracellular calcium dynamics dependent on defined microtopographical features of titanium. Biomaterials 2015, 46, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, A.J.; Karthigeyan, S.; Bhat, R.T.R.; Nageshwarao, M.N.; Murugesan, S.V.; Angamuthu, V. Surface modification techniques for zirconia-based bioceramics: A review. J. Pharm. Bioallied Sci. 2019, 11 (Suppl. S2), S131–S134. [Google Scholar] [CrossRef] [PubMed]
- Bergemann, C.; Duske, K.; Nebe, J.B.; Schöne, A.; Bulnheim, U.; Seitz, H.; Fischer, J. Microstructured zirconia surfaces modulate osteogenic marker genes in human primary osteoblasts. J. Mater. Sci. Mater. Med. 2015, 26, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.; Karsenty, G.; Gundberg, C.; Vashishth, D. Osteocalcin and osteopontin influence bone morphology and mechanical properties. Ann. N. Y. Acad. Sci. 2017, 1409, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Staehlke, S.; Rebl, H.; Nebe, B. Phenotypic stability of the human MG-63 osteoblastic cell line at different passages. Cell Biol. Int. 2019, 43, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Czekanska, E.M.; Stoddart, M.J.; Ralphs, J.R.; Richards, R.G.; Hayes, J.S. A phenotypic comparison of osteoblast cell lines versus human primary osteoblasts for biomaterials testing. J. Biomed. Mater. Res. Part A 2013, 102, 2636–2643. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. 2019, 18, 1–74. [Google Scholar] [CrossRef]
- Duske, K.; Jablonowski, L.; Koban, I.; Matthes, R.; Holtfreter, B.; Sckell, A.; Nebe, J.B.; Von Woedtke, T.; Weltmann, K.-D.; Kocher, T. Cold atmospheric plasma in combination with mechanical treatment improves osteoblast growth on biofilm covered titanium discs. Biomaterials 2015, 52, 327–334. [Google Scholar] [CrossRef]
- Sen, N.; Isler, S. Microstructural, physical, and optical characterization of high-translucency zirconia ceramics. J. Prosthet. Dent. 2020, 123, 761–768. [Google Scholar] [CrossRef]
- Heimann, R. Types and Properties of Biomaterials. In Materials for Medical Applications, 1st ed.; Heimann, R., Ed.; Walter de Gruyter GmbH: Berlin, Germany, 2020; Volume 1, pp. 132–154. ISBN 978-3-11-061919-5. [Google Scholar]
- Hafezeqoran, A.; Koodaryan, R. Effect of Zirconia Dental Implant Surfaces on Bone Integration: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Lee, J.B.; Shin, Y.M.; Kim, W.S.; Kim, S.Y.; Sung, H.J. ROS-Responsive Biomaterial Design for Medical Applications. In Biomimetic Medical Materials. Advances in Experimental Medicine and Biology; Noh, I., Ed.; Springer: Singapore, 2018; p. 1064. [Google Scholar] [CrossRef]
- Wauquier, F.; Leotoing, L.; Coxam, V.; Guicheux, J.; Wittrant, Y. Oxidative stress in bone remodelling and disease. Trends Mol. Med. 2009, 15, 468–477. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staehlke, S.; Springer, A.; Freitag, T.; Brief, J.; Nebe, J.B. The Anchorage of Bone Cells onto an Yttria-Stabilized Zirconia Surface with Mild Nano-Micro Curved Profiles. Dent. J. 2020, 8, 127. https://doi.org/10.3390/dj8040127
Staehlke S, Springer A, Freitag T, Brief J, Nebe JB. The Anchorage of Bone Cells onto an Yttria-Stabilized Zirconia Surface with Mild Nano-Micro Curved Profiles. Dentistry Journal. 2020; 8(4):127. https://doi.org/10.3390/dj8040127
Chicago/Turabian StyleStaehlke, Susanne, Armin Springer, Thomas Freitag, Jakob Brief, and J. Barbara Nebe. 2020. "The Anchorage of Bone Cells onto an Yttria-Stabilized Zirconia Surface with Mild Nano-Micro Curved Profiles" Dentistry Journal 8, no. 4: 127. https://doi.org/10.3390/dj8040127
APA StyleStaehlke, S., Springer, A., Freitag, T., Brief, J., & Nebe, J. B. (2020). The Anchorage of Bone Cells onto an Yttria-Stabilized Zirconia Surface with Mild Nano-Micro Curved Profiles. Dentistry Journal, 8(4), 127. https://doi.org/10.3390/dj8040127