Current Concepts of Laser–Oral Tissue Interaction
Abstract
1. Introduction
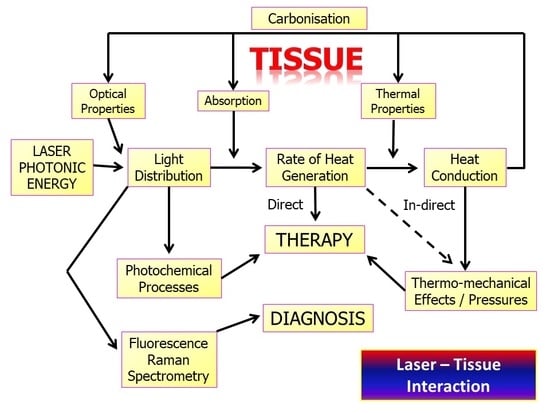
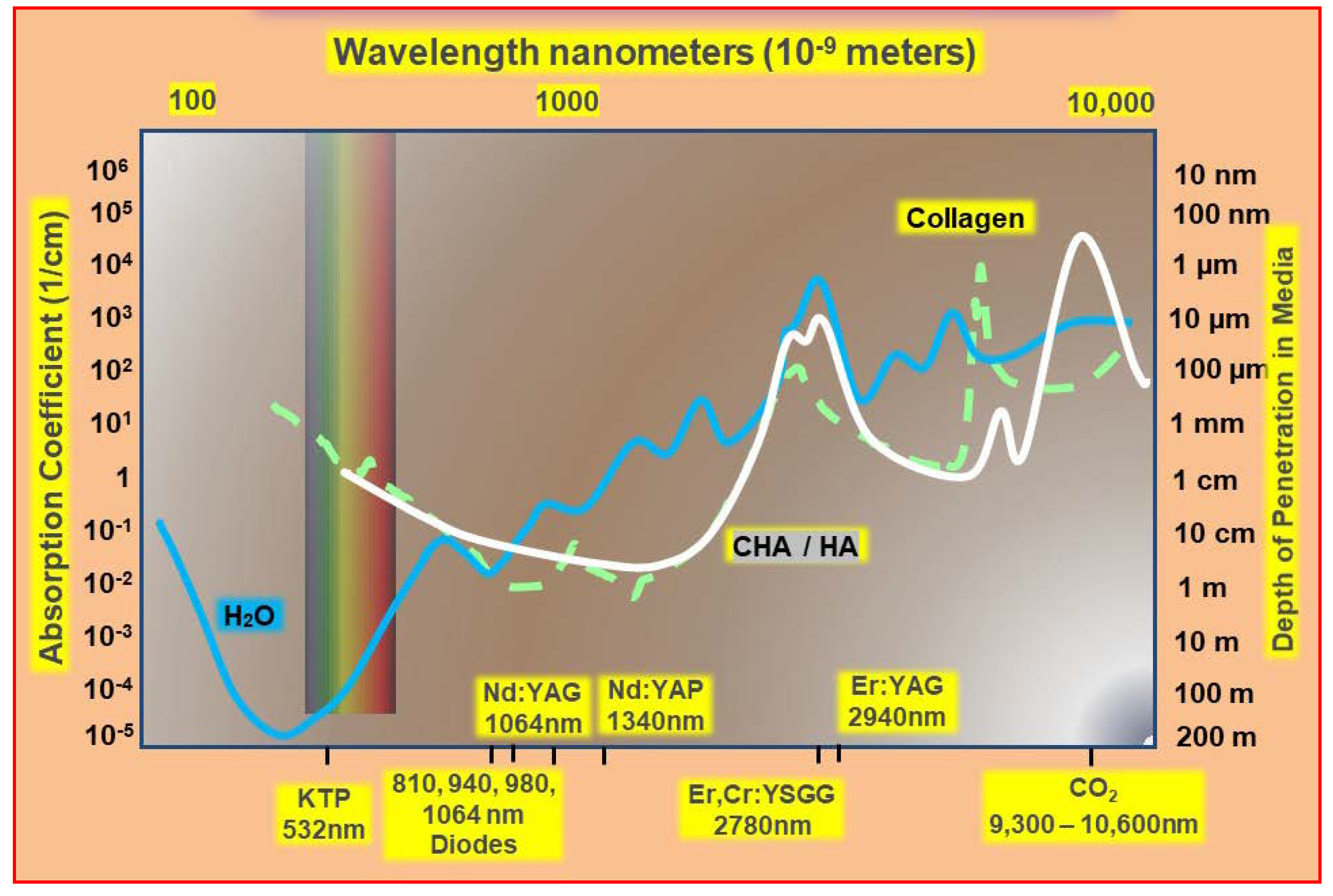
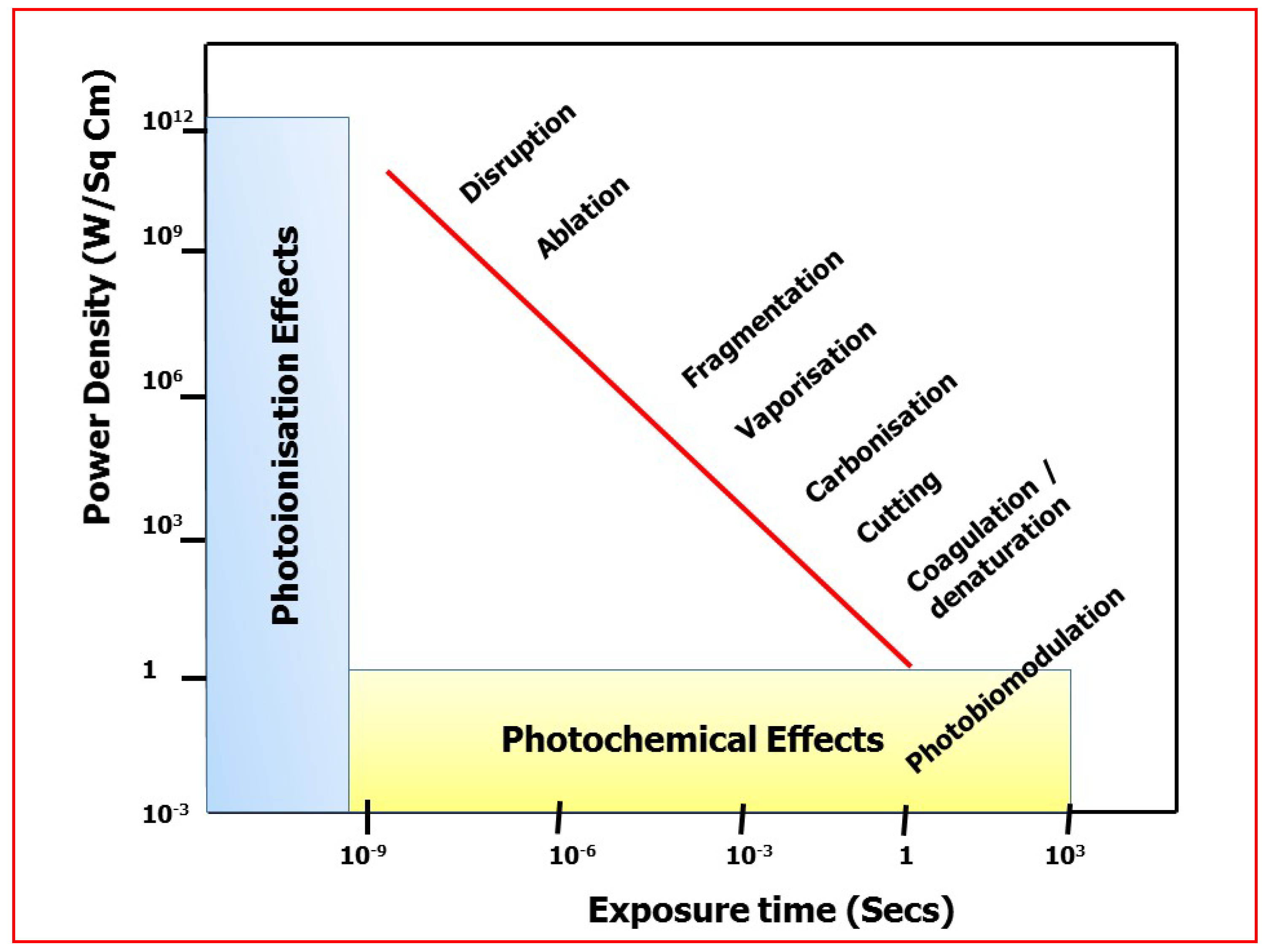
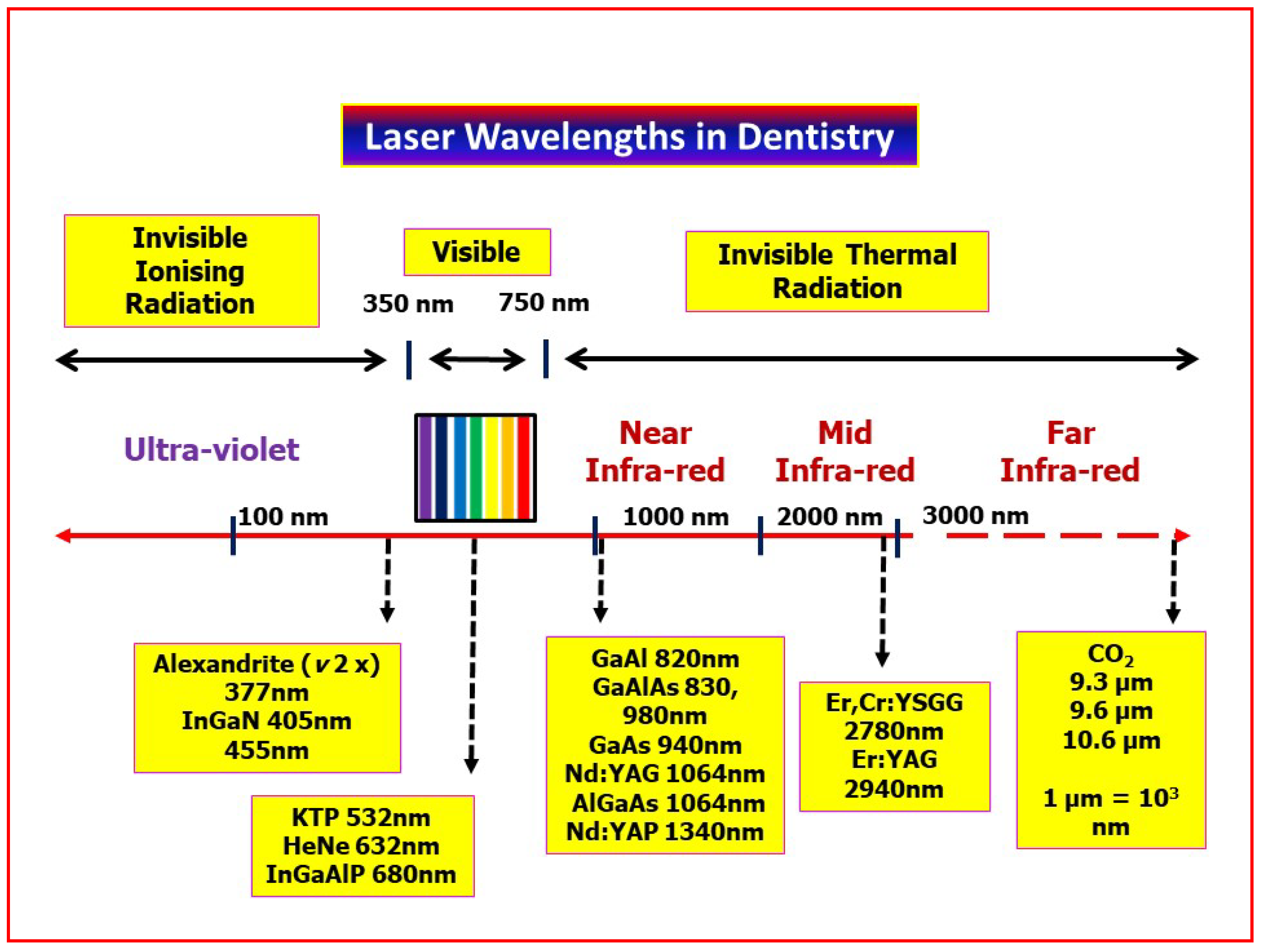
2. Laser Photonic Energy
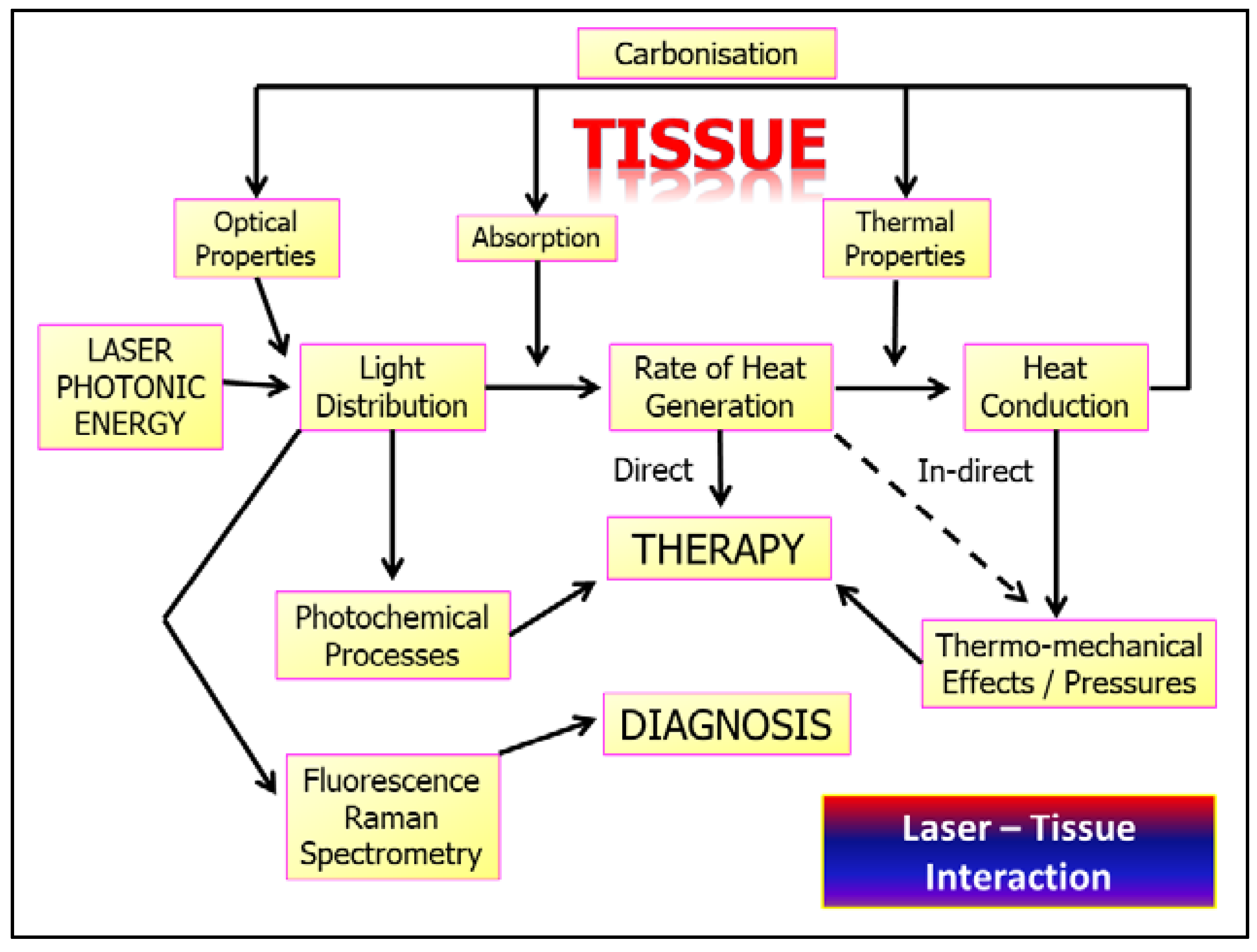
3. Laser–Tissue Interaction: Oral Soft Tissue
3.1. “Uneventful” Healing—Photobiomodulation
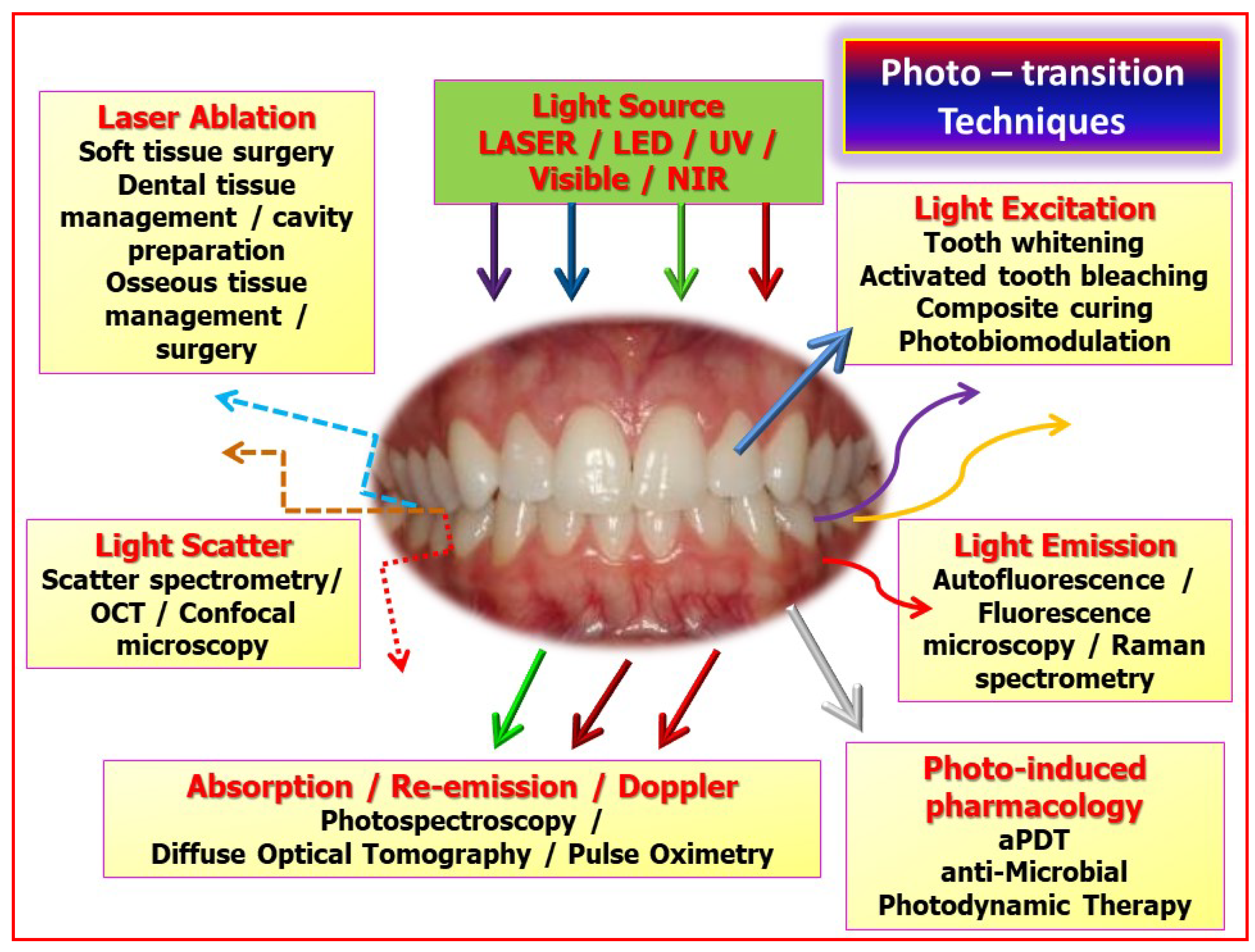
3.2. Laser-Mediated Diagnostics
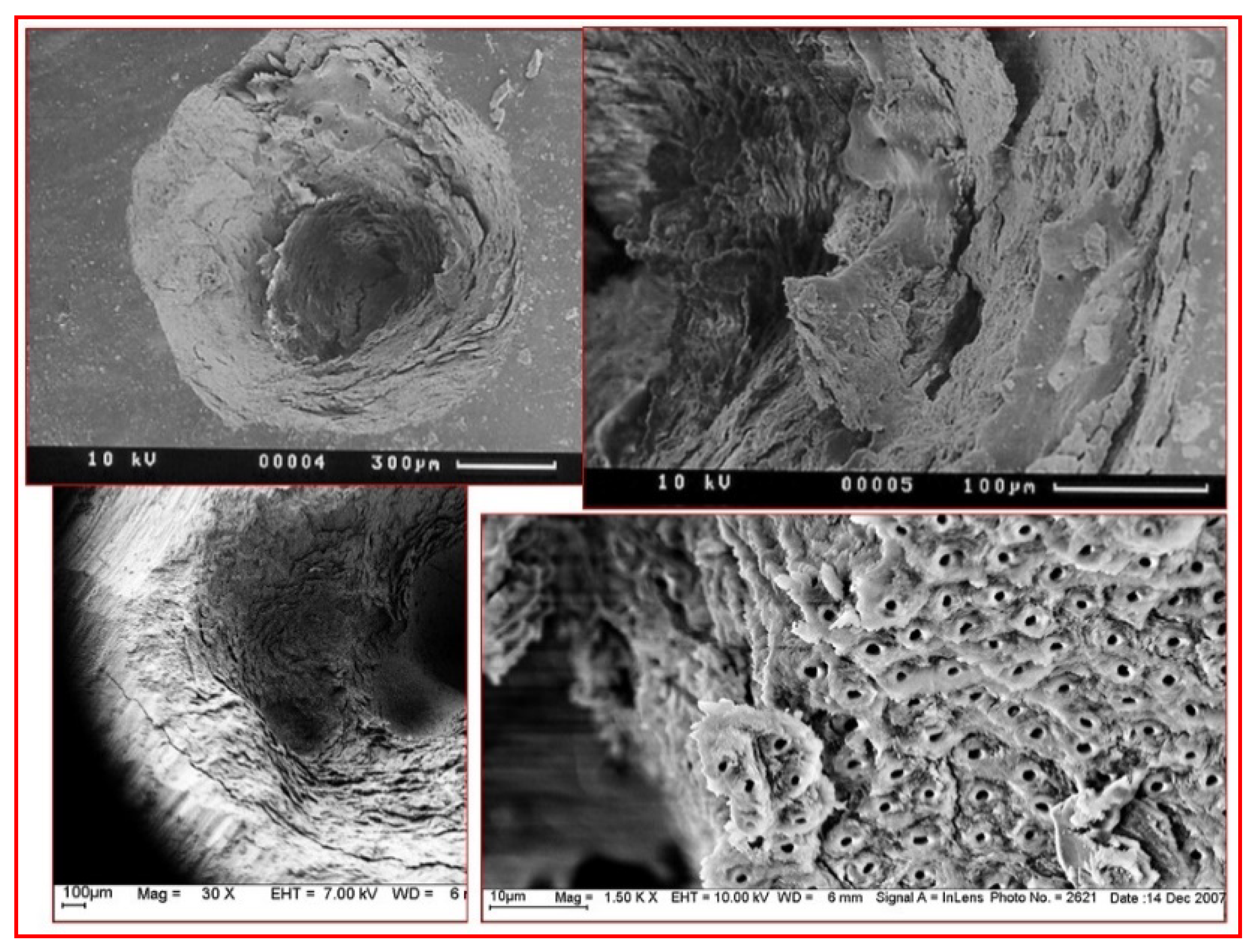
3.3. Laser–Tissue Interactions: Dental and Oral Hard Tissues
4. Operating Parametry of Laser–Tissue Interactions
- Laser wavelength
- Laser emission mode
- Tissue composition
- Tissue thickness
- Surface wetness arising from water or saliva
- Incident angle of the laser beam
- Exposure time.
- Contact versus non-contact modes employed between laser delivery tip and tissue.
- Thermal relaxation factors—Exogenous (water spray, tissue precooling, high-speed suction, pulsing/gating laser emission) and endogenous (tissue type and density, blood supply).
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Parker, S. Lasers in Dentistry-Current Concepts; CH. 3: Laser-Tissue Interaction; Coluzzi, D.J., Ed.; Parker SPA: Cham, Switzerland; Springer Nature: Cham, Switzerland, 2017; pp. 29–53. ISBN 978-3-319-51943-2. [Google Scholar] [CrossRef]
- Parker, S.; Cronshaw, M.; Anagnostaki, E.; Lynch, E. Laser Essentials for the Dental Practitioner: Foundation Knowledge-Construction, Modes of Operation and Safety. EC Dent. Sci. 2019, 18, 2020–2027. [Google Scholar]
- Mó, O.; Yáñez, M.; Eckert-Maksić, M.; Maksić, Z.B.; Alkorta, I.; Elguero, J. Periodic trends in bond dissociation energies. A theoretical study. J. Phys. Chem. A 2005, 109, 4359–4365. [Google Scholar] [CrossRef]
- Fisher, J.C. Photons, physiatrics, and physicians: A practical guide to understanding laser light interaction with living tissue, part I. J. Clin. Laser Med. Surg. 1992, 10, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Boulnois, J.-L. Photophysical processes in recent medical laser developments: A review. Laser Med. Sci. 1986, 1, 47–66. [Google Scholar] [CrossRef]
- Mooney, J.; Kambhampati, P. Get the Basics Right: Jacobian Conversion of Wavelength and Energy Scales for Quantitative Analysis of Emission Spectra. Phys. Chem. Lett. 2013, 4, 3316–3318. [Google Scholar] [CrossRef]
- Sutherland, J.C. Biological effects of polychromatic light. Photochem. Photobiol. 2002, 76, 164–170. [Google Scholar] [CrossRef]
- Knappe, V.; Frank, F.; Rohde, E. Principles of lasers and biophotonic effects. Photomed. Laser Surg. 2004, 22, 411–417. [Google Scholar] [CrossRef]
- Zhang, D.; Tamilselvan, A. Lattice energy and mechanical stiffness of hydroxyapatite. J. Mater. Sci. Mater. Med. 2007, 18, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Parker, S. Laser Tissue Interaction. Brit. Dent. J. 2007, 202, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Karu, T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J. Photochem. Photobiol. B 1999, 49, 1–17. [Google Scholar] [CrossRef]
- Robertson, C.A.; Hawkins Evans, D.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B 2009, 96, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Maisch, T. A new strategy to destroy antibiotic resistant microorganisms: Antimicrobial photodynamic treatment. Mini Rev. Med. Chem. 2009, 9, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Demidova, T.N. Mechanisms of low level light therapy. In Mechanisms for Low-Light Therapy; Hamblin, M.R., Waynant, R.W., Anders, J., Eds.; Proc. SPIE 6140; SPIE-The International Society for Optical Engineering: Bellingham, WA, USA, 2006; p. 614001. [Google Scholar] [CrossRef]
- Crippa, R.; Fruili, S.; Parker, S.; Angiero, F.; Benedicenti, S.; Berne, E. Management of intraoral venous malformations using laser-assisted forced dehydration induced photocoagulation. IL Dent. Mod. 2013, 9, 80–97. [Google Scholar]
- Jain, K.; Gorisch, W. Repair of small blood vessels with the Neodymium-YAG laser: A preliminary report. Surgery 1979, 85, 684–688. [Google Scholar]
- Suragimath, G.; Lohana, M.H.; Varma, S. A Split Mouth Randomised Clinical Comparative Study to Evaluate the Efficacy of Gingival Depigmentation Procedure Using Conventional Scalpel Technique or Diode Laser. J. Lasers Med. Sci. 2016, 7, 227–232. [Google Scholar] [CrossRef]
- Zaffe, D.; Vitale, M.C.; Martignone, A.; Scarpelli, F.; Botticelli, A.R. Morphological, histochemical, and immunocytochemical study of CO2 and Er:YAG laser effect on oral soft tissues. Photomed. Laser Surg. 2004, 22, 185–189. [Google Scholar] [CrossRef]
- Smith, K. The Photobiological Basis of Low Level Laser Radiation Therapy. Laser Ther. 1991, 36, 19–24. [Google Scholar] [CrossRef]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.-Y.; Carroll, J.; Hamblin, M. The nuts and bolts of low level laser therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef]
- Karu, T. Cellular and molecular mechanisms of photobiomodulation (low level laser therapy). IEEE J. Sel. Top. Quantum Electron. 2014, 20, 143–148. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Amaroli, A.; Ravera, S.; Baldini, F.; Benedicenti, S.; Panfoli, I.; Vergani, L. Photobiomodulation with 808-nm diode laser light promotes wound healing of human endothelial cells through increased reactive oxygen species production stimulating mitochondrial oxidative phosphorylation. Lasers Med. Sci. 2019, 34, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, J.; Jang, H.; Park, S.; Na, J.; Myung, J.K.; Kim, M.J.; Jang, W.S.; Lee, S.J.; Kim, H.; et al. Photobiomodulation Enhances the Angiogenic Effect of Mesenchymal Stem Cells to Mitigate Radiation-Induced Enteropathy. Int. J. Mol. Sci. 2019, 20, 1131. [Google Scholar] [CrossRef] [PubMed]
- Heidari, M.; Paknejad, M.; Jamali, R.; Nokhbatolfoghahaei, H.; Fekrazad, R.; Moslemi, N. Effect of laser photobiomodulation on wound healing and postoperative pain following free gingival graft: A split-mouth triple-blind randomized controlled clinical trial. J. Photochem. Photobiol. B 2017, 172, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Arany, P. Craniofacial Wound Healing with Photobiomodulation Therapy: New Insights and Current Challenges. J. Dent. Res. 2016, 95, 977–984. [Google Scholar] [CrossRef]
- Chow, R.T.; Armati, P.J. Photobiomodulation: Implications for Anesthesia and Pain Relief. Photomed. Laser Surg. 2016, 34, 599–609. [Google Scholar] [CrossRef]
- Sugaya, N.; Silva, É.; Kato, I.; Prates, R.; Gallo, C.; Pellegrini, V. Low Intensity laser therapy in patients with burning mouth syndrome: A randomized, placebo-controlled study. Braz. Oral Res. 2016, 30, e108. [Google Scholar] [CrossRef]
- Dias, S.B.F.; Fonseca, M.V.A.; dos Santos, N.C.C.; Mathias, I.F.; Martinho, F.C.; Junior, M.S.; Jardini, M.A.N. Santamaria, M.P. Effect of GaAIAs low-level laser therapy on the healing of human palate mucosa after connective tissue graft harvesting: Randomized clinical trial. Lasers Med. Sci. 2015, 30, 1695–1702. [Google Scholar] [CrossRef]
- Arbabi-Kalati, F.; Arbabi-Kalati, F.; Moridi, T. Evaluation of the effect of low level laser on prevention of chemotherapy-induced mucositis. Acta Med. Iran. 2013, 51, 157–162. [Google Scholar] [PubMed]
- Shirani, A.; Gutknecht, N.; Taghizadeh, M.; Mir, M. Low-level laser therapy and myofacial pain dysfunction syndrome: A randomized controlled clinical trial. Lasers Med. Sci. 2009, 24, 715–720. [Google Scholar] [CrossRef]
- Caccianiga, G.; Paiusco, A.; Perillo, L.; Nucera, R.; Pinsino, A.; Maddalone, M.; Cordasco, G.; Lo Giudice, A. Does Low-Level Laser Therapy Enhance the Efficiency of Orthodontic Dental Alignment? Results from a Randomized Pilot Study. Photomed. Laser Surg. 2017, 35, 421–426. [Google Scholar] [CrossRef]
- Farias, R.; Closs, L.; Miguens, S. Evaluation of the use of low-level laser therapy in pain control in orthodontic patients: A randomized split-mouth clinical trial. Angle Orthod. 2016, 86, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Cronshaw, M.; Parker, S.; Arany, P. Feeling the Heat: Evolutionary and Microbial Basis for the Analgesic Mechanisms of Photobiomodulation Therapy. J. Photobiomodul. Photomed. Laser Surg. 2019, 37, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Vinesh, E.; Jeyapriya, S.M.; Kumar, M.S.; Arunachalam, M. Photobiomodulation and oral wound healing. Indian J. Multidiscip. Dent. 2017, 7, 129–134. [Google Scholar] [CrossRef]
- Hode, L.; Tuner, J. Laser Phototherapy; Prima Books in Sweded AB: Grangesberg, Sweden, 2014; pp. 92–94. ISBN 978-91-976478-5-4. [Google Scholar]
- Kim, A.; Roy, M.; Dadani, F.N.; Wilson, B.C. Topographic mapping of subsurface fluorescent structures in tissue using multiwavelength excitation. J. Biomed. Opt. 2010, 15, 066026. [Google Scholar] [CrossRef] [PubMed]
- Rajan, V.; Varghese, B.; van Leeuwen, T.; Steenbergen, W. Review of methodological developments in laser Doppler flowmetry. Lasers Med. Sci. 2009, 24, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, J.; Nisha, G.; Manju, S.; Philip, E.; Jeemon, P.; Baiju, K.; Beena, V.; Subhash, N. Diffuse reflectance spectroscopy: Diagnostic accuracy of a non-invasive screening technique for early detection of malignant changes in the oral cavity. BMJ Open 2011, 1, e000071. [Google Scholar] [CrossRef] [PubMed]
- Eom, J.; Ahn, J.; Eom, J.; Park, A. Wide field of view optical coherence tomography for structural and functional diagnoses in dentistry. J. Biomed. Opt. 2018, 23, 1–8. [Google Scholar] [CrossRef]
- Torres, C.; Miranda Gomes-Silva, J.; Menezes-Oliveira, M.; Silva Soares, L.; Palma-Dibb, R.; Borsatto, M. FT-Raman spectroscopy, µ-EDXRF spectrometry, and microhardness analysis of the dentin of primary and permanent teeth. Microsc. Res. Tech. 2018, 81, 509–514. [Google Scholar] [CrossRef]
- Driessens, F.; van Dijk, J.; Borggreven, M. Biological calcium phosphates and their role in the physiology of bone and dental tissues I. Composition and solubility of calcium phosphates. Calcif. Tissue Res. 1978, 26, 127–137. [Google Scholar] [CrossRef]
- Poli, R.; Parker, S. Achieving dental analgesia with the erbium chromium yttrium scandium gallium garnet laser (2780nm): A protocol for painless conservative treatment. Photomed. Laser Surg. 2015, 33, 1–8. [Google Scholar] [CrossRef]
- Dederich, D.N. Laser/tissue interaction: What happens to laser light when it strikes tissue? J. Am. Dent. Assoc. 1993, 124, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.; Cronshaw, M.; Anagnostaki, E.; Lynch, E. Parameters for Photobiomodulation Therapy in Clinical Dentistry: A Systematic Review. J. Photobiomodul. Photomed. Laser Surg. 2019, 37, 784–797. [Google Scholar] [CrossRef] [PubMed]




| (eV) | Laser | λ (nm) |
|---|---|---|
| 2.8 2.4 | InGaN KTP | 445 532 |
| 2.0 | He-Ne | 633 |
| 1.6 | Diode | 810 |
| 1.2 | Nd:YAG | 1064 |
| 0.4 | Er:YAG | 2940 |
| 0.1 | CO2 | 10,600 |
| Dissociation Energy of Selected Chemical Bonds | |
|---|---|
| Type of Bond | Dissociation Energy (eV) |
| C = O | 7.1 |
| C = C | 6.4 |
| O-H | 4.8 |
| N-H | 4.1 |
| C-C | 3.6 |
| C-N | 3.0 |
| C-S | 2.7 |
| Fe-OH | 0.35 |
| HA Lattice | 310 |
| Fluorophore | Excitation nm | Fluorescence Peak | Comments |
|---|---|---|---|
| Tryptophan | 275 | 350 | Amino acid |
| Collagen | 335 | 390 | Connective Tissue (CT) |
| Elastin | 360 | 410 | CT |
| Keratin | 370 | 505 | Surface analysis |
| Porphyrins | 405, 630 | 590, 625, 635, 705 | Cell mitochondria/metallo-, copro-, proto-porphyrins |
| Healthy Enamel | 405 | 533 | |
| Caries | 405, 488, 655 | 580–700 | |
| Inorganic composites/GI | 655 | Fluorescence > 700 nm giving rise to false positives | |
| Calculus/plaque | 405, 630 | Fluorescence peaks assoc. with porphyrins giving rise to false positives | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parker, S.; Cronshaw, M.; Anagnostaki, E.; Mylona, V.; Lynch, E.; Grootveld, M. Current Concepts of Laser–Oral Tissue Interaction. Dent. J. 2020, 8, 61. https://doi.org/10.3390/dj8030061
Parker S, Cronshaw M, Anagnostaki E, Mylona V, Lynch E, Grootveld M. Current Concepts of Laser–Oral Tissue Interaction. Dentistry Journal. 2020; 8(3):61. https://doi.org/10.3390/dj8030061
Chicago/Turabian StyleParker, Steven, Mark Cronshaw, Eugenia Anagnostaki, Valina Mylona, Edward Lynch, and Martin Grootveld. 2020. "Current Concepts of Laser–Oral Tissue Interaction" Dentistry Journal 8, no. 3: 61. https://doi.org/10.3390/dj8030061
APA StyleParker, S., Cronshaw, M., Anagnostaki, E., Mylona, V., Lynch, E., & Grootveld, M. (2020). Current Concepts of Laser–Oral Tissue Interaction. Dentistry Journal, 8(3), 61. https://doi.org/10.3390/dj8030061