Oral and Dental Abnormalities Caused by a Pediatric Rhabdomyosarcoma Tumor Treatment: A Clinical Case Report
Abstract
1. Introduction
2. Case Presentation
2.1. Medical History
2.2. Physical Examination
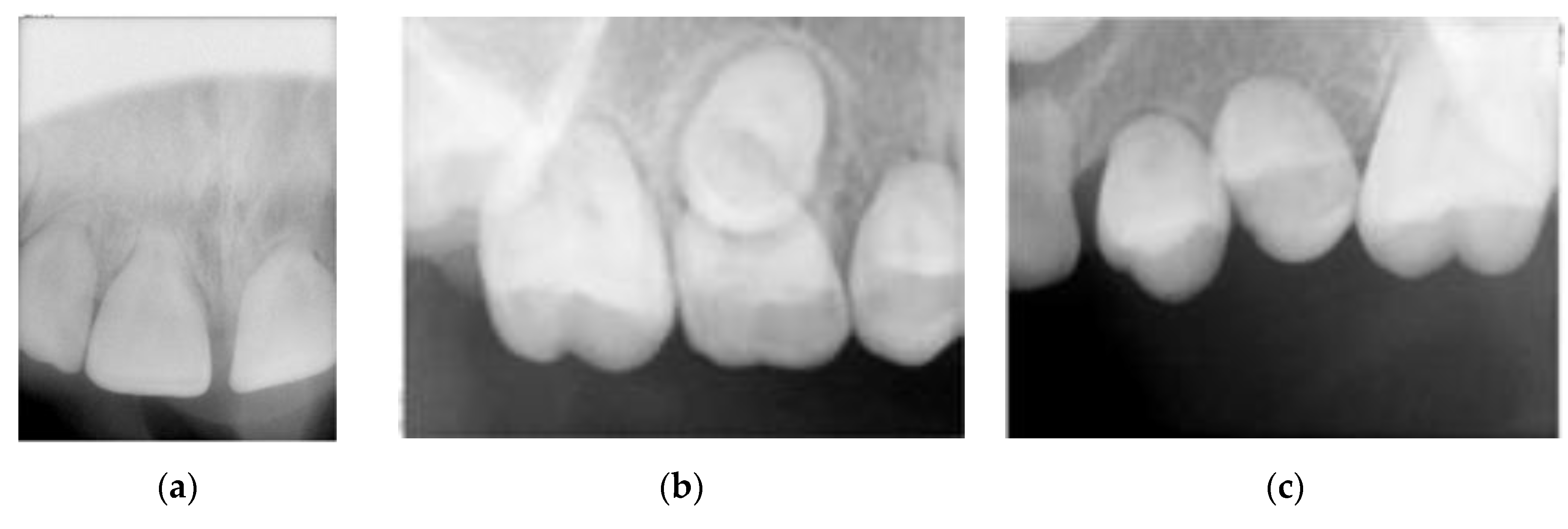
2.3. Intra-Oral Examination
3. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Chen, S.Y.; Thakur, A.; Miller, A.S.; Harwick, R.D. Rhabdomyosarcoma of the oral cavity. Report of four cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1995, 80. [Google Scholar] [CrossRef]
- Hawkins, D.S.; Gupta, A.A.; Rudzinski, E.R. What is new in the biology and treatment of pediatric rhabdomyosarcoma? Curr. Opin. Pediatr. 2014, 26, 50–56. [Google Scholar] [CrossRef]
- Jung, J.-H.; Lee, J.E.; Shin, J.H.; Lim, Y.T.; Choi, H.-Y. Lower eyelid rhabdomyosarcoma in a 3-month-old infant. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2010, 14, 285–287. [Google Scholar] [CrossRef]
- Keller, C.; Guttridge, D.C. Mechanisms of impaired differentiation in rhabdomyosarcoma. FEBS J. 2013, 280, 4323–4334. [Google Scholar] [CrossRef]
- Parham, D.M.; Barr, F.G. Classification of rhabdomyosarcoma and its molecular basis. Adv. Anat. Pathol. 2013, 20, 387–397. [Google Scholar] [CrossRef]
- Sidhom, I.; el Nadi, E.; Taha, H.; Elkinaai, N.; Zaghloul, M.S.; Younes, A.; Labib, R.; Sabry, M.; Bakry, M.S. Clinical significance of anaplasia in childhood rhabdomyosarcoma. J. Egypt. Natl. Cancer Inst. 2015, 27, 83–89. [Google Scholar] [CrossRef][Green Version]
- Zhao, Z.; Yin, Y.; Zhang, J.; Qi, J.; Zhang, D.; Ma, Y.; Wang, Y.; Li, S.; Zhou, J. Spindle cell/sclerosing rhabdomyosarcoma: Case series from a single institution emphasizing morphology, immunohistochemistry and follow-up. Int. J. Clin. Exp. Pathol. 2015, 8, 13814–13820. [Google Scholar] [PubMed]
- Zhu, B.; Davie, J. New insights into signalling-pathway alterations in rhabdomyosarcoma. Br. J. Cancer 2014, 112, 227–231. [Google Scholar] [CrossRef]
- Nangalia, R.; Shah, N.; Sheikh, M.A.; Pal, M. Rhabdomyosarcoma involving maxilla mimicking gingival enlargement: A diagnostic challenge. BMJ Case Rep. 2019, 12, e230692. [Google Scholar] [CrossRef]
- Dury, D.C.; Roberts, M.W.; Miser, J.S.; Folio, J. Dental root agenesis secondary to irradiation therapy in a case of rhabdomyosarcoma of the middle ear. Oral Surg. Oral Med. Oral Pathol. 1984, 57, 595–599. [Google Scholar] [CrossRef]
- Gosiengfiao, Y.; Reichek, J.; Walterhouse, D. What is new in rhabdomyosarcoma management in children? Pediatr. Drugs 2012, 14, 389–400. [Google Scholar]
- Owosho, A.A.; Huang, S.-C.; Chen, S.; Kashikar, S.; Estilo, C.L.; Wolden, S.L.; Wexler, L.H.; Huryn, J.M.; Antonescu, C.R. A clinicopathologic study of head and neck rhabdomyosarcomas showing FOXO1 fusion-positive alveolar and MYOD1-mutant sclerosing are associated with unfavorable outcome. Oral Oncol. 2016, 61, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Rudzinski, E.R. Histology and Fusion Status in Rhabdomyosarcoma. Am. Soc. Clin Oncol. 2013, 33, 425–428. [Google Scholar] [CrossRef]
- Rudzinski, E.R.; Anderson, J.R.; Hawkins, U.S.; Skapek, S.X.; Parham, D.M.; Teot, L.A. The World Health Organization Classification of Skeletal Muscle Tumors in Pediatric Rhabdomyosarcoma: A Report from the Children’s Oncology Group. Arch. Pathol. Lab. Med. 2015, 139, 1281–1287. [Google Scholar] [CrossRef]
- Mod, D.; Mod, H.; Jha, A.K. Oral and dental complications of head and neck radiotherapy and their management. J. Nepal Heal. Res. Counc. 2013, 11. [Google Scholar]
- Ray-Chaudhuri, A.; Shah, K.; Porter, R.J. The oral management of patients who have received radiotherapy to the head and neck region. Br. Dent. J. 2013, 214, 387–393. [Google Scholar] [CrossRef]
- Iatrou, I.; Theologie-Lygidakis, N.; Schoinohoriti, O.; Tzermpos, F.; Vessala, A.-M. Msc Rhabdomyosarcoma of the maxillofacial region in children and adolescents: Report of 9 cases and literature review. J. Cranio-Maxillofacial Surg. 2017, 45, 831–838. [Google Scholar] [CrossRef]
- Choi, D.K.; Schmidt, M.L. Chemotherapy in Children with Head and Neck Cancers. Oral Maxillofac. Surg. Clin. N. Am. 2016, 28, 127–138. [Google Scholar] [CrossRef]
- Bolling, T.; Weege, J.; Eich, H.T.; Timmermann, B.; Meyer, F.-M.; Rübe, C.; Kortmann, R.-D.; Fischedick, K.; Rödel, C.; Koch, R.; et al. Acute and late side effects to salivary glands and oral mucosa after head and neck radiotherapy in children and adolescents. Results of the “Registry for the evaluation of side effects after radiotherapy in childhood and adolescence”. Head Neck 2014, 37, 1137–1141. [Google Scholar] [CrossRef]
- Bonnin, N.; Nezzar, H.; Viennet, A.; Barthelemy, I.; Demeocq, F.; Gabrillargues, J.; Dauplat, M.-M.; Bacin, F. Rhabdomyosarcome palpébral chez un enfant âgé de deux ans. J. Fr. d’Ophtalmol. 2010, 33, 178–184. [Google Scholar] [CrossRef]
- Clement, S.; Schoot, R.; Slater, O.; Chisholm, J.; Abela, C.; Balm, A.; Brekel, M.V.D.; Breunis, W.; Chang, Y.; Fajardo, R.D.; et al. Endocrine disorders among long-term survivors of childhood head and neck rhabdomyosarcoma. Eur. J. Cancer 2016, 54, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hajji, I.; Jellab, B.; Benhaddou, R.; Baki, S. An unusual localization of rhabdomyosarcoma: About a case report. Bull. Soc. Belg. d’Ophtalmol. 2012, 319, 23–28. [Google Scholar]
- Jawad, H.; Hodson, N.A.; Nixon, P.J. A review of dental treatment of head and neck cancer patients, before, during and after radiotherapy: Part 2. Br. Dent. J. 2015, 218, 69–74. [Google Scholar] [CrossRef]
- Paulino, A.C.; Simon, J.H.; Zhen, W.; Wen, B.-C. Long-term effects in children treated with radiotherapy for head and neck rhabdomyosarcoma. Int. J. Radiat. Oncol. 2000, 48, 1489–1495. [Google Scholar] [CrossRef]
- Hoogeveen, R.C.; Hol, M.L.; Pieters, B.R.; Balgobind, B.V.; Berkhout, E.W.; Schoot, R.A.; Smeele, L.E.; Merks, H.J.; Becking, E.A. An overview of radiological manifestations of acquired dental developmental disturbances in paediatric head and neck cancer survivors. Dentomaxillofac. Radiol. 2020, 49, 20190275. [Google Scholar] [CrossRef]
- Borinstein, S.; Steppan, D.; Hayashi, M.; Loeb, D.M.; Isakoff, M.S.; Binitie, O.; Brohl, A.S.; Bridge, J.A.; Stavas, M.; Shinohara, E.T.; et al. Consensus and controversies regarding the treatment of rhabdomyosarcoma. Pediatr. Blood Cancer 2017, 65, e26809. [Google Scholar] [CrossRef] [PubMed]
- Carl, W.; Sako, K.; Schaaf, N.G. Dental complications in the treatment of rhabdomyosarcoma of the oral cavity in children. Oral Surg. Oral Med. Oral Pathol. 1974, 38, 367–371. [Google Scholar] [CrossRef]
- Haupt, R.; Jankovic, M.; Hjorth, L.; Skinner, R. Late effects in childhood cancer survivors and survivorship issues. Epidemiol. Prev. 2013, 37, 266–273. [Google Scholar]
- Gawade, P.L.; Hudson, M.M.; Kaste, S.C.; Neglia, J.; Constine, L.S.; Robison, L.L.; Ness, K.K. A systematic review of dental late effects in survivors of childhood cancer. Pediatr. Blood Cancer 2013, 61, 407–416. [Google Scholar] [CrossRef]
- Effinger, K.E.; Migliorati, C.A.; Hudson, M.M.; McMullen, K.P.; Kaste, S.C.; Ruble, K.; Guilcher, G.M.T.; Shah, A.J.; Castellino, S.M. Oral and dental late effects in survivors of childhood cancer: A Children’s Oncology Group report. Support. Care Cancer 2014, 22, 2009–2019. [Google Scholar] [CrossRef]
- Cubukçu, C.E.; Sevinir, B.; Ercan, I. Disturbed dental development of permanent teeth in children with solid tumors and lymphomas. Pediatr. Blood Cancer 2011, 58, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Kaste, S.C.; Hopkins, K.P.; Bowman, L.C. Dental abnormalities in long-term survivors of head and neck rhabdomyosarcoma. Med Pediatr. Oncol. 1995, 25, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Sonis, A.; Tarbell, N.; Valachovic, R.W.; Gelber, R.; Schwenn, M.; Sallan, S. Dentofacial development in long-term survivors of acute lymphoblastic leukemia: A comparison of three treatment modalities. Cancer 1990, 66, 2645–2652. [Google Scholar] [CrossRef]
- Maciel, J.C.C.; Junior, C.G.D.C.; Brunetto, A.L.; Di Leone, L.P.; Da Silveira, H.E.D. Oral health and dental anomalies in patients treated for leukemia in childhood and adolescence. Pediatr. Blood Cancer 2009, 53, 361–365. [Google Scholar] [CrossRef]
- Mladosievičová, B.; Jurkovič, R.; Izakovičová-Hollá, L. Dentálne abnormality po protinádorovej liečbe v detskom veku. Klin. Onkol. 2015, 28, 20–23. [Google Scholar] [CrossRef] [PubMed]
- de Mattos, V.D.; Ferman, S.; Magalhães, D.M.A.; Antunes, H.S.; Lourenço, S.Q.C. Dental and craniofacial alterations in long-term survivors of childhood head and neck rhabdomyosarcoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 127. [Google Scholar] [CrossRef]
- Gadiwalla, Y.; Patel, V. Osteonecrosis of the jaw unrelated to medication or radiotherapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 446–453. [Google Scholar] [CrossRef]
- Jiang, Y.-M.; Zhu, X.-D.; Qu, S. Incidence of osteoradionecrosis in patients who have undergone dental extraction prior to radiotherapy: A systematic review and meta-analysis. J. Oral Maxillofac. Surg. Med. Pathol. 2014, 26, 269–275. [Google Scholar] [CrossRef]
- Murray, C.G.; Herson, J.; Daly, T.E.; Zimmerman, S. Radiation necrosis of the mandible: A 10-year study. Part II. Dental factors; onset, duration, and management of necrosis. Int. J. Radiat. Oncol. 1980, 6, 549–553. [Google Scholar] [CrossRef]
- Beumer, J.; Harrison, R.; Sanders, B.; Kurrasch, M. Osteoradionecrosis: Predisposing factors and outcomes of therapy. Head Neck Surg. 1984, 6, 819–827. [Google Scholar] [CrossRef]
- Nabil, S.; Samman, N. Incidence and prevention of osteoradionecrosis after dental extraction in irradiated patients: A systematic review. Int. J. Oral Maxillofac. Surg. 2011, 40, 229–243. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, L.B.; Themudo, R.; Botelho, J.; Machado, V. Oral and Dental Abnormalities Caused by a Pediatric Rhabdomyosarcoma Tumor Treatment: A Clinical Case Report. Dent. J. 2020, 8, 59. https://doi.org/10.3390/dj8020059
Lopes LB, Themudo R, Botelho J, Machado V. Oral and Dental Abnormalities Caused by a Pediatric Rhabdomyosarcoma Tumor Treatment: A Clinical Case Report. Dentistry Journal. 2020; 8(2):59. https://doi.org/10.3390/dj8020059
Chicago/Turabian StyleLopes, Luísa Bandeira, Rodrigo Themudo, João Botelho, and Vanessa Machado. 2020. "Oral and Dental Abnormalities Caused by a Pediatric Rhabdomyosarcoma Tumor Treatment: A Clinical Case Report" Dentistry Journal 8, no. 2: 59. https://doi.org/10.3390/dj8020059
APA StyleLopes, L. B., Themudo, R., Botelho, J., & Machado, V. (2020). Oral and Dental Abnormalities Caused by a Pediatric Rhabdomyosarcoma Tumor Treatment: A Clinical Case Report. Dentistry Journal, 8(2), 59. https://doi.org/10.3390/dj8020059







