Photoinhibition of Streptococcus mutans Biofilm-Induced Lesions in Human Dentin by Violet-Blue Light
Abstract
1. Introduction
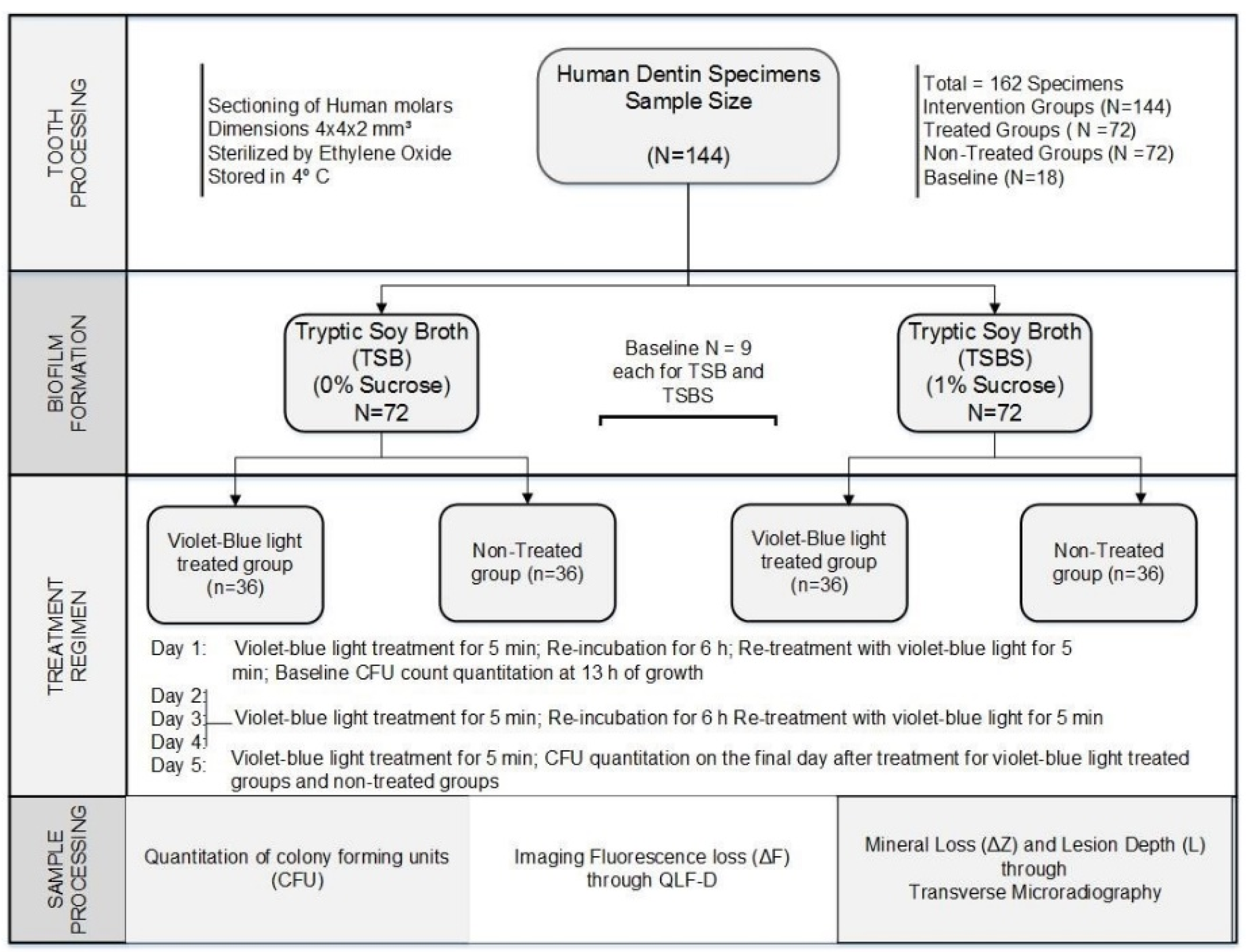
2. Materials and Methods
2.1. Study Design
2.2. Bacterial Strain and Culture Conditions
2.3. Light Source
2.4. Selection of Tooth Specimens
2.5. Quantification of Biofilm Formation
2.6. Quantitative Light-Induced Fluorescence
2.7. Transverse Microradiography
2.8. Statistical Methods
3. Results
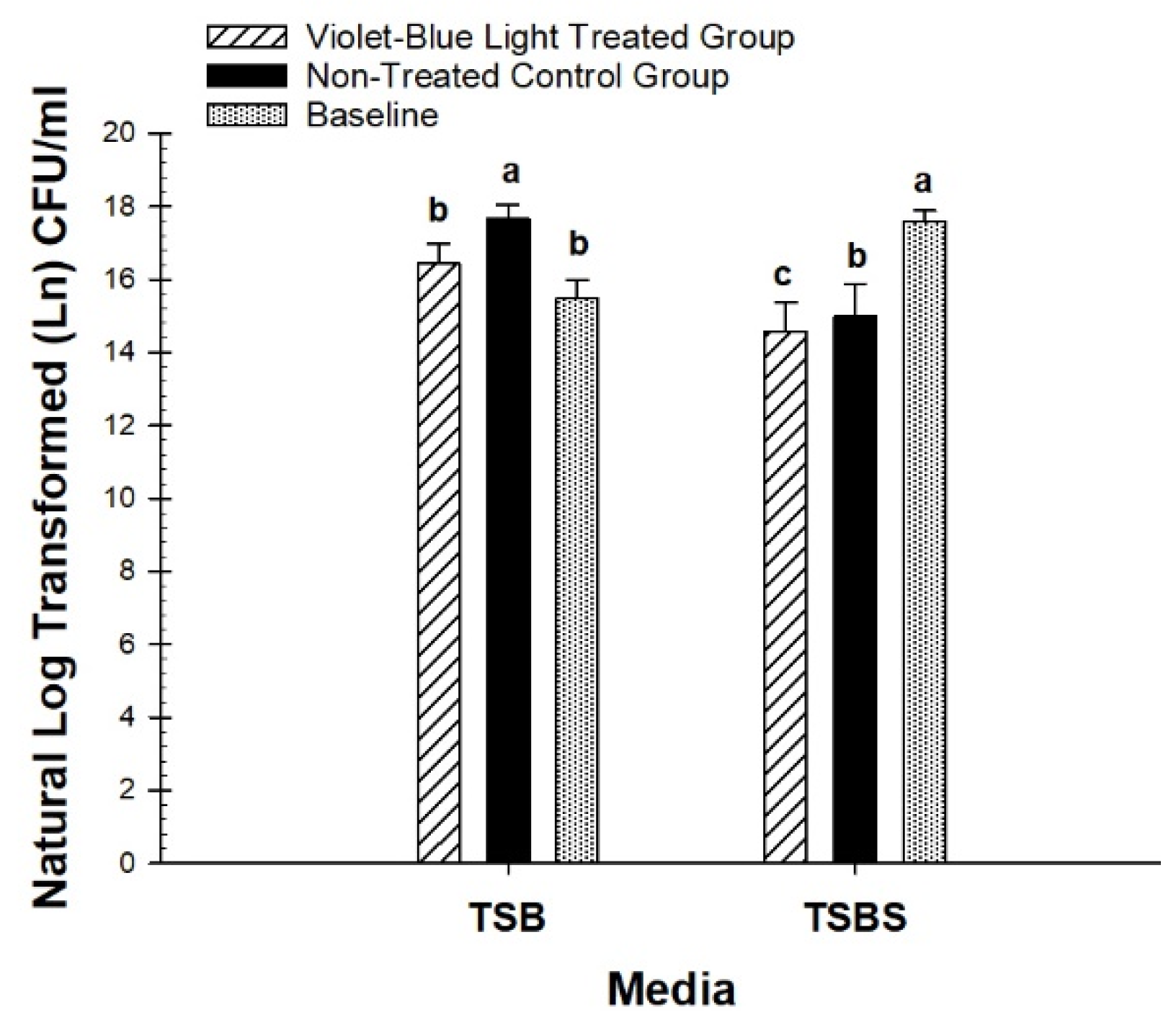
3.1. Photo Inhibitory Effect on Colony Forming Units
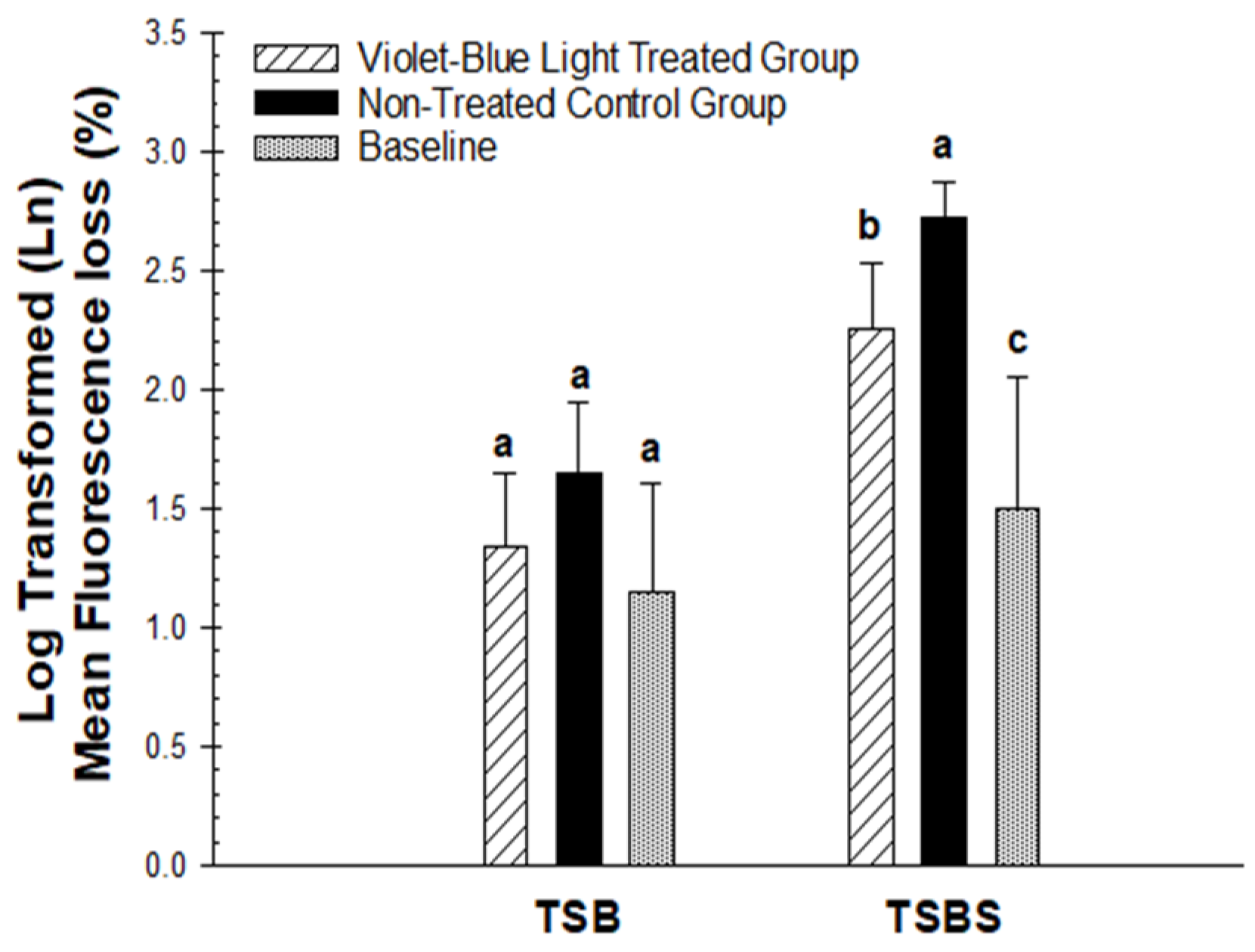
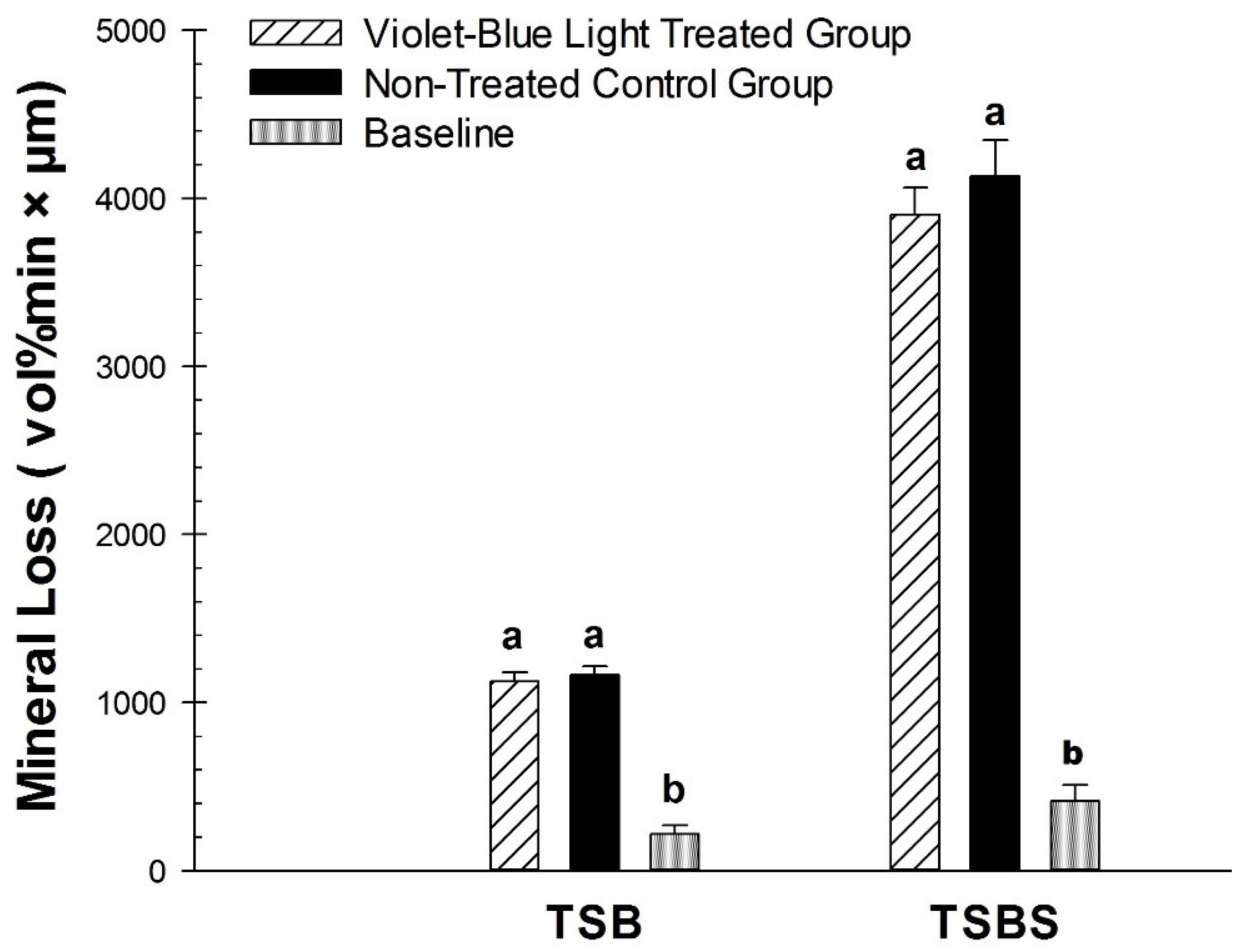
3.2. Photo Inhibitory Effect on Lesion Fluorescence Loss (∆F), Depth (L), and Integrated Mineral Loss (∆Z)
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fejerskov, O. Changing paradigms in concepts on dental caries: consequences for oral health care. Caries Res. 2004, 38, 182–191. [Google Scholar] [CrossRef]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Edelstein, B.L. The dental caries pandemic and disparities problem. BMC Oral Health 2006, 6, S2. [Google Scholar] [CrossRef]
- ten Cate, J.M.; Zaura, E. The numerous microbial species in oral biofilms: how could antibacterial therapy be effective? Adv. Dent. Res. 2012, 24, 108–111. [Google Scholar] [CrossRef]
- Soukos, N.S.; Goodson, J.M. Photodynamic therapy in the control of oral biofilms. Periodontology 2000 2011, 55, 143–166. [Google Scholar] [CrossRef]
- Paulino, T.P.; Ribeiro, K.F.; Thedei, G., Jr.; Tedesco, A.C.; Ciancaglini, P. Use of hand held photopolymerizer to photoinactivate Streptococcus mutans. Arch. Oral Biol. 2005, 50, 353–359. [Google Scholar] [CrossRef]
- Pereira, C.A.; Costa, A.C.; Carreira, C.M.; Junqueira, J.C.; Jorge, A.O. Photodynamic inactivation of Streptococcus mutans and Streptococcus sanguinis biofilms in vitro. Lasers Med. Sci. 2013, 28, 859–864. [Google Scholar] [CrossRef]
- Misba, L.; Khan, A.U. Enhanced photodynamic therapy using light fractionation against Streptococcus mutans biofilm: type I and type II mechanism. Future Microbiol. 2018, 13, 437–454. [Google Scholar] [CrossRef]
- de Freitas, M.T.M.; Soares, T.T.; Aragao, M.G.B.; Lima, R.A.; Duarte, S.; Zanin, I.C.J. Effect of Photodynamic Antimicrobial Chemotherapy on Mono- and Multi-Species Cariogenic Biofilms: A Literature Review. Photomed. Laser Surg. 2017, 35, 239–245. [Google Scholar] [CrossRef]
- Fontana, C.R.; Song, X.; Polymeri, A.; Goodson, J.M.; Wang, X.; Soukos, N.S. The effect of blue light on periodontal biofilm growth in vitro. Lasers Med. Sci. 2015, 30, 2077–2086. [Google Scholar] [CrossRef]
- Chebath-Taub, D.; Steinberg, D.; Featherstone, J.D.; Feuerstein, O. Influence of blue light on Streptococcus mutans re-organization in biofilm. J. Photochem. Photobiol. B 2012, 116, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, O.; Moreinos, D.; Steinberg, D. Synergic antibacterial effect between visible light and hydrogen peroxide on Streptococcus mutans. J. Antimicrob. Chemother. 2006, 57, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.; Moreinos, D.; Featherstone, J.; Shemesh, M.; Feuerstein, O. Genetic and physiological effects of noncoherent visible light combined with hydrogen peroxide on Streptococcus mutans in biofilm. Antimicrob. Agents Chemother. 2008, 52, 2626–2631. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, D.L.; Lima, R.A.; Zanin, I.C.; Klein, M.I.; Janal, M.N.; Duarte, S. Effect of Twice-Daily Blue Light Treatment on Matrix-Rich Biofilm Development. PLoS ONE 2015, 10, e0131941. [Google Scholar] [CrossRef] [PubMed]
- Gomez, G.F.; Huang, R.; MacPherson, M.; Ferreira Zandona, A.G.; Gregory, R.L. Photo Inactivation of Streptococcus mutans Biofilm by Violet-Blue light. Curr. Microbiol. 2016, 73, 426–433. [Google Scholar] [CrossRef]
- Gomez, G.F.; Huang, R.; Eckert, G.; Gregory, R.L. Effect of phototherapy on the metabolism of Streptococcus mutans biofilm based on a colorimetric tetrazolium assay. J. Oral Sci. 2018, 60, 242–246. [Google Scholar] [CrossRef]
- Felix Gomez, G.G.; Lippert, F.; Ando, M.; Zandona, A.F.; Eckert, G.J.; Gregory, R.L. Effect of Violet-Blue Light on Streptococcus mutans-Induced Enamel Demineralization. Dent. J. 2018, 6, 6. [Google Scholar] [CrossRef]
- Switalski, L.M.; Butcher, W.G.; Caufield, P.C.; Lantz, M.S. Collagen mediates adhesion of Streptococcus mutans to human dentin. Infect. Immun. 1993, 61, 4119–4125. [Google Scholar]
- Araujo, P.V.; Correia-Silva Jde, F.; Gomez, R.S.; Massara Mde, L.; Cortes, M.E.; Poletto, L.T. Antimicrobial effect of photodynamic therapy in carious lesions in vivo, using culture and real-time PCR methods. Photodiagnosis Photodyn. Ther. 2015, 12, 401–407. [Google Scholar] [CrossRef]
- Pinheiro, S.L.; Segatti, B.; Pucca, D.S.; Dutra, P.T. Dental acid etchant as a sensitizing agent in photodynamic therapy to reduce S. mutans in dentinal carious lesions. Lasers Med. Sci. 2019, 34, 305–309. [Google Scholar] [CrossRef]
- Steiner-Oliveira, C.; Longo, P.L.; Aranha, A.C.; Ramalho, K.M.; Mayer, M.P.; de Paula Eduardo, C. Randomized in vivo evaluation of photodynamic antimicrobial chemotherapy on deciduous carious dentin. J. Biomed. Opt. 2015, 20, 108003. [Google Scholar] [CrossRef]
- Lima, J.P.; Sampaio de Melo, M.A.; Borges, F.M.; Teixeira, A.H.; Steiner-Oliveira, C.; Nobre Dos Santos, M.; Rodrigues, L.K.; Zanin, I.C. Evaluation of the antimicrobial effect of photodynamic antimicrobial therapy in an in situ model of dentine caries. Eur. J. Oral Sci. 2009, 117, 568–574. [Google Scholar] [CrossRef]
- Melo, M.A.; Rolim, J.P.; Passos, V.F.; Lima, R.A.; Zanin, I.C.; Codes, B.M.; Rocha, S.S.; Rodrigues, L.K. Photodynamic antimicrobial chemotherapy and ultraconservative caries removal linked for management of deep caries lesions. Photodiagnosis Photodyn. Ther. 2015, 12, 581–586. [Google Scholar] [CrossRef]
- Huang, R.; Li, M.; Gregory, R.L. Effect of nicotine on growth and metabolism of Streptococcus mutans. Eur. J. Oral Sci. 2012, 120, 319–325. [Google Scholar] [CrossRef]
- Gursoy, H.; Ozcakir-Tomruk, C.; Tanalp, J.; Yilmaz, S. Photodynamic therapy in dentistry: A literature review. Clin. Oral Investig. 2013, 17, 1113–1125. [Google Scholar] [CrossRef]
- Dai, T.; Gupta, A.; Murray, C.K.; Vrahas, M.S.; Tegos, G.P.; Hamblin, M.R. Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist. Updat. 2012, 15, 223–236. [Google Scholar] [CrossRef]
- Tavares, A.; Carvalho, C.M.; Faustino, M.A.; Neves, M.G.; Tome, J.P.; Tome, A.C.; Cavaleiro, J.A.; Cunha, A.; Gomes, N.C.; Alves, E.; et al. Antimicrobial photodynamic therapy: study of bacterial recovery viability and potential development of resistance after treatment. Mar. Drugs 2010, 8, 91–105. [Google Scholar] [CrossRef]
- de Josselin de Jong, E.; Sundstrom, F.; Westerling, H.; Tranaeus, S.; ten Bosch, J.J.; Angmar-Mansson, B. A new method for in vivo quantification of changes in initial enamel caries with laser fluorescence. Caries Res. 1995, 29, 2–7. [Google Scholar] [CrossRef]
- al-Khateeb, S.; ten Cate, J.M.; Angmar-Mansson, B.; de Josselin de Jong, E.; Sundstrom, G.; Exterkate, R.A.; Oliveby, A. Quantification of formation and remineralization of artificial enamel lesions with a new portable fluorescence device. Adv. Dent. Res. 1997, 11, 502–506. [Google Scholar] [CrossRef]
- Heinrich-Weltzien, R.; Kuhnisch, J.; van der Veen, M.; de Josselin de Jong, E.; Stosser, L. Quantitative light-induced fluorescence (QLF)—A potential method for the dental practitioner. Quintessence Int. 2003, 34, 181–188. [Google Scholar]
- Lippert, F.; Juthani, K. Fluoride dose-response of human and bovine enamel artificial caries lesions under pH-cycling conditions. Clin. Oral Investig. 2015, 19, 1947–1954. [Google Scholar] [CrossRef]
- Fontana, M.; Buller, T.L.; Dunipace, A.J.; Stookey, G.K.; Gregory, R.L. An In vitro microbial-caries model used to study the efficacy of antibodies to Streptococcus mutans surface proteins in preventing dental caries. Clin. Diagn. Lab. Immunol. 2000, 7, 49–54. [Google Scholar] [CrossRef][Green Version]
- Li, M.; Huang, R.; Zhou, X.; Qiu, W.; Xu, X.; Gregory, R.L. Effect of nicotine on cariogenic virulence of Streptococcus mutans. Folia Microbiol. Praha 2016, 61, 505–512. [Google Scholar] [CrossRef]
- Waller, E.; van Daelen, C.J.; van der Veen, M.H. Application of QLFTM for Diagnosis and Quality Assessment in Clinical Practice; Inspektor Research Systems: Amsterdam, The Netherlands, 2012. [Google Scholar]
- van der Veen, M.; de Josselin de jong, E. Application of quantitative light-induced fluorescence for assessing early caries lesions. Monogr. Oral Sci. 2000, 17, 144–162. [Google Scholar]
- Miller, J.H.; Aviles-Reyes, A.; Scott-Anne, K.; Gregoire, S.; Watson, G.E.; Sampson, E.; Progulske-Fox, A.; Koo, H.; Bowen, W.H.; et al. The collagen binding protein Cnm contributes to oral colonization and cariogenicity of Streptococcus mutans OMZ175. Infect. Immun. 2015, 83, 2001–2010. [Google Scholar] [CrossRef]
- Paes Leme, A.F.; Koo, H.; Bellato, C.M.; Bedi, G.; Cury, J.A. The role of sucrose in cariogenic dental biofilm formation--new insight. J. Dent. Res. 2006, 85, 878–887. [Google Scholar] [CrossRef]
- Xiao, J.; Koo, H. Structural organization and dynamics of exopolysaccharide matrix and microcolonies formation by Streptococcus mutans in biofilms. J. Appl. Microbiol. 2010, 108, 2103–2113. [Google Scholar]
- Cohen-Berneron, J.; Steinberg, D.; Featherstone, J.D.; Feuerstein, O. Sustained effects of blue light on Streptococcus mutans in regrown biofilm. Lasers Med. Sci. 2016, 31, 445–452. [Google Scholar] [CrossRef]
- Monod, J. The growth of bacterial cultures. Annu. Rev. Microbiol. 1949, 3, 371–394. [Google Scholar] [CrossRef]
- Ferreira Zandona, A.; Ando, M.; Gomez, G.F.; Garcia-Corretjer, M.; Eckert, G.J.; Santiago, E.; Katz, B.P.; Zero, D.T. Longitudinal analyses of early lesions by fluorescence: An observational study. J. Dent. Res. 2013, 92, 84S–89S. [Google Scholar] [CrossRef]
- Buchalla, W. Comparative fluorescence spectroscopy shows differences in noncavitated enamel lesions. Caries Res. 2005, 39, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Konig, K.; Flemming, G.; Hibst, R. Laser-induced autofluorescence spectroscopy of dental caries. Cell. Mol. Biol. Noisy-le-grand 1998, 44, 1293–1300. [Google Scholar] [PubMed]
- Fyrestam, J.; Bjurshammar, N.; Paulsson, E.; Johannsen, A.; Ostman, C. Determination of porphyrins in oral bacteria by liquid chromatography electrospray ionization tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 7013–7023. [Google Scholar] [CrossRef] [PubMed]
- Konopka, K.; Goslinski, T. Photodynamic therapy in dentistry. J. Dent. Res. 2007, 86, 694–707. [Google Scholar] [CrossRef]
- van der Veen, M.H.; ten Bosch, J.J. The influence of mineral loss on the auto-fluorescent behaviour of in vitro demineralised dentine. Caries Res. 1996, 30, 93–99. [Google Scholar] [CrossRef]
- van der Veen, M.H.; ten Bosch, J.J. Autofluorescence of bulk sound and in vitro demineralized human root dentin. Eur J. Oral Sci 1995, 103, 375–381. [Google Scholar] [CrossRef]
- Kuhnisch, J.; Ifland, S.; Tranaeus, S.; Angmar-Mansson, B.; Hickel, R.; Stosser, L.; Heinrich-Weltzien, R. Establishing quantitative light-induced fluorescence cut-offs for the detection of occlusal dentine lesions. Eur. J. Oral Sci. 2006, 114, 483–488. [Google Scholar] [CrossRef]
- Diniz, M.B.; Campos, P.H.; Wilde, S.; Cordeiro, R.C.L.; Zandona, A.G.F. Performance of light-emitting diode device in detecting occlusal caries in the primary molars. Lasers Med. Sci. 2019, 34, 1235–1241. [Google Scholar] [CrossRef]
- Ko, H.Y.; Kang, S.M.; Kim, H.E.; Kwon, H.K.; Kim, B.I. Validation of quantitative light-induced fluorescence-digital (QLF-D) for the detection of approximal caries in vitro. J. Dent. 2015, 43, 568–575. [Google Scholar] [CrossRef]
- Lenzi, T.L.; Piovesan, C.; Mendes, F.M.; Braga, M.M.; Raggio, D.P. In vitro performance of QLF system and conventional methods for detection of occlusal caries around tooth-colored restorations in primary molars. Int. J. Paediatr. Dent. 2016, 26, 26–34. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felix Gomez, G.G.; Lippert, F.; Ando, M.; Zandona, A.F.; Eckert, G.J.; Gregory, R.L. Photoinhibition of Streptococcus mutans Biofilm-Induced Lesions in Human Dentin by Violet-Blue Light. Dent. J. 2019, 7, 113. https://doi.org/10.3390/dj7040113
Felix Gomez GG, Lippert F, Ando M, Zandona AF, Eckert GJ, Gregory RL. Photoinhibition of Streptococcus mutans Biofilm-Induced Lesions in Human Dentin by Violet-Blue Light. Dentistry Journal. 2019; 7(4):113. https://doi.org/10.3390/dj7040113
Chicago/Turabian StyleFelix Gomez, Grace Gomez, Frank Lippert, Masatoshi Ando, Andrea F. Zandona, George J. Eckert, and Richard L. Gregory. 2019. "Photoinhibition of Streptococcus mutans Biofilm-Induced Lesions in Human Dentin by Violet-Blue Light" Dentistry Journal 7, no. 4: 113. https://doi.org/10.3390/dj7040113
APA StyleFelix Gomez, G. G., Lippert, F., Ando, M., Zandona, A. F., Eckert, G. J., & Gregory, R. L. (2019). Photoinhibition of Streptococcus mutans Biofilm-Induced Lesions in Human Dentin by Violet-Blue Light. Dentistry Journal, 7(4), 113. https://doi.org/10.3390/dj7040113





