Characterization and Antimicrobial Susceptibility of Lactococcus lactis Isolated from Endodontic Infections in Ouagadougou, Burkina Faso
Abstract
1. Introduction
2. Materials and Methods
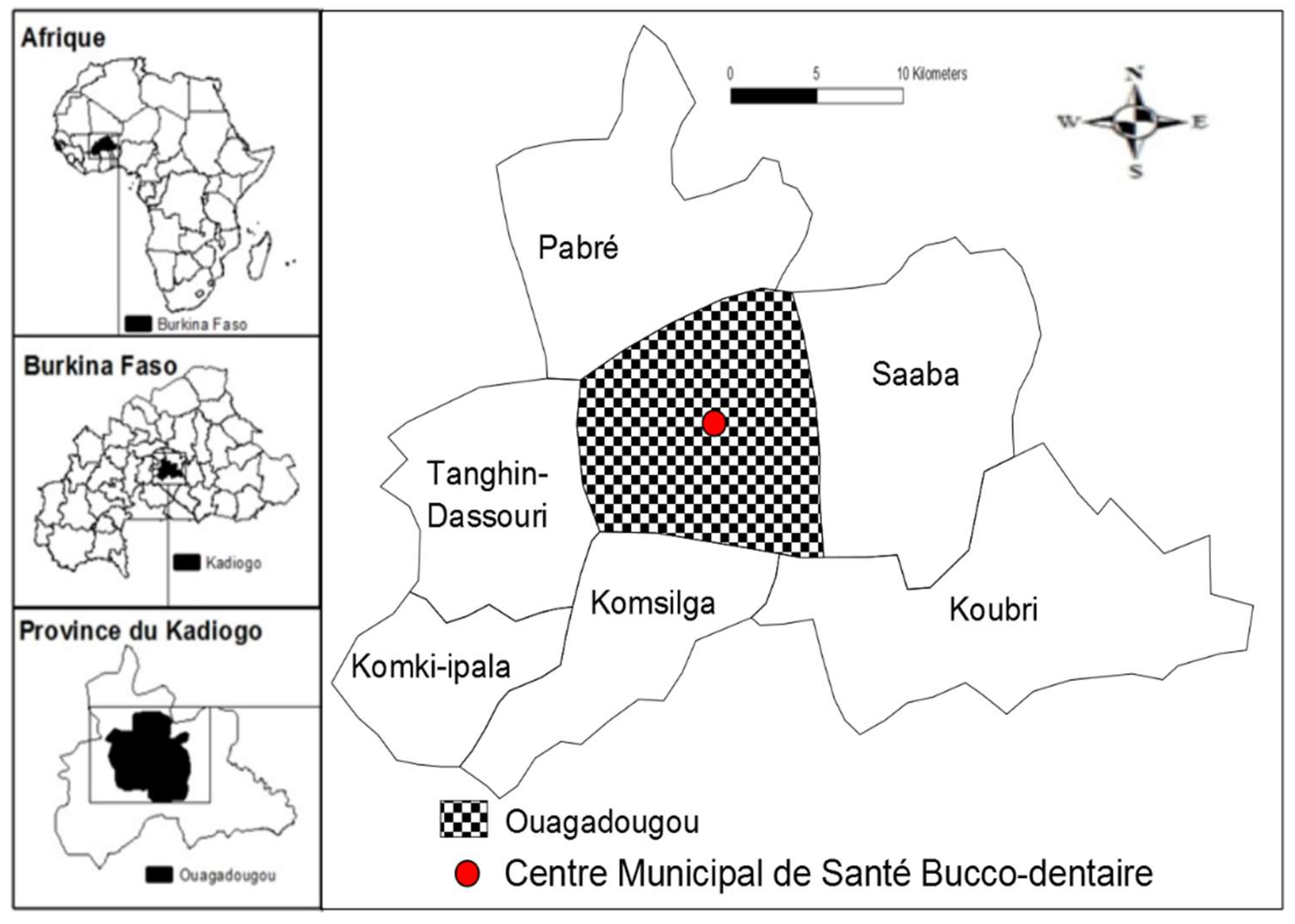
2.1. Study Design, Period and Settings
2.2. Diagnostic Criteria for Endodontic Infections
2.3. Inclusion and Non-Inclusion Criteria
3. Experimental Procedures
3.1. Patient Data Collection
3.2. Sample Collection
3.3. Isolation and Identification of Lactococcus lactis
3.4. Antimicrobial Susceptibility Testing
3.5. Phenotypic Detection of Extended Spectrum ß-Lactamase (ESBL)
3.6. Conservation of Isolated Strains
3.7. Statistical Analyses
3.8. Ethical Considerations
4. Results
4.1. Characteristics of Patients
4.2. Prevalence of Isolated Bacteria
4.3. Antibiotic Susceptibility Profile
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mussano, F.; Ferrocino, I.; Gavrilova, N.; Genova, T.; Dell’Acqua, A.; Cocolin, L.; Carossa, L. Apical periodontitis: Preliminary assessment of microbiota by 16S rRNA high throughput amplicon target sequencing. BMC Oral Health 2018, 18, 55–63. [Google Scholar] [CrossRef]
- Ogle, O.E. Odontogenic Infections. Dent. Clin. N. Am. 2018, 61, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Hellwig, E.; Vespermann, R.; Wittmer, A.; Schmid, M.; Karygianni, L.; Al-Ahmad, A. Comprehensive Analysis of Secondary Dental Root Canal Infections: A Combination of Culture and Culture-Independent Approaches Reveals New Insights. PLoS ONE 2012, 7, e49576. [Google Scholar] [CrossRef] [PubMed]
- Ricucci, D.; Pascon, E.A.; Pitt Ford, T.R.; Langeland, K. Epithelium and bacteria in periapical lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, 239–429. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F.; Rôças, I.N.; Alves, F.R.F.F.; Silva, M.G. Bacteria in the apical root canal of teeth with primary apical periodontitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 107, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hsiao, W.W.L.; Nandakumar, R.; Barbuto, S.M.; Mongodin, E.F.; Paster, B.J.; Fraser-Liggett, C.M.; Fouad, A.F. Analyzing endodontic infections by deep coverage pyrosequencing. J. Dent. Res. 2010, 89, 980–984. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Vannini, L.; La Storia, A.; Laghi, L.; Piombino, P.; Stellato, G.; Serrazanetti, D.I.; Gozzi, G.; Turroni, S.; Ferrocino, I.; et al. The same microbiota and a potentially discriminant metabolome in the saliva of omnivore, ovo-lacto-vegetarian and vegan individuals. PLoS ONE 2014, 9, e112373. [Google Scholar] [CrossRef]
- De Filippis, F.; Parente, E.; Ercolini, D. Metagenomics insights into food fermentations. Microb. Biotechnol. 2017, 10, 91–102. [Google Scholar] [CrossRef]
- Taalé, E.; Savadogo, A.; Zongo, C.; Tapsoba, F.; Karou, S.D.; Traoré, A.S. Les peptides antimicrobiens d’origine microbienne: Cas des bactériocines. Int. J. Biol. Chem. Sci. 2016, 10, 384–399. [Google Scholar] [CrossRef]
- Karaaslan, A.; Soysal, A.; Kadayifci, E.K.; Yakut, N.; Demir, S.O.; Akkoc, G.; Atici, S.; Sarmis, A.; Toprak, N.U.; Bakir, M. Lactococcus lactis spp lactis infection in infants with chronic diarrhea: Two cases report and literature review in children. J. Infect. Dev. Ctries. 2016, 10, 304–307. [Google Scholar] [CrossRef]
- Cristea, A.D.; Preoteasa, C.T.; Popa, M.; Marutescu, L.; Chifiriuc, M.C.; Gheorghe, I.; Suciu, I. In Vitro Testing of Susceptibility to Endodontic Irrigants and Disinfectants of Bacterial Strains Isolated from Chronic Apical Periodontitis. ROM Biotechnol. Lett. 2016, 21, 11218–11225. [Google Scholar]
- Karygianni, L.; Anderson, A.C.; Tennert, C.; Kollmar, K.; Altenburger, M.J.; Hellwig, E.; Al-Ahmad, A. Supplementary sampling of obturation materials enhances microbial analysis of endodontic treatment failures: A proof of principle study. Clin. Oral Investig. 2015, 19, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Vengerfeldt, V.; Špilka, K.; Saag, M.; Preem, J.K.; Oopkaup, K.; Truu, J.; Mändar, R. Highly diverse microbiota in dental root canals in cases of apical periodontitis (data of illumina sequencing). J. Endod. 2014, 40, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, M.; Acuner, I.C.; Uyar, M. Deep neck infection due to Lactococcus lactis cremoris: A case report. Eur. Arch. Otorhinolaryngol. Head Neck 2005, 262, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Ismaili, M.A.; Guilal, J.; Hamama, A.; Saidi, B.; Zah, M. Identification de bactéries lactiques du lait cru de chamelle du sud du Maroc. IJMS 2016, 1, 81–94. [Google Scholar]
- Topçu, Y.; Akıncı, G.; Bayram, E.; Hız, S.; Türkmen, M. Brain abscess caused by Lactococcus lactis cremoris in a child. Eur. J. Pediatr. 2011, 170, 1603–1605. [Google Scholar] [CrossRef]
- Björby, A.; Löe, H. The relative significance of different local factors in the initiation and development of periodontal inflammation. J. Periodontal Res. 1967, 2, 76–77. [Google Scholar]
- Rôcas, I.N.; Siqueira, J.F., Jr. Detection of antibiotic resistance genes in samples from acute and chronic endodontic infections and after treatment. Arch. Oral Biol. 2013, 58, 1123–1128. [Google Scholar] [CrossRef]
- Sharpe, M.E.; Fryer, T.F.; Smith, D.G. Identification of the lactic acid bacteria. In Identification Methods for Microbiologists for Applied Bacteriology Technical Series; Accadmic Press: London, UK, 1979; Volume 2, pp. 233–259. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.M.; Sherries, J.C.; Turck, M. Antibiotic susceptibility testing. Am. J. Pathol. 1966, 45, 493–496. [Google Scholar]
- Comité de l’Antibiogramme de la Société Française de Microbiologie; Recommendations 2017; European Committee on Antimicrobial Susceptibility Testing (CASFM/EUCAST): Basel, Switzerland, 2017; pp. 1–128.
- Kivanç, S.A.; Takim, M.; Kivanç, M.; Güllülü, G. Bacillus Spp. isolated from the conjunctiva and their potential antimicrobial activity against other eye pathogens. Afr. Health. Sci. 2014, 14, 364–371. [Google Scholar] [CrossRef]
- Tarazanova, M.; Huppertz, T.; Kok, J.; Bachmann, H. Altering textural properties of fermented milk by using surface-engineered Lactococcus lactis. Microb. Biotechnol. 2018, 11, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Anas, M.; Zinedine, B.A.; Rizk, H.A.; Eddine, H.J.; Mebrouk, K. Screening of autochthonous Lactobacillus species from Algerian raw goats’ milk for the production of bacteriocin-like compounds against Staphylococcus aureus. Afr. J. Biotechnol. 2012, 11, 4595–4607. [Google Scholar]
- Pierre, T.; Aurélien, L.; Matthieu, R. Innovative treatments for multidrug-resistant bacteria. Bull. Acad. Natl. Med. 2015, 198, 439–456. [Google Scholar]
- Uchida, Y.; Morita, H.; Adachi, S.; Asano, T.; Taga, T.; Kondo, N. Bacteriuml meningitis and septicemia of neonate due to Lactococcus lactis. Pediatr. Int. 2011, 53, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Ouédraogo, Y.; Kaboré, W.A.D.; Konsem, T.; Fall, M.; Millogo, M.; Ouattara, S.; Ouédraogo, D. La carie dentaire: Aspects épidémiologies et thérapeutiques aux services de chirurgie dentaire du Centre Hospitalier Universitaire Yalgado Ouédraogo et du Centre Municipal de Santé Bucco-Dentaire. Odontostomatol Tropicale 2015, 38, 49–55. [Google Scholar]
- Slaoui Hasnaoui, J.; Bendelha, A.; Rahmani, E.L.M.; Chala, S.; Benfdil, F. Perception du «bon» médecin dentiste: Enquête auprès des patients marocains. AOS 2015, 273, 1–10. [Google Scholar] [CrossRef]
- Kaboré, W.A.D.; Ouédraogo, C.D.W.; Millogo, M.; Traoré, A.S.; Barro, N.; Sangaré, L. Les motifs de consultation d’odontologie à Ouagadougou. Rev. Ivoir. Odonto-Stomatol. 2016, 18, 21–27. [Google Scholar]
- Azogui-Levy, S.; Rochereau, T. Pourquoi s’intéresser à la santé bucco-dentaire? Repères épidémiologiques et économiques. La Santé de l’Homme 2012, 417, 5–6. [Google Scholar]
- Hescot, P.; China, E.; Bourgeois, D.; Maina, S.; Monteiro da Silva, O.; Luc Eiselé, J.; Simpson, C.; Horn, V. The FDI African Strategy for Oral Health: Addressing the specific needs of the continent. Int. Dent. J. 2013, 63, 113–120. [Google Scholar] [CrossRef]
- Georgountzos, G.; Michopoulos, C.; Grivokostopoulos, C.; Kolosaka, M.; Vlassopoulou, N.; Lekkou, A. Infective Endocarditis in a Young Adult due to Lactococcus lactis: A Case Report and Review of the Literature G. Case Rep. Med. 2018, 2018, 5091456. [Google Scholar] [CrossRef]
- Lin, K.H.; Sy, C.L.; Chen, C.S.; Lee, C.H.; Lin, Y.T.; Li, J.Y. Infective endocarditis complicated by intracerebral hemorrhage due to Lactococcus lactis subsp. cremoris. Infection 2010, 38, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Rostagno, C.; Pecile, P.; Stefano, P.L. Early Lactococcus lactis endocarditis after mitral valve repair: A case report and literature review. Infection 2013, 41, 897–899. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Nakayama, M.; Nakahira, K.; Nakura, Y.; Kanagawa, N.; Yanagihara, I.; Miyaishi, S. Sudden infant death due to Lactococcal infective endocarditis. Legal Med. 2016, 19, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Mansour, B.; Habib, A.; Asli, N.; Geffen, Y.; Miron, D.; Elias, N. A case of infective endocarditis and pulmonary septic emboli caused by Lactococcus lactis. Case Rep. Pediatr. 2016, 2016, 1024054. [Google Scholar] [PubMed]
- Elliott, J.A.; Facklam, R.R. Antimicrobial susceptibilities of Lactococcus lactis and Lactococcus garvieae and a proposed method to discriminate between them. J. Clin. Microbiol. 1996, 34, 1296–1298. [Google Scholar]
- Mofredj, A.; Bahloul, H.; Chanut, C. Lactococcus lactis: An opportunistic bacterium? Med. Mal. Infect. 2007, 37, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Adjagodo, A.; Tchibozo, M.A.D.; Kelome, N.C.; Lawani, R. Flux des polluants liés aux activités anthropiques, risques sur les ressources en eau de surface et la chaine trophique à travers le monde: Synthèse bibliographique. Int. J. Biol. Chem. Sci. 2016, 10, 1459–1472. [Google Scholar] [CrossRef]
- Bagré, T.S.; Samandoulougou, S.; Traoré, M.; Illy, D.; Bsadjo-Tchamba, G.; Bawa-Ibrahim, H.; Bouda, S.C.; Traoré, A.S.; Barro, N. Détection biologique des résidus d’antibiotiques dans le lait et produits laitiers de vache consommés à Ouagadougou, Burkina Faso. J. Appl. Biosci. 2015, 87, 8105–8112. [Google Scholar] [CrossRef]
- Guessennd, N.K.; Ouattara, M.B.; Ouattara, N.D.; Nevry, R.K.; Gbanon, V.; Tiekoura, K.B.; Dosso, M.; Ger, B.M.R. Étude des bactéries multirésistantes des effluents hospitaliers d’un centre hospitalier et universitaire (CHU) de la ville d’Abidjan (Côte d’Ivoire). J. Appl. Biosci. 2013, 69, 5456–5464. [Google Scholar] [CrossRef]
- Ouédraogo, A.S.; Jean Pierre, H.; Banûls, A.L.; Ouédraogo, R.; Godreuil, S. Émergence et diffusion de la résistance aux antibiotiques en Afrique de l’Ouest: Facteurs favorisants et évaluation de la menace. Med. Sante Trop. 2017, 27, 147–154. [Google Scholar]
- Prasanna, N. Endodontic Microbiology—A Special Issue of Dentistry Journal. Dent. J. 2018, 6, 14. [Google Scholar]
- Wisniewska-Spychala, B.; Sokaiski, J.; Grajek, S.; Jemiellti, M.; Trojnarska, O.; Choroszy-Król, I.; Sójka, A.; Maksymiuk, T. Dentinogenous infectiuos foci-a risk factor of infective endocarditis. Med. Sci. Monit. 2012, 18, 93–104. [Google Scholar] [CrossRef]
- Persac, S.; Prévost, R.; Gigon, H.S.; Peron, J.M. Point actuel sur l’infection focale d’origine buccodentaire. Revue de Stomatologie et de Chirurgie Maxillo-Faciale 2011, 112, 353–359. [Google Scholar] [CrossRef] [PubMed]
| 0 | 1 | 2 | 3 |
|---|---|---|---|
| Absence of tartar, tooth decay or fillings | Caria, scale or shutter close to the gum | Caria, tartar, or filling in contact with the marginal gingiva, a degree of subgingival calculus | Caria, tartar, or filling in the marginal gingiva, abundant subgingival calculus |
| Antibiotics | Susceptibility of Isolates N (%) | |
|---|---|---|
| Resistant (R + I) | Sensitive | |
| Amoxicillin-clavulanic acid | 3 (60) | 2 (40) |
| Ceftriaxone | 4 (80) | 1 (20) |
| Cefixime | 5 (100) | 0 (0) |
| Cefuroxime | 4 (80) | 1 (20) |
| Cefotaxime | 4 (80) | 1 (20) |
| Gentamycin | 2 (40) | 3 (60) |
| Clindamycin | 2 (40) | 3 (60) |
| Metronidazole | 5 (100) | 0 (0) |
| Piperacillin-tazobactam | 2 (40) | 3 (60) |
| Oxacillin | 4 (80) | 1 (20) |
| Spiramycin | 3 (60) | 2 (40) |
| Lincomycin | 2 (40) | 3 (60) |
| Piperacillin | 2 (40) | 3 (60) |
| Tobramycin | 2 (40) | 3 (60) |
| Netilmicin | 3 (60) | 2 (40) |
| Erythromycin | 3 (60) | 2 (40) |
| Trimethoprim-sulfamethoxazole | 3 (60) | 2(40) |
| Chloramphenicol | 4 (80) | 1 (20) |
| Ciprofloxacin | 3 (60) | 2 (40) |
| Penicillin G | 3 (60) | 2 (40) |
| Amoxicillin | 3 (60) | 2 (40) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaboré, W.A.D.; Dembélé, R.; Bagré, T.S.; Konaté, A.; Boisramé, S.; Chevalier, V.; Konsem, T.; Traoré, A.S.; Barro, N. Characterization and Antimicrobial Susceptibility of Lactococcus lactis Isolated from Endodontic Infections in Ouagadougou, Burkina Faso. Dent. J. 2018, 6, 69. https://doi.org/10.3390/dj6040069
Kaboré WAD, Dembélé R, Bagré TS, Konaté A, Boisramé S, Chevalier V, Konsem T, Traoré AS, Barro N. Characterization and Antimicrobial Susceptibility of Lactococcus lactis Isolated from Endodontic Infections in Ouagadougou, Burkina Faso. Dentistry Journal. 2018; 6(4):69. https://doi.org/10.3390/dj6040069
Chicago/Turabian StyleKaboré, Wendpoulomdé Aimé Désiré, René Dembélé, Touwendsida Serge Bagré, Ali Konaté, Sylvie Boisramé, Valérie Chevalier, Tarcissus Konsem, Alfred S. Traoré, and Nicolas Barro. 2018. "Characterization and Antimicrobial Susceptibility of Lactococcus lactis Isolated from Endodontic Infections in Ouagadougou, Burkina Faso" Dentistry Journal 6, no. 4: 69. https://doi.org/10.3390/dj6040069
APA StyleKaboré, W. A. D., Dembélé, R., Bagré, T. S., Konaté, A., Boisramé, S., Chevalier, V., Konsem, T., Traoré, A. S., & Barro, N. (2018). Characterization and Antimicrobial Susceptibility of Lactococcus lactis Isolated from Endodontic Infections in Ouagadougou, Burkina Faso. Dentistry Journal, 6(4), 69. https://doi.org/10.3390/dj6040069





